Development of Fluorous Lewis Acid-Catalyzed Reactions
Abstract
:Contents
- Introduction
- Fluorous Lewis Acid Catalysts
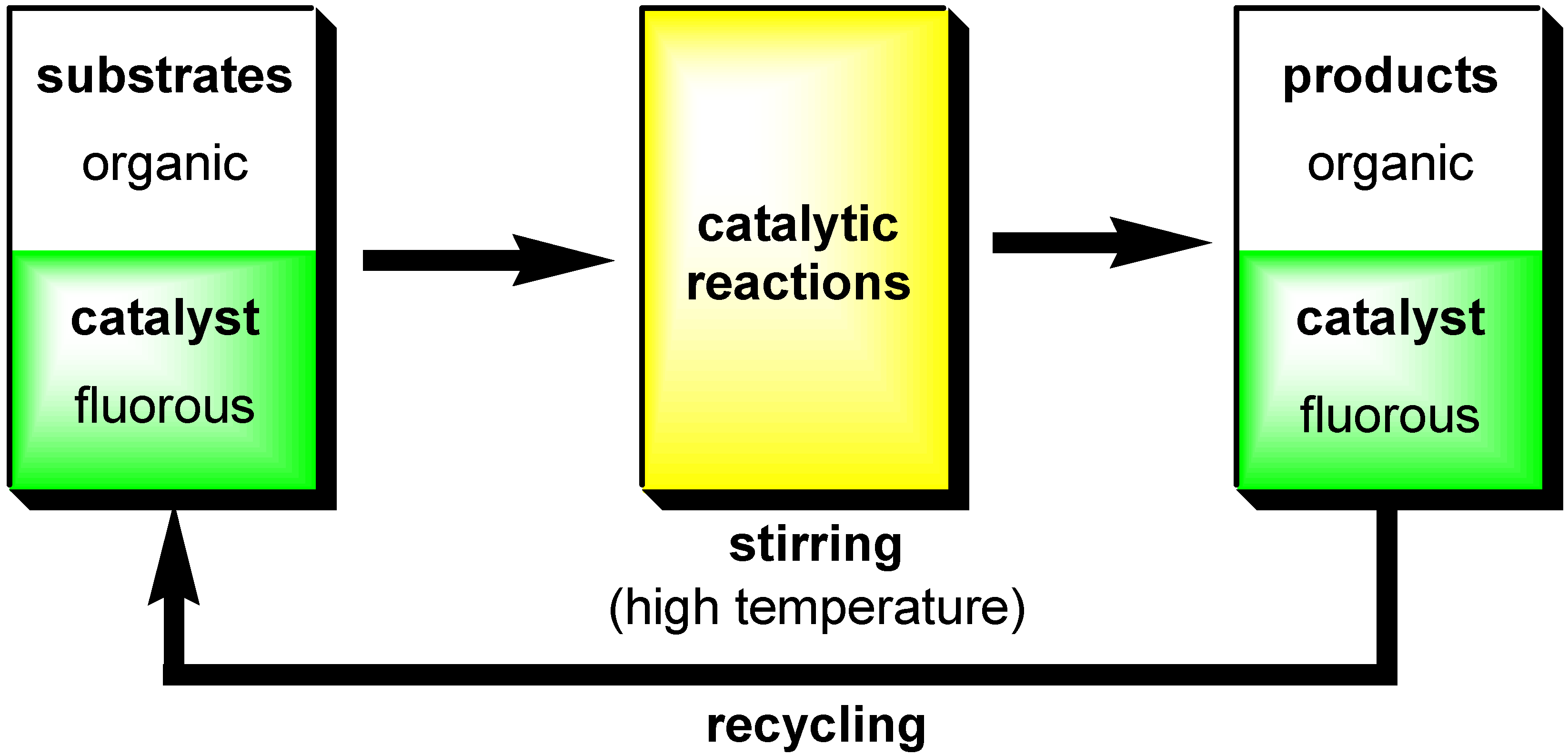
- Fluorous Biphasic Catalysis by Fluorous Lewis Acids
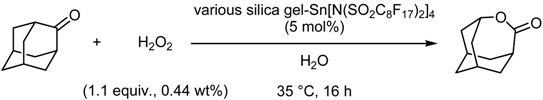
- Fluorous Biphasic Reactions Containing Water
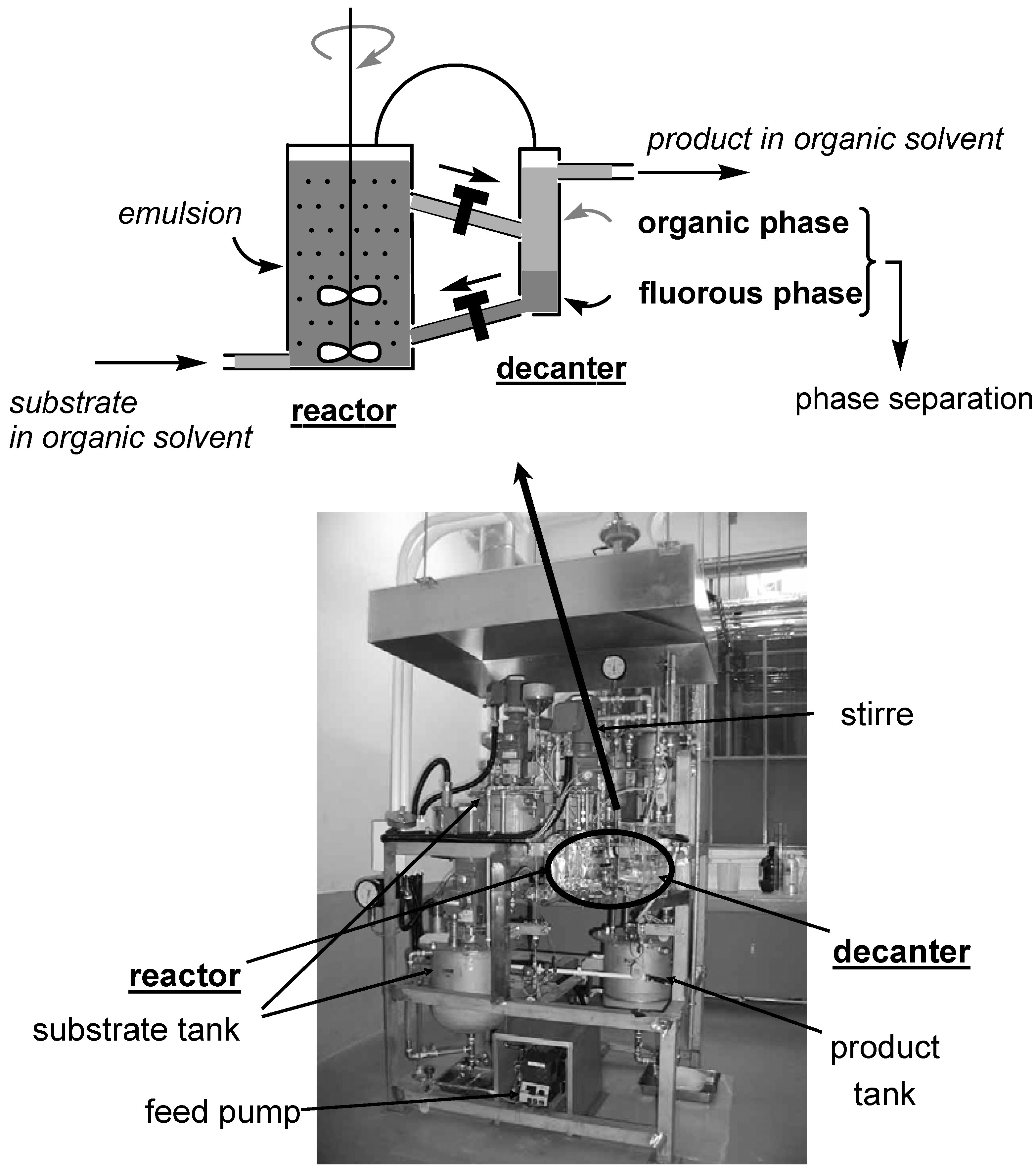
- Continuous-flow Reaction in FBS
- Fluorous Silica Gel-Supported Lewis Acid Catalysts
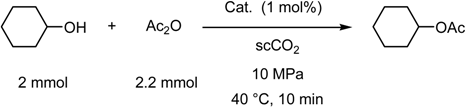
- Supercritical Carbon Dioxide as a Reaction Medium
- Conclusions
Introduction
Fluorous Lewis Acid Catalysts
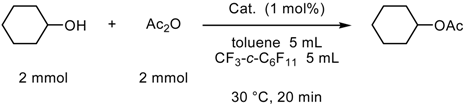
Fluorous Biphasic Catalysis by Fluorous Lewis Acids
| % yieldb,c | ||
| cyclea | Yb[C(SO2-n-C8F17)3]3 | Sc[C(SO2-n-C8F17)3]3 |
| 1 | 99 | 99 (98) |
| 2 | 99 (96) | 100 (98) |
| 3 | 98 | 99 |
| 4 | 99 | 99 |
| 5 | 99 (96) | 100 (98) |
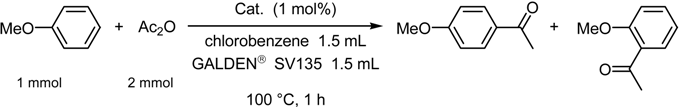
| catalyst | % yielda | ratio (p / o)a |
| Hf[N(SO2-n-C8F17)2]4 | 80 | 100 : 0 |
| Hf[N(SO2-n-C8F17)2]4b | 92 | >99 : <1 |
| Hf(OTf)4 | 49 | 97 : 3 |
| Sc(OTf)3 | 45 | 98 : 2 |
| Yb(OTf)3 | 16 | 97 : 3 |
| AlCl3c | 2 | 100 : 0 |
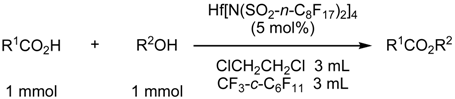
Fluorous Biphasic Reactions Containing Water
| R1CO2H | R2OH | conditions | % yielda | % selectivityb |
| AcOH | c-C6H11OH | 50 °C, 8 h | 82 | 98 |
| c-C6H11CO2H | n-C4H9OH | 70 °C, 15 h | 92 | 97 |
| c-C6H11CO2H | PhCH2OH | 50 °C, 24 h | 89 | 98 |
| c-C6H11CO2H | c-C6H11OH | 50 °C, 24 h | 55 | 98 |
| PhCO2H | n-C4H9OH | 90 °C, 15 hc | 85 | 96 |
| CH2=CH(CH3)CO2H | CH3OHd | 60 °C, 8 h | 86 | 98 |
| catalyst | cyclea | % yieldb,c | % selectivityd |
| Sn[N(SO2-n-C8F17)2]4 | 1 | 93 (91) | 99 |
| 4 | 93 | 99 | |
| Sn[N(SO2-n-C8F17)2]4e | 1 | 68 | 80 |
| Sn[N(SO2-n-C8F17)2]2 | 1 | 48 | 83 |
| Sn(OTf)2 | 1 | 37 | 87 |
| Hf[N(SO2-n-C8F17)2]4 | 1 | 82 | 92 |
| Hf(OTf)4 | 1 | 41 | 91 |
| Sc[N(SO2-n-C8F17)2]3 | 1 | 53 | 69 |
| Sc(OTf)3 | 1 | 31 | 66 |
Continuous-flow Reaction in FBS

Fluorous Silica Gel-Supported Lewis Acid Catalysts
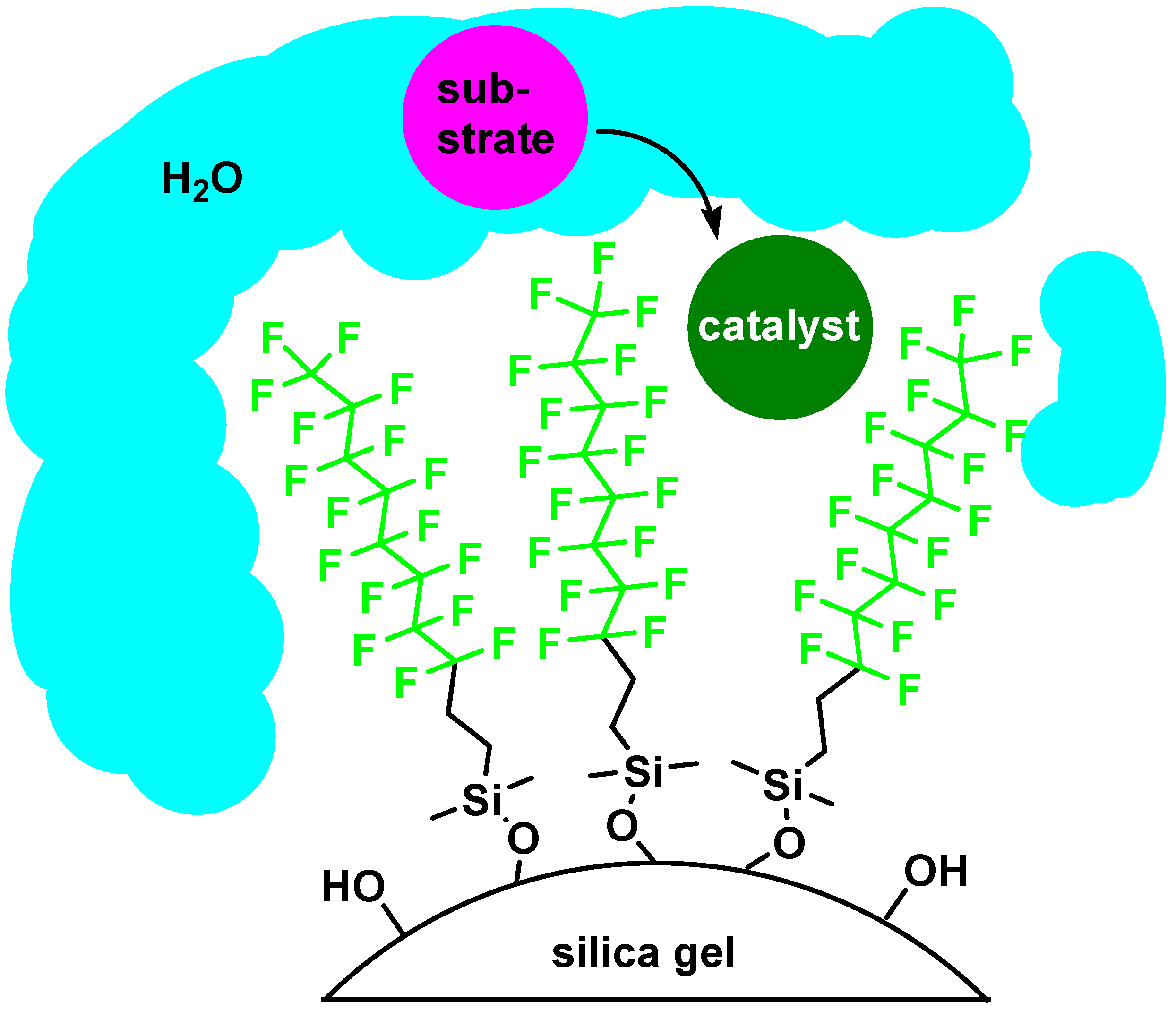
| catalyst | cyclea | % yieldb,c | % selectivityc,d |
| fluorous SiO2-Sn[N(SO2-n-C8F17)2]4 | 1 | 79 (87) | 100 (89) |
| 2 | 75 (89) | 97 (92) | |
| 3 | 73 (86) | 96 (90) | |
| 4 | 71 (88) | 91 (93) | |
| ODS SiO2-Sn[N(SO2-n-C8F17)2]4 | 1 | 60 | 88 |
| 2 | 41 | 84 | |
| 3 | 28 | 82 | |
| SiO2-Sn[N(SO2-n-C8F17)2]4 | 1 | 43 | 93 |
| 2 | 34 | 100 | |
| 3 | 24 | 86 |
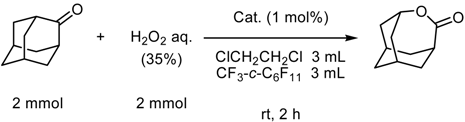
Supercritical Carbon Dioxide as a Reaction Medium
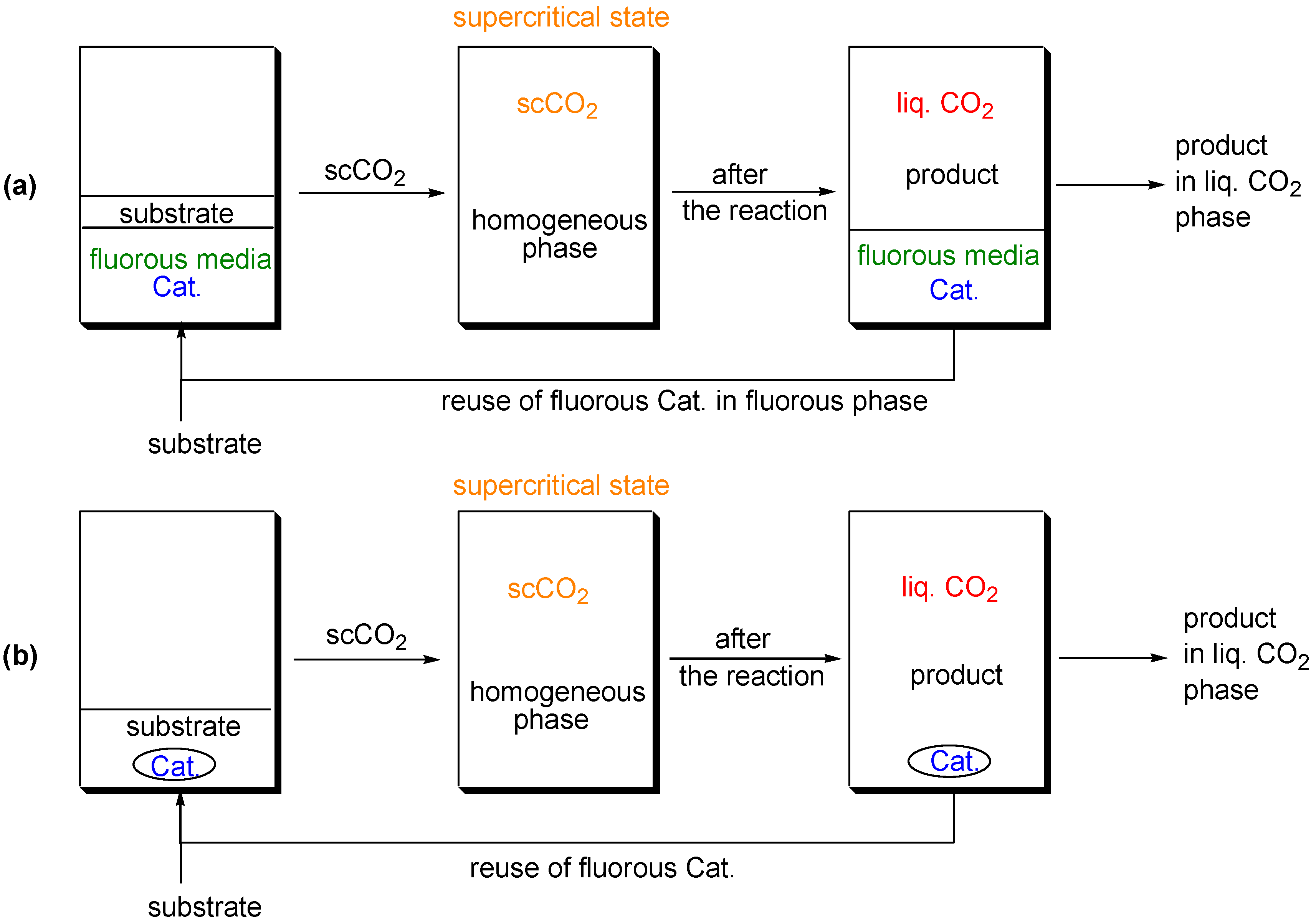
(a) Supercritical carbon dioxide/fluorous solvent system
| cyclea | % yieldb,c |
| 1 | 99 (98) |
| 2 | 99 |
| 3 | 99 |
| 4 | 99 |
| 5 | 99 |
(b) Supercritical carbon dioxide system
| % yieldb,c | ||
| cyclea | Yb[N(SO2-n-C8F17)2]3 | Sc[C(SO2-n-C8F17)3]3 |
| 1 | 99 (98) | 99 (98) |
| 2 | 99 | 100 (98) |
| 3 | 98 | 99 |
| % yieldb,c | ||
| cyclea | Yb[N(SO2-n-C8F17)2]3 | Sc[C(SO2-n-C8F17)3]3 |
| 1 | 79 (77) | 82 (79) |
| 2 | 77 | 80 |
| 3 | 77 | 79 |
Conclusions
Acknowledgements
References and Notes
- For reviews see: a)Handbook of Fluorous Chemistry; Gladysz, J. A.; Curran, D. P.; Horváth, I. T. (Eds.) Wiley-VCH: Weinheim, 2004.; b)Fluorous Chemistry; Otera, J. (Ed.) CMC Publishing: Tokyo, 2005..
- Horváth, I. T.; Rábai, J. Science 1994, 266, 72–75.
- Chem. Eng. News 2005, 39.
- For review: Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W. W. L. Chem. Rev. 2002, 102, 2227–2302..
- Koppel, I. A.; Taft, R. W.; Anvia, F.; Zhu, S.-Z.; Hu, L.-Q.; Sung, K.-S.; DesMarteau, D. D.; Yagupolskii, L. M.; Yagupolskii, Y. L.; Ignat’ev, N. V.; Kondratenko, N. V.; Volkonskii, A. Y.; Vlasov, V. M.; Notario, R.; Maria, P.-C. J. Am. Chem. Soc. 1994, 116, 3047–3057.
- Nishikido, J.; Nakajima, H.; Saeki, T.; Ishii, A.; Mikami, K. Synlett 1998, 1347–1348.Nishikido, J.; Yamamoto, F.; Nakajima, H.; Mikami, Y.; Matsumoto, Y.; Mikami, K. Synlett 1999, 1990–1992.
- For reviews: Nishikido, J.; Yoshida, A. J. Synth. Org. Chem., Jpn. 2005, 63, 144–153.; b)Yoshida, A.; Hao, X.; Yamazaki, O.; Yamada, I.; Nishikido, J. Fluorous Chemistry; Otera, J., Ed.; CMC Publishing: Tokyo, 2005; pp. 185–199. [Google Scholar].
- Mikami, K.; Mikami, Y.; Matsumoto, Y.; Nishikido, J.; Yamamoto, F.; Nakajima, H. Tetrahedron Lett. 2001, 42, 289–292.Mikami, K.; Mikami, Y.; Matsuzawa, H.; Nishikido, J.; Yamamoto, F.; Nakajima, H. Tetrahedron 2002, 58, 4015–4021.Nishikido, J.; Yamamoto, F.; Nakajima, H. U. S. Patent 6436866, 2002.
- Hao, X.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2005, 46, 2697–2700.
- GALDEN® SV135 is a fluorous solvent containing ether oxygen atoms, purchased from Solvay Solexis K.K. (http://www.solvaysolexis.com/).
- Yoshida, A.; Hao, X.; Nishikido, J. Green Chem. 2003, 5, 554–557.
- For reviews: a)Otera, J. Esterification; Wiley-VCH: Weinheim, 2003. [Google Scholar]; Otera, J. Chem. Rev. 1993, 93, 1449–1470..
- Hao, X.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2004, 45, 781–785.
- Hao, X.; Yamazaki, O.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2003, 44, 4977–4980.Hao, X.; Yamazaki, O.; Yoshida, A.; Nishikido, J. Green Chem. 2003, 5, 524–528.
- Yoshida, A.; Hao, X.; Nishikido, J. 1st International Symposium on Fluorous Technologies (ISoFT’05), Bordeaux, July 2005; p. 59.
- Yamazaki, O.; Hao, X.; Yoshida, A.; Nishikido, J. Tetrahedron Lett. 2003, 44, 8791.Yoshida, A.; Hao, X.; Yamazaki, A.; Nishikido, J. 1st International Symposium on Fluorous Technologies (ISoFT’05), Bordeaux, July 2005; p. 8.
- Nishikido, J.; Nanbo, M.; Yoshida, A.; Nakajima, H.; Matsumoto, Y.; Mikami, K. Synlett 2002, 1613–1616.
- Tzschucke, C. H.; Markert, C.; Glatz, H.; Bannwarth, W. Angew. Chem. Int. Ed. 2002, 41, 4500–4503. [CrossRef]
- For reviews: Oakes, R. S.; Clifford, A. A.; Rayner, C. M. J. Chem. Soc. Perkin Trans. 1 2001, 917–941.; Jessop, P. G.; Ikariya, T.; Noyori, R. Chem. Rev. 1999, 99, 475–494.; Ikariya, T.; Noyori, R. Transition Metal Catalysed Reactions 1999, 1–28.; d)Jessop, P. G.; Leitner, W. Chemical Synthesis Using Supercritical Fluids; Wiley-VCH: Weinheim, 1999. [Google Scholar]
- For recent examples: Franciò, G.; Wittmann, K.; Leitner, W. J. Organomet. Chem. 2001, 621, 130–142.; Osuna, A. M. B.; Chen, W.; Hope, E. G.; Kemmitt, R. D. W.; Paige, D. R.; Stuart, A. M.; Xiao, J.; Xu, J. J. Chem. Soc., Dalton Trans. 2000, 4052–4055.; Sellin, M. F.; Cole-Hamiltone, D. J. J. Chem. Soc., Dalton Trans. 2000, 1681–1683.; Meehan, N. J.; Sandee, A. J.; Kamer, P. C. J.; van Leeuwen, P. W. N.; Poliakoff, M. Chem. Commum. 2000, 1497–1498.; Palo, D. R.; Erkey, C. Organometallics 2000, 19, 81–86.; Franciò, G.; Leitner, W. Chem. Commun. 1999, 1663.; Koch, D.; Leitner, W. J. Am. Chem. Soc. 1998, 120, 13398–13404..
- Brown, R. A.; Pollet, P.; McKoon, E.; Eckert, C. A.; Liotta, C. L.; Jessop, P. G. J. Am. Chem. Soc. 2001, 123, 1254–1255.Lange, S.; Trautner, P.; Woelk, K.; Leitner, W. Chirality 2000, 12, 450–457.Kainz, S.; Brinkmann, A.; Leitner, W.; Pfaltz, A. J. Am. Chem. Soc. 1999, 121, 6421–6429.Xiao, J.; Nefkens, S. C. A.; Jessop, P. G.; Ikariya, T.; Noyori, R. Tetrahedron Lett. 1996, 37, 2813–2816.Burk, M. J.; Feng, S.; Gross, M. F.; Tumas, W. J. Am. Chem. Soc. 1995, 117, 8277–8278.
- For Lewis acid-catalyzed reactions in supercritical fluids, see: Komoto, I.; Kobayashi, S. Org. Lett. 2002, 4, 1115–1118.; Fukuzawa, S.-I.; Metoki, K.; Komuro, Y.; Funazukuri, T. Synlett 2002, 134–136.; Komoto, I.; Kobayashi, S. Chem. Commum. 2001, 1842–1843.; Fukuzawa, S.-I.; Matsuzawa, H.; Metoki, K. Synlett 2001, 709–711.; Tsuchiya, T.; Odashima, K.; Kobayahi, S. Chem. Lett. 2000, 178–179.; Kawada, A.; Mitamura, S.; Matsuo, J.-I.; Tsuchiya, T.; Kobayashi, S. Bull. Chem. Soc. Jpn. 2000, 73, 2325–2333.; Mikami, K.; Matsukawa, S.; Kayaki, Y.; Ikariya, T. Tetrahedron Lett. 2000, 41, 1931–1934.; Oakes, R. S.; Matsukawa, S.; Heppenstall, T. J.; Shezad, N.; Clifford, A.A.; Rayner, C. M. Chem. Commum. 1999, 1459–1460..
- Nishikido, J.; Kamishima, M.; Matsuzawa, H.; Mikami, K. Tetrahedron 2002, 58, 8345–8349. [CrossRef]
- Fluorous catalysts as a solid in organic solvents were reviewed, see: Wende, M.; Meier, R.; Gladysz, J. A. J. Am. Chem. Soc. 2001, 123, 11490–11491.; Ishihara, K.; Kondo, S.; Yamamoto, H. Synlett 2001, 1371–1374..
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yoshida, A.; Hao, X.; Yamazaki, O.; Nishikido, J. Development of Fluorous Lewis Acid-Catalyzed Reactions. Molecules 2006, 11, 627-640. https://doi.org/10.3390/11080627
Yoshida A, Hao X, Yamazaki O, Nishikido J. Development of Fluorous Lewis Acid-Catalyzed Reactions. Molecules. 2006; 11(8):627-640. https://doi.org/10.3390/11080627
Chicago/Turabian StyleYoshida, Akihiro, Xiuhua Hao, Osamu Yamazaki, and Joji Nishikido. 2006. "Development of Fluorous Lewis Acid-Catalyzed Reactions" Molecules 11, no. 8: 627-640. https://doi.org/10.3390/11080627