Formal Synthesis of the ACE Inhibitor Benazepril·HCl via an Asymmetric Aza-Michael Reaction
Abstract
:Introduction
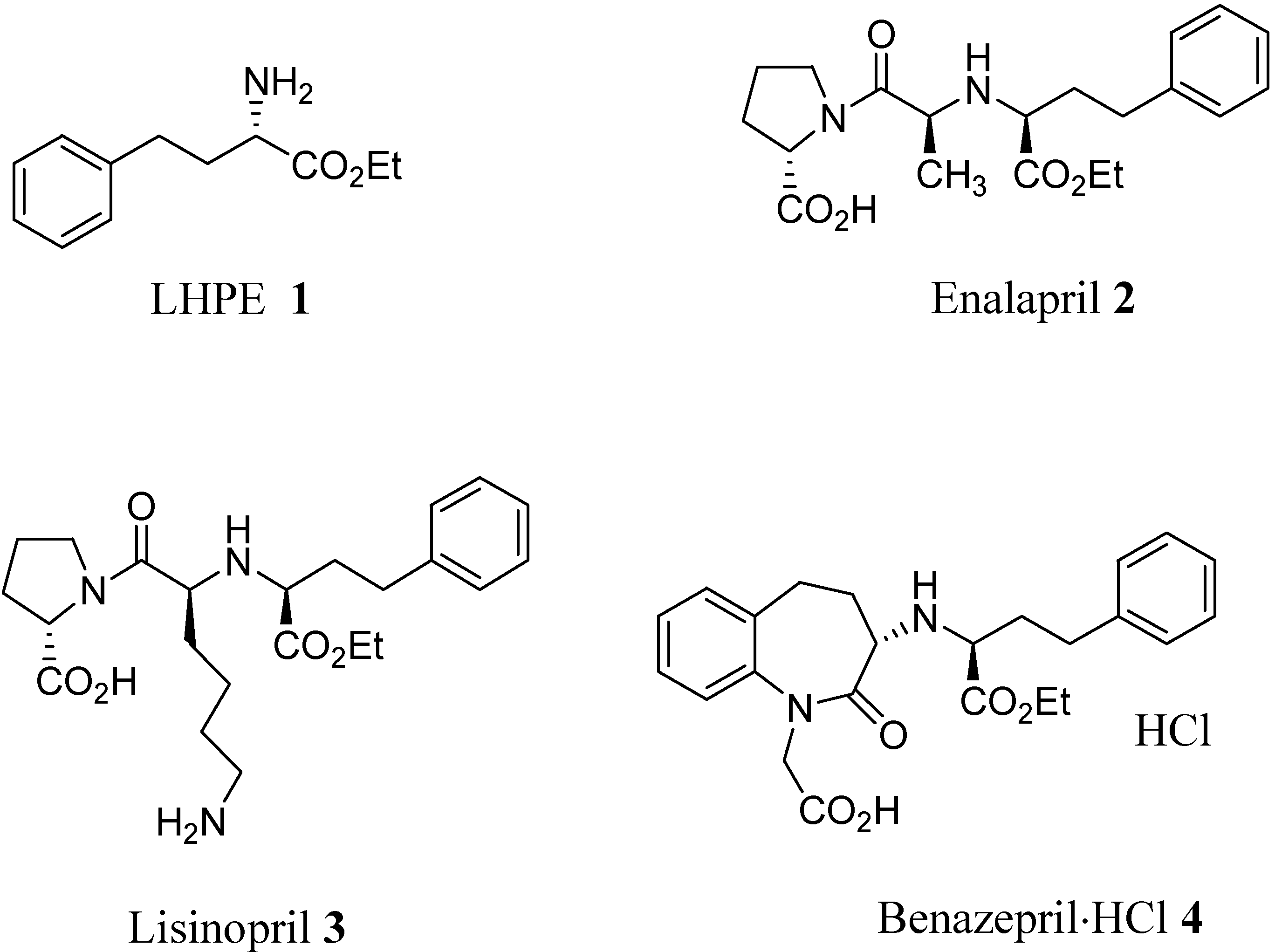
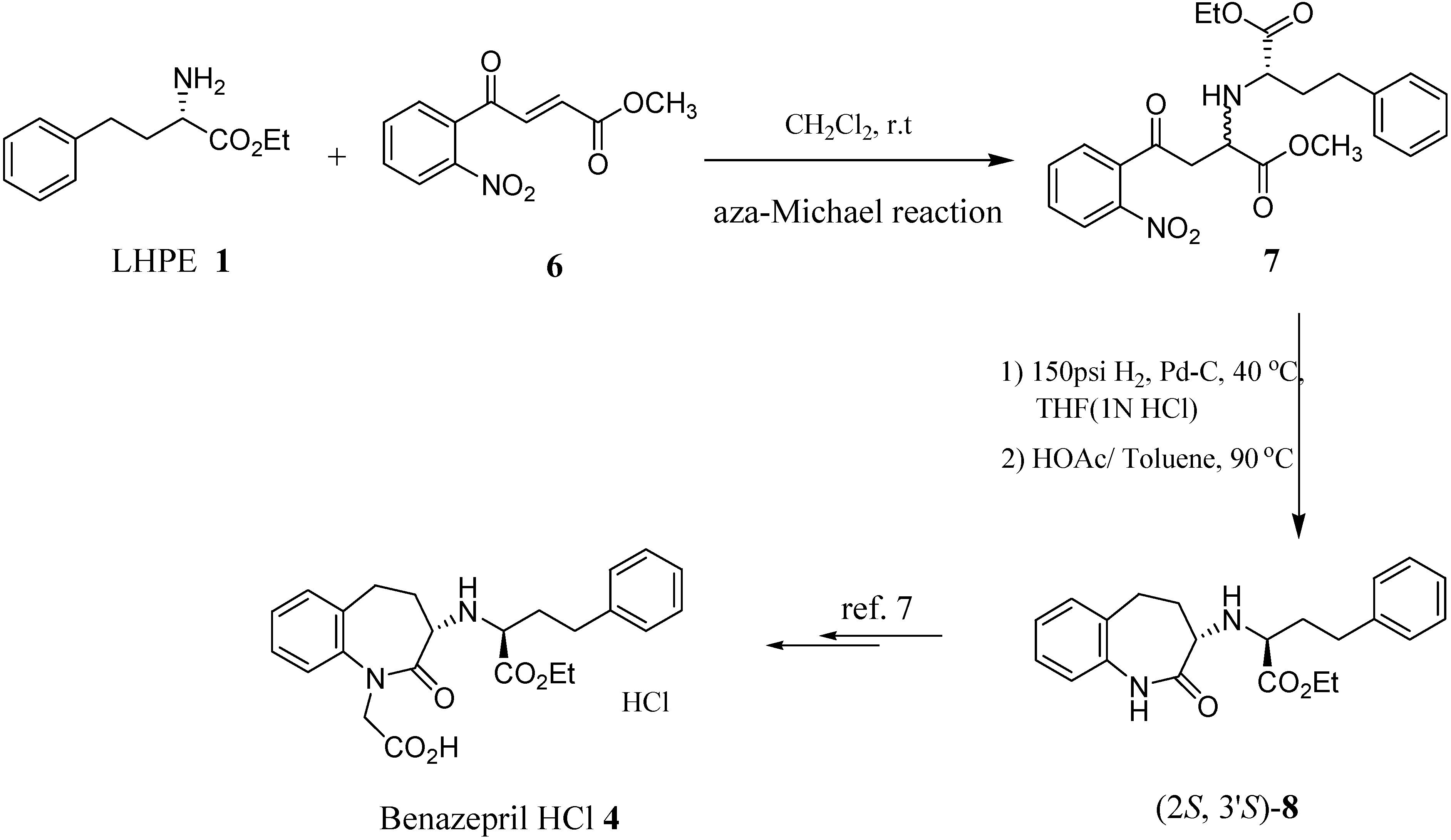
Results and Discussion
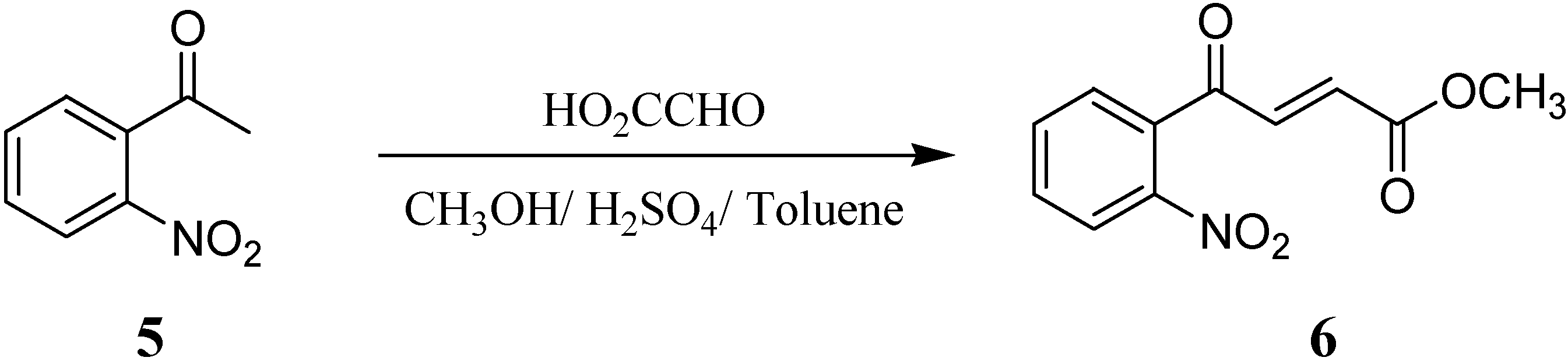
| Entrya | Solvent | Reaction time (hr) | Diastereomeric ratio of 7 (S, S) : (R, S)b | Conversion (%)c,d |
| 1 | Dichloromethane | 17 | 4.20 : 1 | 96.0 |
| 2 | Acetonitrile | 26 | 3.75 : 1 | 99.0 |
| 3 | Ethanol | 18 | 2.15 : 1 | 98.0 |
| 4 | Isopropanol | 16 | 1.91 : 1 | 98.8 |
| 5 | Xylene | 16 | 1.88 : 1 | 99.3 |
| 6 | Pyridine | 33 | 1.87 : 1 | 98.2 |
| 7 | Ether | 25 | 1.86 : 1 | 98.2 |
| 8 | Benzene | 16 | 1.83 : 1 | 98.6 |
| 9 | Acetonitrile/H2O | 35 | 1.81 : 1 | 99.2 |
| 10 | Isobutanol | 33 | 1.80 : 1 | 96.9 |
| 11 | tert-Butanol | 18 | 1.69 : 1 | 96.7 |
| 12 | DME | 25 | 1.68 : 1 | 97.5 |
| 13 | Ethyl acetate | 18 | 1.67 : 1 | 95.2 |
| 14 | Toluene | 16 | 1.60 : 1 | 99.1 |
| 15 | THF | 16 | 1.53 : 1 | 98.6 |
| Entry | Solvent a /addictive | LHPE (equiv.) | Reaction time (hr) | Reaction temperature (°C) | Proportion of diastereomers (S, S) : (R, S) | Conversionb (%) |
| 1 | CH2Cl2 | 0.50 | 53 | 20 | 2.89 : 1 | 86.7 |
| 2 | CH2Cl2 | 0.90 | 53 | 20 | 3.10 : 1 | 92.5 |
| 3 | CH2Cl2 | 1.05 | 16 | 20 | 4.15 : 1 | 96.0 |
| 4 | CH2Cl2 | 1.10 | 16 | 20 | 4.50 : 1 | 96.0 |
| 5 | CH2Cl2 | 1.50 | 16 | 20 | 3.85 : 1 | 98.0 |
| 6 | CH2Cl2 | 2.00 | 16 | 20 | 3.07 : 1 | 97.5 |
| 7 | CH2Cl2 | 1.05 | 35 | 0 | 2.70 : 1 | 90.8 |
| 8 | CH2Cl2 | 1.05 | 16 | 40 | 3.75 : 1 | 96.2 |
| 9 | CH2Cl2 | 1.05 | 16 | 60 | 2.20 : 1 | 98.0 |
| 10 | CH2Cl2 | 1.05 | 10 | 20 | 3.36 : 1 | 90.5 |
| 11 | CH2Cl2 | 1.05 | 26 | 20 | 3.68 : 1 | 95.3 |
| 12 | CH2Cl2 | 1.05 | 35 | 20 | 3.76 : 1 | 96.2 |
| 13 | CH2Cl2 | 1.05 | 40 | 20 | 3.60 : 1 | 96.0 |
| 14 | CH2Cl2 | 1.05 | 53 | 20 | 3.29 : 1 | 94.4 |
| 15 | CH2Cl2 | 1.10 | 120 | 20 | 0.62 : 1 | [b] |
| 16 | CH2Cl2 | 1.10 | 168 | 20 | 0.54 : 1 | [b] |
| 17 | CH2Cl2 | 0.95 | 10 | 20 | 3.82 : 1 | 88.7 |
| 18 | CH2Cl2 | 0.95 | 26 | 20 | 3.87 : 1 | 88.5 |
| 19 | CH2Cl2 | 0.95 | 35 | 20 | 3.79 : 1 | 91.3 |
| 20 | CH2Cl2 | 0.95 | 40 | 20 | 3.56 : 1 | 91.6 |
| 21 | CH2Cl2 | 1.05c | 24 | 20 | 2.56 : 1 | 98.0 |
| 22 | CH2Cl2 | 1.05d | 24 | 20 | - | - |
| 23 | CH2Cl2/Et3N | 1.15 | 12 | 20 | 1.59 : 1 | 99.0 |
| 24 | C2H5OH | 1.15 | 18 | 20 | 2.15 : 1 | 98.3 |
| 25 | C2H5OH/Et3N | 1.15 | 12 | 20 | 2.11 : 1 | 100.0 |
Conclusions
Experimental
General
Preparation of 4-(2-nitrophenyl)-4-oxo-but-2-enoic acid methyl ester (6)
General procedure for Aza-Michael addition reactions
Preparation of (2S,3’S)-2-(2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-ylamino)-4-phenylbutyric acid ethyl ester (8).
References
- Kori, M.; Itoh, K.; Inada, Y.; Katoh, T.; Sumino, Y.; Nishikawa, K.; Sugihara, H. Chem. Pharm. Bull. 1994, 42, 580–585. [CrossRef]Kubota, H.; Nunami, K.; Yamagishi, M.; Nishimoto, S.; Hayashi, K. Chem. Pharm. Bull. 1991, 39, 1374–1377. [CrossRef]
- Patchett, A. A.; Harris, E; Tristram, E. W.; Wyvratt, M. J.; Wu, M. T.; Taub, D.; Peterson, E. R.; Ikeler, T. J.; Broeke, J. T.; Payne, L. G.; Ondeyka, D. L.; Thorsett, E. D.; Greenlee, W. J.; Lohr, N. S.; Hoffsommer, R. D.; Joshua, H.; Ruyle, W. V.; Rothrock, J. W.; Aster, S. D.; Maycock, A. L.; Robinson, F. M.; Hirschmann, R.; Sweet, C. S.; Ulm, E. H.; Gross, D. M.; Vassil, T. C.; Stone, C. A. Nature 1980, 288, 280–283. [CrossRef]
- Barton, J. N.; Piwinski, J. J.; Skiles, J. W.; Regan, J. R.; Menard, P. R.; Desai, R.; Golec, F. S.; Reilly, L. W.; Goetzen, T.; Ueng, S. N.; Warus, J. D.; Schwab, A.; Samuels, A. I.; Neiss, E. S.; Suh, J. T. J. Med. Chem. 1990, 33, 1600–1606. [CrossRef]Iwasaki, G.; Kimura, R.; Numao, N.; Kondo, K. Chem. Pharm. Bull. 1989, 37, 280–283. [CrossRef]Blacklock, T. J.; Shuman, R. F.; Butcher, J. W.; Shearin, Jr. W. E.; Budavari, J.; Grenda, V. J. J. Org. Chem. 1988, 53, 836–844. [CrossRef]Iwasaki, G.; Kimura, R.; Numao, N.; Kondo, K. Chem. Lett. 1988, 1691–1694. [CrossRef]
- Watthey, J. W. H.; Stanton, J. L.; Desai, M.; Babiarz, J. E.; Finn, B. M. J. Med. Chem. 1985, 28, 1511–1516. [CrossRef]
- Boyer, S. K.; Pfund, R. A.; Portmann, R. E.; Sedelmeier, G. H.; Wetter, H. F. Helv. Chim. Acta 1988, 71, 337–343.
- Urbach, H.; Henning, R. Tetrahedron Letters 1984, 25, 1143–1146. [CrossRef]Yamada, M; Nagashima, J.; Takahashi, S. Tetrahedron Lett. 1998, 39, 9019–9022. [CrossRef]Knollmüller, M.; Ferencic, M.; Gärtner, P.; Girreser, U.; Klinge, M.; Gaischin, L.; Mereiter, K.; Noe, C. R. Monstsh. Chem. 1999, 130, 769–782.
- Chang, C.-Y.; Yang, T.-K. Tetrahedron: Asymmetr. 2003, 14, 2239–2245. [CrossRef]
- Yanagida, Y.; Matsumoto, S.; Takahashi, S. U.S. Patent 5264611, 1993.
- Hawkins, J.M.; Gregory, C.F. J. Org. Chem. 1986, 52, 2820–2822. [CrossRef]
- Tan, K.; Alvarez, R.; Nour, M.; Cave, C.; Chiaroni, A.; Riche, C.; d'Angelo, J. Tetrahedron Lett. 2001, 42, 5021–5023. [CrossRef]
- Kobayashi, S.; Kakumoto, K.; Sugiura, M. Org. Lett. 2002, 4, 1319–1322. [CrossRef]
- Sample availability: Samples of compounds 1 and 4 are available on request from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yu, L.-T.; Huang, J.-L.; Chang, C.-Y.; Yang, T.-K. Formal Synthesis of the ACE Inhibitor Benazepril·HCl via an Asymmetric Aza-Michael Reaction. Molecules 2006, 11, 641-648. https://doi.org/10.3390/11080641
Yu L-T, Huang J-L, Chang C-Y, Yang T-K. Formal Synthesis of the ACE Inhibitor Benazepril·HCl via an Asymmetric Aza-Michael Reaction. Molecules. 2006; 11(8):641-648. https://doi.org/10.3390/11080641
Chicago/Turabian StyleYu, Luo-Ting, Ji-Ling Huang, Ching-Yao Chang, and Teng-Kuei Yang. 2006. "Formal Synthesis of the ACE Inhibitor Benazepril·HCl via an Asymmetric Aza-Michael Reaction" Molecules 11, no. 8: 641-648. https://doi.org/10.3390/11080641




