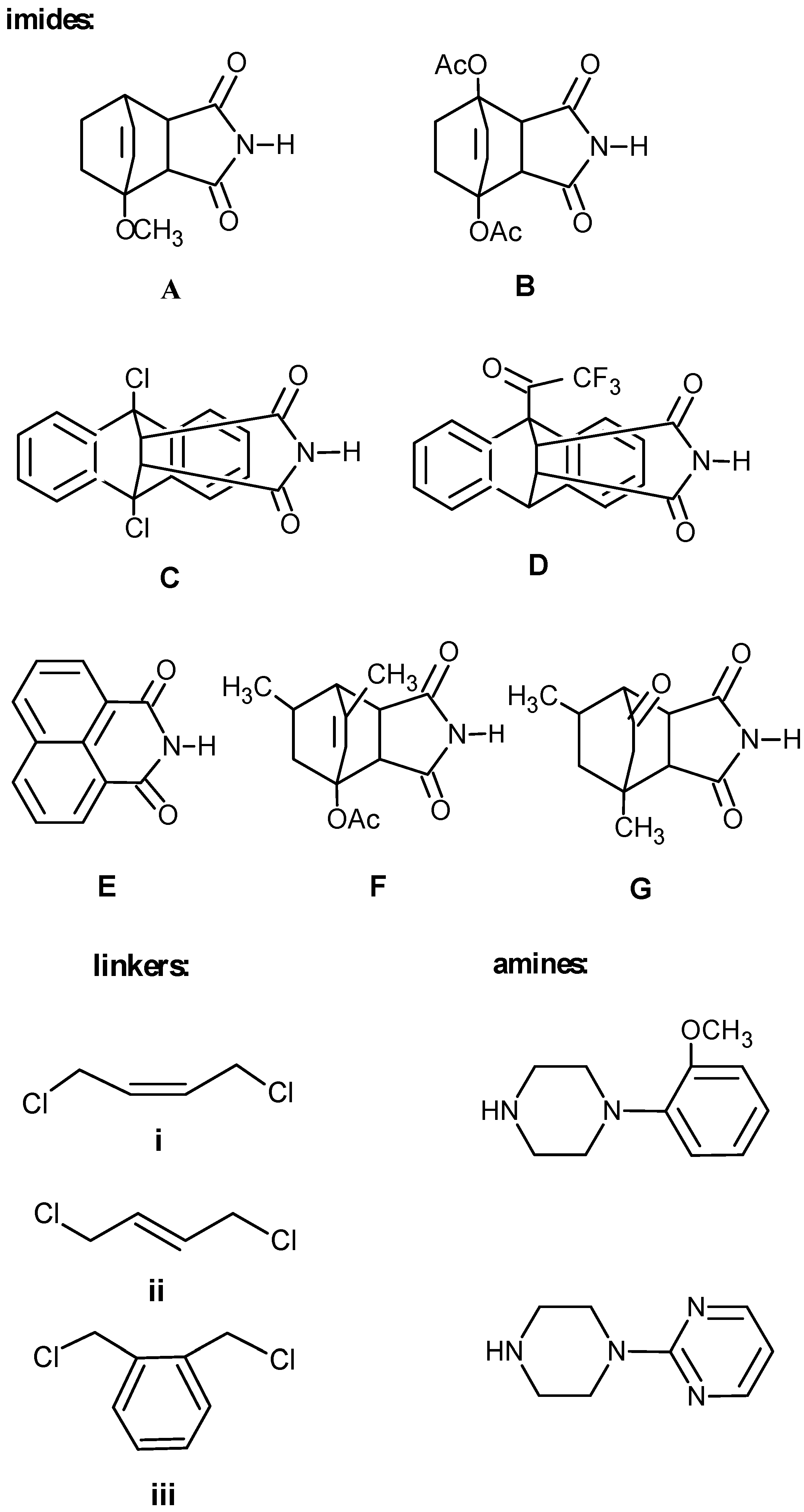
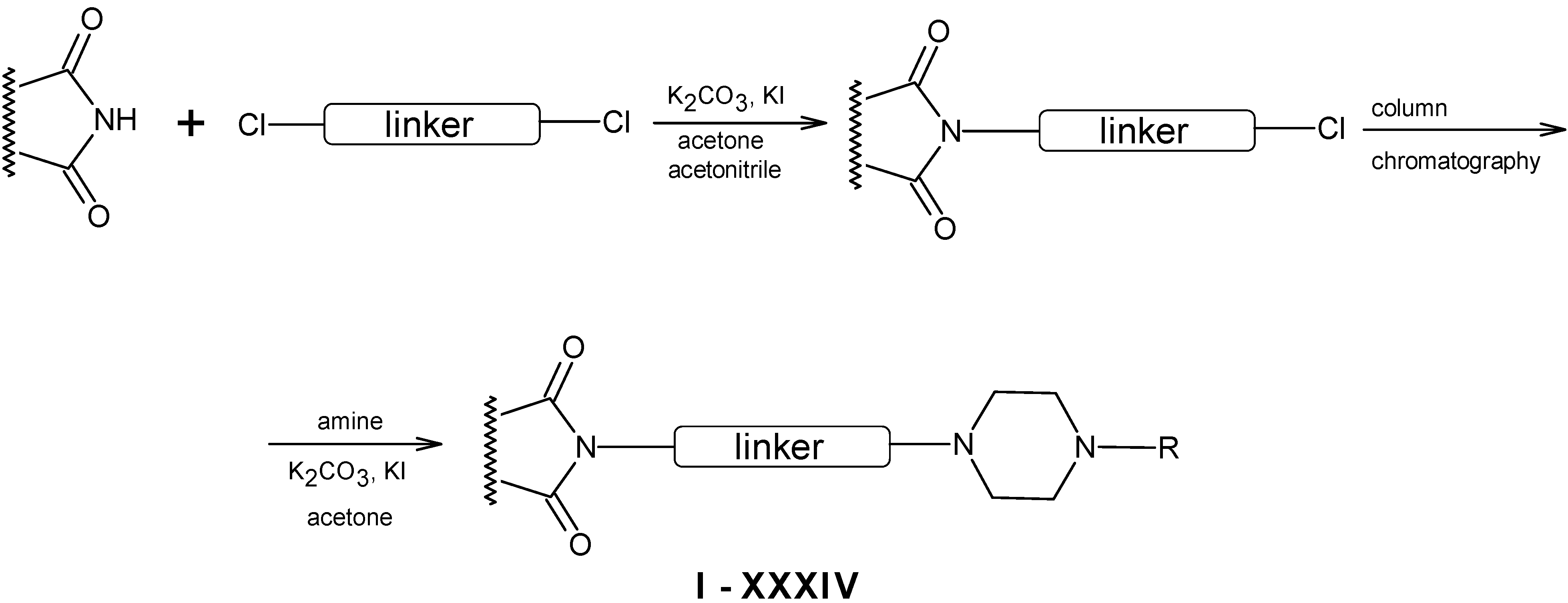
Synthesis of aryl- and heteroarylpiperazinyl N-substituted imide derivatives
1-(2-Methoxyphenyl)piperazine or 1-(2-pyrimidinyl)piperazine (0.01 mol) were added to a mixture of N-chloroalkenylimide (0.01 mol), powdered anhydrous K2CO3 (0.01 mol), and a catalytic amount of KI in acetone (30 mL) The reaction mixture was stirred at a room temperature for 24 h. After the reaction completion the inorganic residue was filtered off and the solvent was evaporated. The crude compound obtained was purified by flash chromatography (eluents: chloroform or chloroform-methanol 100:0.2). All new derivatives were converted into their corresponding hydrochlorides with ethereal HCl and recrystallized from methanol.
4-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (I): m.p. 166 °C; 1H-NMR δ (ppm): 10.63 (s, 1H, NH+), 7.04-6.09 (m, 4H, Harom.), 6.07-6.01 (m, 2H, C8-H, C9-H), 5.76-5.74 (m, 1H, C2’-H), 5.60-5.54 (m, 1H, C3’-H), 4.05 (d, 2H, C4’-H, 3J=8.0), 3.95 (m, 2H, C1’-H), 3.79 (s, 3H, OCH3), 3.52-3.45 (m, 4H, C5’-H, C7’-H), 3.36 (s, 3H, C1‑OCH3), 3.27-3.29 (m, 1H, C2-H), 3.21-3.16 (m, 2H, C6’-H), 3.08-3.06 (m, 1H, C6-H), 3.02-2.92 (m, 3H, C7-H, C8’-H), 1.94-1.87 (m, 1H, C10-H), 1.74-1.71 (m, 1H, C10-H), 1.41-1.34 (m,1H, C11‑H), 1.23-1.20 (m, 1H, C11-H); Anal. Calc. for C26H33N3O4·HCl·2H2O: 59.59 % C, 7.31 % H, 8.01% N; found: 59.01 % C, 6.86 % H, 7.97 % N.
4-{(2Z)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (II): m.p. 230 °C; 1H-NMR δ (ppm): 11.53 (s, 1H, NH+), 8.45 (d, 2H, 3J=4.0, C9’-H, C11’-H), 6.77 (t, 1H, 3J=4.4, 4.8, C10’-H), 6.08-6.00 (m, 2H, C8-H, C9-H), 5.80-5.75 (m, 1H, C2’-H), 5.59-5.55 (m, 1H, C3’-H), 4.72 (d, 2H, 3J=14, C4’-H), 4.01 (d, 2H, 3J=6.8, C1’-H), 3.89 (m, 2H, C5’‑H, C7’-H), 3.47-3.38 (m, 4H, C5’-H, C6’-H, C7’-H), 3.35 (s, 3H, C1-OCH3), 3.28-3.26 (m, 1H, C2-H), 3.07-3.01 (m, 1H, C6-H, C8’-H), 2.91 (m, 1H, C7-H), 1.91-1.87 (m, 1H, C10-H), 1.73-1.68 (m, 1H, C10-H), 1.40-1.33 (m, 1H, C11-H), 1.22-1.16 (m, 1H, C11-H); Anal. Calc. for C23H29N5O3· HCl·2H2O: 60.06 % C, 6.57 % H, 15.23% N; found: 59.98 % C, 6.63 % H, 15.07 % N.
4-{(2Z)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (III): m.p. 153 °C; 1H-NMR δ (ppm): 11.21 (s, 1H, NH+), 7.03-6.88 (m, 4H, Harom.), 6.18-6.12 (m, 2H, C8-H, C9-H), 5.86-5.79 (m, 1H, C2’-H), 5.65-5.57 (m, 1H, C3’-H), 3.94-3.93 (d, 2H, 3J=4.4, C4’-H), 3.78 (s, 3H, OCH3), 3.73 (m, 2H, C5’-H, C7’-H), 3.48 (m, 2H, C1’-H), 3.36 (s, 3H, C1‑OCH3), 3.33-3.28 (m, 4H, C5’-H, C6’-H, C7’-H), 3.09-3.07 (m, 4H, C2-H, C6-H, C8’-H), 3.00 (m, 1H, C7-H), 1.92-1.97 (m, 1H, C10-H), 1.74-1.69 (m, 1H, C10-H), 1.41-1.35 (m, 1H, C11-H), 1.24-1.18 (m, 1H, C11-H); ESI MS: m/z = 451.56:452.2 (100%).
4-{(2E)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (IV): m.p.147-152 °C; 1H-NMR δ (ppm): 11.29 (s, 1H, NH+), 8.45-8.44 (d, 2H, 3J=4.4, C9’‑H, C11’-H), 6.76 (t, 1H, 3J=4.8, C10’-H), 6.15-6.09 (m, 2H, C8-H, C9-H), 5.82-5.76 (m, 1H, C2’‑H), 5.64-5.56 (m, 1H, C3’-H), 4.68 (d, 2H, 3J=14, C4’-H), 3.93-3.92 (m, 2H, C1’-H), 3.70 (m, 2H, C5’-H, C7’-H), 3.40-3.27 (m, 7H, C1-OCH3, C5’-H, C6’-H, C7’-H), 3.29-3.27 (m, 1H, C2-H), 3.08-3.06 (m, 1H, C6-H), 2.92-2.88 (m, 2H, C7-H, C8’-H), 1.93-1.87 (m, 1H, C10-H), 1.74-1.69 (m, 1H, C10-H), 1.41-1.35 (m, 1H, C11-H), 1.24-1.17 (m, 1H, C11-H); ESI MS: m/z = 423.51:424.4 (100%), 446.6 (5%).
4-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (V): m.p.189 °C (for HCl); 1H-NMR (free base) δ (ppm): 7.17 (m, 4H, C7’-H, C8’-H, C9’-H, C10’-H), 7.01-6.96 (m, 1H, C11’-H), 6.91-6.90 (m, 2H, C12’-H, C13’-H), 6.84 (m, 1H, C14’‑H), 6.18-6.08 (m, 1H, C8-H), 6.05-6.02 (m, 1H, C9-H), 4.88-4.79 (m, 4H, C1’-H, C2’-H), 3.85 (s, 3H, OCH3), 3.69 (m, 2H, C3’-H, C5’-H), 3.50 (s, 3H, C1-OCH3), 3.14-2.88 (m, 5H, C2-H, C6-H, C7-H, C3’-H, C5’-H), 2.61 (m, 4H, C4’-H, C6’-H), 1.85-1.68 (m, 2H, C10-H), 1.55-1.47 (m, 2H, C11-H); Anal. Calc. for C30H35N3O4·6H2O (free base): 59.12 % C, 5.78 % H, 6.89% N; found: 59.35 % C, 5.44 % H, 6.45 % N; ESI MS: m/z = 501.2:502.2 (100%).
4-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-1-methoxy-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (VI): m.p. 163 °C; 1H-NMR δ (ppm): 10.32 (s, 1H, NH+), 8.01 (d, 2H, 3J=4.4, C7’-H, C10’‑H), 7.26-7.25 (d, 1H, 3J=7.2, C11’-H), 7.00-6.92 (m, 2H, C8’-H, C9’-H), 6.70-6.68 (d, 1H, 3J=7.2, C13’‑H), 6.32 (t, 1H, 3J=4.8, C12’-H), 5.61-5.53 (m, 2H, C8-H, C9-H), 4.30-4.24 (m, 4H, C1’‑H, C2’‑H), 4.08 (m, 2H, C3’-H, C5’-H), 3.17 (m, 3H, C1-OCH3, C3’-H, C5’-H), 2.99-2.91 (m, 4H, C4’‑H, C6’-H), 2.77-2.69 (m, 3H, C2-H, C6-H, C7-H), 1.49-1.45 (m, 1H, C10-H), 1.31-1.25 (m, 1H, C10-H), 0.97-0.91 (m, 1H, C11-H), 0.79-0.74 (m, 1H, C11-H); Anal. Calc. for C27H31N5O3· HCl·½H2O: 63.58 % C, 6.32 % H, 13.73% N; found: 62.27 % C, 6.43 % H, 13.41 % N; ESI MS: m/z = 473.5:474.2 (100%).
4-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (VII): m.p. 206 °C; 1H-NMR δ (ppm): 10.88 (s, 1H, NH+), 7.00-6.90 (m, 4H, Harom.), 6.14 (s, 2H, C8-H, C9-H), 5.78 (m, 1H, C2’-H), 5.60 (m, 1H, C3’-H), 4.08 (d, 2H, 2J=8.0, C4’‑H), 3.96 (m, 4H, C2-H, C6-H, C5’-H, C6’-H), 3.79 (s, 3H, OCH3), 3.49 (m, 4H, C1’-H, C5’-H, C7’‑H), 3.18 (m, 2H, C6’-H), 3.02 (m, 2H, C8’-H), 2.41 (d, 1H, 2J=5.6, C10-H), 2.07 (m, 6H, C1‑OAc, C7-OAc), 1.72 (d, 1H, 2J=6.8, C11-H); ESI MS: m/z = 537.6:532.5 (100%).
4-{(2Z)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (VIII): m.p. 215 °C; 1H-NMR δ (ppm): 11.83 (s, 1H, NH+), 8.46-8.45 (m, 2H, C9’-H, C11’‑H), 6.79-6.77 (m, 1H, C10’-H), 6.13 (s, 2H, C8-H, C9-H), 5.89-5.79 (m, 1H, C2’-H), 5.64-5.5 (m, 1H, C3’-H), 4.72-4.69 (m, 2H, C5’-H, C7’-H), 4.03-4.02 (m, 2H, C4’-H), 3.96 (m, 2H, C2-H, C6‑H), 3.89 (m, 2H, C1’-H), 3.46-4.34 (m, 4H, C6’-H, C8’-H), 3.04-3.01 (m, 2H, C5’-H, C7’-H), 2.4-2.39 (m, 2H, C10-H), 2.07 (m, 6H, C1-OAc, C7-OAc), 1.73-1.71 (m, 2H, C11-H); ESI MS: m/z = 509.5:510.2 (100%).
4-{(2Z)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (IX): m.p. 168 °C; 1H-NMR δ (ppm): 11.48 (s, 1H, NH+), 7.04-6.88 (m, 4H, Harom.), 6.26 (s, 2H, C8-H, C9-H), 5.86-5.80 (m, 1H, C2’-H), 5.66-5.59 (m, 1H, C3’-H), 3.97 (s, 2H, C2-H, C6-H), 3.94 (m, 2H, C4’-H), 3.78 (s, 3H, OCH3), 3.72 (m, 2H, C1’-H), 3.49-3.47 (m, 2H, C5’‑H, C7’-H), 3.38-3.33 (m, 2H, C5’-H, C7’-H), 3.15-3.05 (m, 4H, C6’-H, C8’-H), 2.40 (d, 2H, 3J=6.4, C10-H), 2.07 (s, 6H, C1-OAc, C7-OAc), 2.72 (d, 2H, 3J=6.8, C11-H); ESI MS: m/z = 537.3:538.2 (100%).
4-{(2E)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (X): m.p. 198 °C; 1H-NMR δ (ppm): 11.80 (s, 1H, NH+), 8.45-8.44 (d, 2H, 3J=4.4, C9’‑H, C11’-H), 6.77 (t, 1H, 3J=4.8, C10’-H), 6.24 (s, 2H, C8-H, C9-H), 5.83-5.76 (m, 1H, C2’-H), 5.65-5.58 (m, 1H, C3’-H), 3.97 (s, 2H, C2-H, C6-H), 4.67 (d, 2H, 3J=14, C4’-H), 3.96 (s, 2H, C2-H, C6-H), 3.98-3.82 (m, 2H, C1’-H), 3.70 (m, 2H, C5’-H, C7’-H), 3.45-3.34 (m, 4H, C6’-H, C8’-H), 2.94-2.87 (m, 2H, C5’-H, C7’-H), 2.39 (d, 2H, 3J=6.8, C10-H), 2.07 (s, 6H, C1-OAc, C7-OAc), 2.72 (d, 2H, 3J=6.8, C11-H); ESI MS: m/z = 509.5:510.2 (100%), 532.2 (15%).
4-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (XI): m.p. 215 °C; 1H-NMR δ (ppm): 7.58-7.56 (m, 1H, C7’-H), 7.46-7.38 (m, 1H, C10’-H), 7.22-7.19 (m, 1H, C8’-H), 7.13-7.11 (m, 1H, C9’-H), 7.03-6.95 (m, 2H, C11’-H, C14’‑H), 6.92-6.87 (m, 2H, C12’, C13’-H), 6.15-6.12 (m, 2H, C8-H, C9-H), 4.70-4.68 (m, 1H, C2’-H), 4.55-4.48 (m, 2H, C1’-H), 4.07 (s, 1H, C2-H), 4.01 (s, 1H, C6-H), 3.77 (s, 3H, OCH3), 3.55-3.33 (m, 6H, C3’-H, C4’-H, C5’-H, C6’-H), 2.96-2.90 (m, 1H, C2’-H), 2.49-2.39 (m, 2H, C10-H), 2.05 (s, 6H, C1-OAc, C7-OAc), 1.73-1.71 (m, 2H, C11-H); ESI MS: m/z = 587.6:588.3 (100%).
4-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-ene-1,7-diyl diacetate (XII): m.p. 197 °C; 1H-NMR δ (ppm): 11.22 (s, 1H, NH+), 8.45 (m, 2H, C11’-H, C13’-H), 7.76-7.74 (m, 1H, C10’-H), 7.44-7.35 (m, 2H, C8’-H, C9’-H), 7.14-7.12 (m, 1H, C7’-H), 6.76 (t, 1H, 3J= 4.8, C12’), 6.12 (s, 2H, C8-H, C9-H), 4.70-4.68 (m, 4H, C3’-H, C5’-H), 4.49-4.48 (m, 2H, C2’-H), 4.06-4.04 (m, 2H, C2-H, C6-H), 3.51-3.35 (m, 4H, C4’-H, C6’-H), 3.20-3.16 (m, 2H, C1’-H), 2.42-2.41 (m, 2H, C10-H), 2.06 (s, 6H, C1-OAc, C7-OAc), 1.73-1.71 (m, 2H, C11-H); ESI MS: m/z = 559.6:560.4 (100%).
2-{(2Z)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetra-hydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XIII): m.p. 175 °C (for HCl); 1H-NMR (free base) δ (ppm): 7.77 (m, 2H, C8-H, C11-H), 7.57 (m, 2H, C12-H, C15-H), 7.43 (m, 2H, C9-H, C10-H), 7.37 (m, 2H, C13-H, C14-H), 6.90-6.85 (m, 4H, C9’-H-C12’-H), 5.25-5.23 (m, 1H, C2’-H), 4.34-4.32 (m, 1H, C3’-H), 3.75 (s, 3H, OCH3), 3.68 (d, 2H, 3J=6.4, C1’-H), 3.60 (s, 2H, C2-H, C6-H), 2.89 (m, 6H, C4’-H, C6’-H, C8’-H), 2.40 (m, 4H, C5’-H, C7’-H); Anal. Calc. for C33H31Cl2N3O3·11/2H2O (free base): 64.40 % C, 5.55 % H, 6.83% N; found: 68.62 % C, 5.35 % H, 6.75 % N; ESI MS: m/z = 588.5:589.2 (100%).
2-{(2Z)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XIV): m.p. 188 °C; 1H-NMR (DMSO-d6) δ (ppm): 11.73 (s, 1H, NH+), 8.46 (d, 2H, 3J=4.4, C9’-H, C11’-H), 7.82-7.80 (m, 2H, C8-H, C11-H), 7.61-7.59 (m, 2H, C12‑H, C15-H), 7.47-7.46 (m, 2H, C9-H, C10-H), 7.42-7.40 (m, 2H, C13-H, C14-H), 6.78 (t, 1H, 3J=4.4, 4.8, C10’-H), 5.59-5.53 (m, 1H, C2’-H), 4.72-4.69 (m, 2H, C4’-H), 4.63-4.57 (m, 1H, C3’-H), 3.77-3.76 (m, 4H, C5’-H, C7’-H), 3.68-3.64 (s, 2H, C2-H, C6-H), 3.46-3.36 (m, 4H, C6’-H, C8’-H), 2.98-2.95 (m, 2H, C1’-H); ESI MS: m/z = 559.5:560.1 (100%), 562.1 (65%).
2-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetra-hydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XV): m.p. 240 °C; 1H-NMR δ (ppm): 10.40 (s, 1H, NH+), 7.83-7.81 (m, 2H, C11-H, C12-H), 7.65-7.63 (m, 2H, C8-H, C15-H), 7.48-7.45 (m, 4H, C9-H, C10-H, C13-H, C14-H), 7.05-6.90 (m, 4H, C9’-H-C12’-H), 5.28 (m, 1H, C3’-H), 5.02-4.95 (m, 2H, C4’-H), 4.61-4.57 (m, 1H, C2’-H), 3.80 (s, 3H, OCH3), 3.71 (d, 2H, 3J= 5.6, C1’-H), 3.66 (s, 2H, C2‑H, C6-H), 3.55-3.52 (m, 2H, C5’-H, C7’-H), 3.35-3.33 (m, 2H, C5’-H, C7’-H), 3.09-2.96 (m, 4H, C6’-H, C8’-H); ESI MS: m/z = 588.5:589.2 (100%).
2-{(2E)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XVI): m.p. 210 °C; 1H-NMR δ (ppm): 11.48 (s,1H, NH+), 8.47 (d, 2H, 3J=4.4, C9’-H, C11’-H), 7.81-7.80 (m, 2H, C8-H, C15-H), 7.62-7.61 (m, 2H, C9-H, C10‑H), 7.47-7.44 (m, 4H, C13-H, C14-H, C11-H, C-12H), 6.78 (t, 1H, 3J=4.4, C10’-H), 5.35-5.27 (m, 1H, C3’-H), 4.97-4.92 (m, 2H, C2’-H), 4.74-4.71 (m, 1H, C4’-H), 3.70-3.65 (s, 4H, C1’-H, C2-H, C6-H), 3.49 (m, 2H, C5’-H, C7’-H), 3.43-3.32 (m, 4H, C6’-H, C8’-H), 2.91-2.88 (m, 2H, C5’-H, C7’‑H); ESI MS: m/z = 559.5:560.2 (100%).
2-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XVII): m.p. 225 °C; 1H-NMR δ (ppm): 7.83-7.80 (m, 2H, C11-H, C12-H), 7.67-7.65 (m, 2H, C8-H, C15-H), 7.52-7.50 (m, 2H, C10-H, C9-H), 7.48-7.46 (m, 2H, C13-H, C14-H), 7.13-7.10 (m, 2H, C8’-H, C9’-H), 6.91-6.84 (m, 5H, C7’-H, C10’-H, C11’-H C12’-H, C14’-H), 4.99-4.97 (m, 1H, C13’-H), 4.60 (s, 2H, C2’-H), 3.75 (s, 3H, OCH3), 3.72 (m, 2H, C1’-H), 3.46 (m, 2H, C2-H, C6-H), 2.87 (m, 4H, C3’-H, C5’-H), 2.33 (m, 4H, C4’-H, C6’-H); Anal. Calc. for C37H33Cl2N3O3·2HCl·5H2O: 58.35 % C, 5.65 % H, 5.52% N; found: 58.61 % C, 5.68 % H, 5.10 % N; ESI MS: m/z = 637.5:638.1 (100%), 640.2 (60%).
2-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-4,7-dichloro-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XVIII): m.p. 273 °C; 1H-NMR (for HCl) δ (ppm): 8.33-8.30 (m, C11’-H, C13’-H), 7.81-7.79 (m, 2H, C11-H, C8-H), 7.65-7.63 (m, 2H, C12-H, C15-H), 7.49-7.44 (m, 4H, C10-H, C9-H, C13-H, C14-H), 7.13-7.07 (m, 2H, C8’-H, C9’-H), 6.86-6.83 (m, 1H, C10’-H), 6.61-6.58 (m, 1H, C7’-H), 5.03-5.01 (d, 1H, C12’-H), 4.60 (m, 2H, C2’-H), 3.71 (m, 2H, C1’-H), 3.63 (m, 4H, C3’-H, C6’-H), 3.44 (m, 2H, C2-H, C6-H), 2.33 (m, 4H, C4’-H, C5’-H); Anal. Calc. for C34H29Cl2N5O2·21/2H2O (free base): 62.29 % C, 4.45 % H, 10.68% N; found: 62.72 % C, 4.79 % H, 10.76 % N; ESI MS: m/z = 609.5:610.1 (100%), 612.1 (60%).
2-{(2Z)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XIX): m.p. 168 °C; 1H-NMR δ (ppm): 11.39 (s, 1H, NH+), 7.66-7.58 (m, 2H, C8-H, C11-H), 7.34-7.26 (m, 5H, C10-H, C12-H, C13-H, C14-H, C15‑H), 7.05-6.89 (m, 5H, C9-H, C9’-H, C10’-H, C11’-H, C12’-H), 5.56-5.50 (m, 1H, C2’-H), 4.92 (m, 1H, C7-H), 4.50-4.45 (m, 1H, C6-H), 4.16-4.14 (m, 1H, C3’-H), 3.80 (s, 3H, OCH3), 3.76 (m, 3H, C2-H, C5’-H, C7’-H ), 3.49-3.48 (m, 2H, C4’-H), 3.40-3.32 (m, 2H, C5’-H, C7’-H), 3.16-3.03 (m, 4H, C1’-H, C6’-H), 2.08 (m, 2H, C8’-H); ESI MS: m/z = 615.6:616.3 (100%).
2-{(2Z)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XX): m.p. 163 °C; 1H-NMR δ (ppm): 8.33-8.31 (m, 2H, C9’‑H, C11’-H), 7.64 (m, 1H, C11-H), 7.47-7.45 (m, 1H, C12-H), 7.31-7.21 (m, 5H, C8-H, C9-H, C10-H,C13-H, C14-H), 7.05-7.03 (m, 1H, C15-H), 6.51 (m, 1H, C10’-H), 5.44 (m, 1H, C2’-H), 4.78 (m, 1H, C7-H), 4.51 (m, 1H, C3’-H), 3.98 (d, 1H, 3J=8.4, C2-H), 3.83 (m, 4H, C5’-H, C7’-H), 3.72-3.65 (m, 2H, C4’-H), 3.30-3.28 (m, 1H, C6-H), 3.04 (m, 2H, C1’-H), 2.48 (m, 4H, C6’-H, C8’-H); ESI MS: m/z = 587.5:588.2 (100%), 610.2 (2%).
2-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XXI): m.p. 180 °C; 1H-NMR δ (ppm): 11.47 (s, 1H, NH+), 7.59 (m, 2H, C11-H, C12-H), 7.34-7.26 (m, 5H, C8-H, C10-H, C13-H, C14-H, C15-H), 7.05-6.91 (m, 5H, C9-H, C9’-H, C10’-H, C11’-H, C12’-H), 5.56-5.48 (m, 1H, C3’-H), 4.93 (s, 1H, C7-H), 4.82-4.75 (m, 1H, C2’-H), 4.15 (d, 1H, 3J=8.4, C2-H), 3.80 (s, 3H, -OCH3), 3.63 (m, 2H, C4’-H), 3.52-3.49 (m, 5H, C6-H, C5’-H, C7’-H), 3.38-3.33 (m, 2H, C1’-H), 3.07 (m, 4H, C6’-H, C8’-H); ESI MS: m/z = 615.6:616.3 (100%).
2-{(2E)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XXII): m.p. 204 °C; 1H-NMR δ (ppm): 11.67 (s, 1H, NH+), 8.45 (d, 2H, 3J=4.4, C9’-H, C11’-H) 7.66-7.57 (m, 2H, C11-H, C12-H), 7.36-7.28 (m, 5H, C8-H, C9‑H, C10-H, C13-H, C14-H), 6.97-6.95 (m, 1H, C15-H), 5.52-5.48 (m, 1H, C3’-H), 4.92 (s, 1H, C7‑H), 4.79-4.69 (m, 1H, C2’-H), 4.14 (d, 1H, 3J=8, C2-H), 3.62-3.61 (m, 2H, C4’-H), 3.49-3.35 (m, 9H, C6-H, C5’-H, C7’-H), 2.92-2.89 (m, 2H, C1’-H); ESI MS: m/z = 587.5:588.2 (100%), 610.2 (2%).
2-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetra-hydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XXIII): m.p. 190 °C; 1H-NMR δ (ppm): 10.67 (s, 1H, NH+), 7.65-7.59 (m, 3H, C8-H, C11-H, C12-H), 7.36-7.25 (m, 5H, C9-H, C10-H, C13-H, C14-H, C15-H), 7.20-7.14 (m, 3H, C7’-H, C10’-H, C14-H), 7.02-6.95 (m, 3H, C8’-H, C9’-H, C11’-H), 6.91 (m, 2H, C12’-H, C14’-H), 5.93-5.91 (m, 1H, C13’-H), 4.94-4.93 (m, 1H, C7-H), 4.49 (m, 2H, C2’-H), 4.38-4.37 (m, 2H, C1’-H), 4.28-4.26 (m, 1H, C2-H), 4.12 (m, 4H, C3’-H, C5’-H), 3.79 (s, 3H, OCH3), 3.58-3.56 (m, 1H, C6-H), 3.47-3.44 (m, 2H, C4’-H), 3.08 (m, 2H, C6’-H); ESI MS: m/z = 665.7:666.2 (100%).
2-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-4-trifluoroacetyl-4,7-ethano-3a,4,9,9a-tetrahydro-1H-dibenzo[fi]isoindole-1,3-(2H)-dione (XXIV): m.p. 196 °C; 1H-NMR δ (ppm): 11.16 (s, 1H, NH+), 8.45-8.43 (m, 2H, C11’-H, C13’-H), 7.65-7.63 (m, 2H, C11-H, C12-H), 7.58-7.56 (m, 1H, C15-H), 7.35-7.25 (m, 4H, C9-H, C10-H, C13-H, C14-H), 7.20-7.13 (m, 3H, C7’-H, C9’-H, C10’-H), 6.96-6.94 (m, 1H, C8-H), 6.77 (m, 1H, C12’-H), 5.91-5.89 (d, 1H, 3J=7.6, C8’-H), 4.93 (m, 1H, C7-H), 4.70-4.66 (m, 4H, C3’-H, C5’-H), 4.53-4.49 (m, 2H, C2’-H), 4.33-4.32 (m, 2H, C1’-H), 4.26-4.24 (m, 1H, C2-H), 3.56-3.54 (m, 1H, C6-H), 3.35-3.31 (m, 2H, C4’-H), 3.16-3.11 (m, 2H, C6’-H); Anal. Calc. for C36H30F3N5O3·HCl·4H2O: 57.95 % C, 5.27 % H, 9.39% N; found: 58.47 % C, 5.12 % H, 9.34 % N.
2-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-3a,6-dihydro-1H-benzo[de]isoquinoline-1,3-(2H)-dione (XXV): m.p. 239 °C; 1H-NMR δ (ppm): 10.32 (s, 1H, NH+), 8.54 (t, 4H, 3J=9.6, 8.0, C6‑H, C8-H, C10-H, C12-H), 7.91 (t, 2H, 3J=7.6, C7-H, C11-H), 7.74 (d, 1H, 3J=6.4, C10’-H), 7.38-7.32 (m, 2H, C8’-H, C9’-H), 7.26 (m, 1H, C7’-H), 7.05-6.98 (m, 4H, C12’-H, C13’-H, C14’-H, C15’-H), 5.49 (m, 2H, C2’-H), 4.76 (m, 2H, C1’-H), 3.81 (s, 3H, OCH3), 3.56-3.46 (m, 6H, C3’-H, C4’-H, C5’-H), 3.15-3.09 (m, 2H, C6’-H); Anal. Calc. for C31H29N3O3·HCl·4H2O: 61.84 % C, 6.64 % H, 6.98% N; found: 61.43 % C, 5.81 % H, 6.74 % N.
2-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-3a,6-dihydro-1H-benzo[de]isoquinoline-1,3(2H)-dione (XXVI): m.p. 258 °C; 1H-NMR δ (ppm): 10.48 (s, 1H, NH+), 8.54 (t, 4H, 3J=8.0, C6-H, C8-H, C10-H, C12-H), 8.46 (d, 2H, 3J=4.8, C7-H, C11-H), 7.91 (t, 1H, 3J=7.6, C8’-H, C9’-H), 7.72 (m, 1H, C7’-H), 7.35 (m, 2H, C11’-H, C13’-H), 7.25 (m, 1H, C10’-H), 6.78 (t, 1H, 3J=4.8, C12’-H), 5.45 (s, 2H, C2’‑H), 4.80 (m, 2H, C1’-H), 4.70 (m, 2H, C3’-H, C5’-H), 3.56-3.53 (m, 2H, C3’-H, C5’-H), 3.47-3.31 (m, 4H, C4’-H, C6’-H); Anal. Calc. for C28H25N5O2·HCl·H2O: 64.61 % C, 5.38 % H, 13.47% N; found: 64.34 % C, 5.32 % H, 13.50 % N.
4-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-7,11-dimethyl-3,5-dioxo-4-azatricyclo [5.2.2.02,6]undec-8-en-1-yl acetate (XXVII): m.p. 144 °C; 1H-NMR δ (ppm): 7.04-6.91 (m, 2H, C9’‑H, C12’-H), 5.99-5.94 (m, 2H, C10’-H, C11’-H), 5.72 (s, 1H, C9-H), 5.63-5.60 (m, 1H, C3‘-H), 4.15-4.13 (m, 2H, C4‘-H), 4.06-3.94 (m, 3H, C1’-H, C2’-H), 3.81-3.79 (m, 8H, C2-H, C5’-H, C7’-H, OCH3), 3.40-3.35 (m, 4H, C6’-H, C8’-H), 3.20-3.18 (m, 1H, C6-H), 2.61-2.54 (m, 2H, C7-H, C10-H), 2.04 (m, 4H, C1-OAc, C11-H), 1.53 (s, 3H, C8-CH3), 0.98-0.94 (m, 1H, C10-H), 0.79 (d, 3H, 3J=16.8, C11-CH3); ESI MS: m/z = 507.6:508.3 (100%).
4-{(2Z)-[4-pirimidylpiperazin-1-yl]but-2-en-1-yl}-7,11-dimethyl-3,5-dioxo-4-azatricyclo[5.2.2.02,6] undec-8-en-1-yl acetate (XXVIII): m.p. 190 °C; 1H-NMR δ (ppm): 11.79 (s, 1H, NH+), 8.45 (d, 2H, 3J=4.8, C9’-H, C11’-H), 6.77 (t, 1H, 3J=4.8, C10‘-H), 5.70 (s, 1H, C9-H), 5.55-5.49 (m, 1H, C2‘-H), 4.72-4.68 (m, 2H, C4‘-H), 4.02-4.01 (m, 2H, C5’-H, C7’-H), 3.90-3.88 (m, 2H, C4’-H), 3.78-3.76 (m, 1H, C3’-H), 3.47-3.41 (m, 4H, C6’-H, C8’-H), 3.17-3.14 (m, 1H, C6-H), 3.03 (m, 2H, C5’-H, C6’-H), 2.59-2.52 (m, 2H, C7-H, C10-H), 2.08-2.03 (m, 5H, C1-OAc, C2-H, C11-H), 1.65 (s, 3H, C8-CH3), 0.97-0.93 (m, 1H, C10-H), 0.79 (d, 3H, 3J=6.4, C11-CH3); ESI MS: m/z = 479.5:480.3 (100%).
4-{(2Z)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-7,11-dimethyl-3,5-dioxo-4-azatricyclo [5.2.2.02,6]undec-8-en-1-yl acetate (XXIX): m.p. 149°C; 1H-NMR δ (ppm): 11.50 (s, 1H, NH+), 7.02-6.90 (m, 4H, Harom.), 5.89-5.60 (m, 3H, C9-H, C2’-H, C3‘-H), 3.94 (m, 2H, C4’-H), 3.78 (s, 3H, OCH3), 3.73 (m, 2H, C5‘-H), 3.48 (m, 2H, C7’-H), 3.40-3.35 (m, 3H, C2-H, C1’-H), 3.17 (m, 1H, C6-H), 3.07 (m, 4H, C6’-H, C8’-H), 2.60-2.55 (m, 2H, C7-H, C10-H), 2.04 (s, 4H, C1-OAc, C11-H), 1.71 (s, 3H, C8-CH3), 0.98-0.95 (m, 1H, C10-H), 0.81 (d, 3H, 3J=6.4, C11-CH3); ESI MS: m/z = 507.6:508.4 (100%).
4-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-7,11-dimethyl-3,5-dioxo-4-azatricyclo [5.2.2.02,6]undec-8-en-1-yl acetate (XXX): m.p. 153 °C; 1H-NMR δ (ppm): 10.95 (s, 1H, NH+), 7.77-7.76 (m, 1H, C10’-H), 7.44-7.35 (m, 2H, C8’-H, C9’-H), 7.09-7.07 (m, 1H, C7’-H), 7.03-6.91 (m, 4H, C11’-H, C12’-H, C13’-H, C14’-H), 5.67 (s, 1H, C9-H), 4.78-4.68 (m, 2H, C2’-H), 4.59-4.50 (m, 2H, C1’-H), 3.88-3.86 (m, 1H, C2-H), 3.78 (s, 3H, OCH3), 3.49-3.35 (m, 6H, C3’-H, C4’-H, C5’-H), 3.25-3.23 (m, 1H, C6-H), 3.18-3.13 (m, 2H, C6’-H), 2.59-2.54 (m, 2H, C7-H, C10-H), 2.04 (m, 4H, C1‑OAc, C11-H), 1.53 (s, 3H, C8-CH3), 0.98-0.94 (m, 1H, C10-H), 0.79 (d, 3H, 3J=6.4, C11- CH3); ESI MS: m/z = 557.6:558.3 (100%).
4-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-7,11-dimethyl-3,5-dioxo-4-azatricyclo[5.2.2.02,6]undec-8-en-1-yl acetate (XXXI): m.p. 173°C; 1H-NMR δ (ppm): 8.43 (d, 2H, 3J=4.0, C11’-H, C13’-H), 7.67 (d, 1H, 3J=5.6, C10’-H), 7.46-7.34 (m, 2H, C8’-H, C9’-H), 7.05 (d, 1H, 3J=8.0, C7’-H), 6.76 (t, 1H, 3J=4.0, C12’-H), 5.67 (s, 1H, C9-H), 4.72-4.62 (m,4H, C3’-H, C5’-H), 4.56-4.42 (m, 2H, C2’-H), 3.84 (d, 1H, 3J=8.0, C2-H), 3.40-3.33 (m, 6H, C1’-H, C4’-H, C6’-H), 3.23-3.20 (m, 2H, C6-H, C7-H), 2.55 (m, 1H, C10-H), 2.01 (m, 4H, C1-OAc, C11-H), 1.50 (s, 3H, C8-CH3), 0.95-0.92 (m, 1H, C10‑H), 0.77 (d, 3H, 3J=8.2, C11- CH3); Anal. Calc. for C30H35N5O4·HCl·31/2H2O: 57.23 % C, 6.83 % H, 11.13% N; found: 57.44 % C, 6.41 % H, 11.08 % N.
4-{(2E)-[4-(2-methoxyphenyl)piperazin-1-yl]but-2-en-1-yl}-1,11-dimethyl-4-azatricyclo[5.2.2.02,6] un-decane-3,5,8-trione (XXXII): m.p. 175 °C; 1H-NMR δ (ppm): 10.94 (s, 1H, NH+), 7.03-6.90 (m, 4H, Harom.), 5.84-5.78 (m, 1H, C2’-H), 5.64-5.58 (m, 1H, C3’-H), 4.16-4.07 (m, 2H, C5’-H, C7’-H), 3.96 (m, 2H, C4’-H), 3.79 (s, 3H, OCH3), 3.49-3.43 (m, 4H, C6’-H, C8’-H), 3.37-3.34 (m, 1H, C6-H), 3.21-3.16 (m, 2H, C5’-H, C7’-H), 3.05-2.99 (m, 2H, C1’-H), 2.84-2.82 (m, 1H, C2-H), 2.44 (m, 1H, C7-H), 2.19 (m, 1H, C11-H), 2.09-2.05 (m, 1H, C9-H), 1.94-1.88 (m, 1H, C10-H), 1.77-1.71 (m, 1H, C9-H), 1.99 (s, 3H, C1-CH3), 1.09-1.01 (m, 1H, C10-H), 0.85 (d, 3H, 3J=7.2, C11-CH3); ESI MS: m/z = 465.5:466.2 (100%).
4-{2-[4-(2-methoxyphenyl)-piperazin-1-yl]methylbenzyl}-1,11-dimethyl-4-azatricyclo[5.2.2.02,6]-undecane-3,5,8-trione (XXXIII): m.p. 186 °C; 1H-NMR δ (ppm): 7.70 (m, 1H, C7’-H), 7.46-7.43 (m, 2H, C8’-H, C9’-H), 7.10 (m, 1H, C10’-H), 7.03-6.82 (m, 4H, C11’-H, C12’-H, C13’-H, C14’-H), 4.82-4.64 (m, 2H, C5-H, C3’-H), 4.61 (s, 2H, C2’-H), 3.86 (s, 3H, OCH3), 3.50-3.33 (m, 7H, C6-H, C3’-H, C4’-H, C5’-H, C6’-H), 3.11-3.05 (m, 2H, C1’-H), 2.87 (d, 1H, 3J=9.2, C2-H), 2.49-2.45 (m, 1H, C7-H), 2.17 (m, 1H, C11-H), 2.00 (d, 1H, 3J=20, C9-H), 1.89 (t, 1H, 3J=12, C10-H), 1.60 (d, 1H, 3J=20, C9-H), 1.14 (s, 3H, C1-CH3), 1.05-0.97 (m, 1H, C10-H), 0.84 (d, 3H, 3J=8, C11-CH3); Anal. Calc. for C31H37N3O4 ·HCl·31/2H2O: 60.49 % C, 7.32 % H, 6.83% N; found: 60.98 % C, 7.08 % H, 6.31 % N.
4-[2-(4-pirimidylpiperazin-1-yl)methylbenzyl]-1,11-dimethyl-4-azatricyclo[5.2.2.02,6]undecane-3,5,8-trione (XXXIV): m.p. 176 °C; 1H-NMR δ (ppm): 8.43 (d, 2H, 3J=4.6, C11’-H, C13’-H), 8.29-8.12 (m, 1H, C10’-H), 7.35 (m, 2H, C8’-H, C9’-H), 7.09 (m, 1H, C7’-H), 6.75 (t, 1H, 3J=4.8, C12’-H), 4.78-4.57 (m, 4H, C3’-H, C5’-H,), 4.53 (s, 2H, C2’-H), 3.44-3.33 (m, 4H, C4’-H, C6’-H), 3.23-3.14 (m, 3H, C6-H, C1’-H), 2.87 (d, 1H, 3J=8, C2-H), 2.43 (m, 1H, C7-H), 2.18 (m, 1H, C11-H), 2.10-1.95 (m, 1H, C9-H), 1.93-1.81 (m, 1H, C10-H), 1.60-1.57 (m, 1H, C9-H), 1.13 (s, 3H, C1-CH3), 1.05-0.97 (m, 1H, C10-H), 0.84 (d, 3H, 3J=8, C11-CH3); Anal. Calc. for C28H33N5O3·HCl·31/2H2O: 57.24 % C, 6.98 % H, 11.93% N; found: 57.23 % C, 6.39 % H, 11.89 % N.