Abstract
Five novel Schiff bases have been prepared from o-formylphenoxyacetic acid and a series of aminothiazoles to form a number of potentially biologically active compounds. The structures of these Schiff bases have been characterized using IR and 1H- and 13C-NMR spectroscopy.
Introduction
Schiff bases are used as substrates in the preparation of a number of industrial and biologically active compounds via ring closure, cycloaddition, and replacement reactions [1]. Moreover, Schiff bases are also known to have biological activities such as antimicrobial [2,3,4,5], antifungal [4,5,6], antitumor [7,8,9], and as herbicides [10]. Schiff bases have also been employed as ligands for complexation of metal ions [11]. On the industrial scale, they have wide range of applications such as dyes and pigments [12]. Keeping in view the facts mentioned, we decided to synthesize new Schiff bases which were predicted to have useful biological activity. The synthesis of other similar Schiff bases, their biological activity, and complex formation are under study.
Results and Discussion
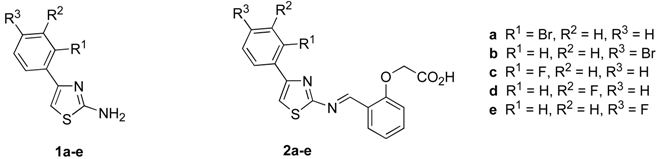
The Schiff bases 2a-e were synthesized by condensation of o-formylphenoxyacetic acid and aryl aminothiazoles 1a-e by reaction in hot ethanol or dioxane using sodium sulfate as a dehydrating agent. The aminothiazoles were prepared by the known [13] reaction of thiourea with substituted acetophenones in the presence of iodine as oxidant. Good yields were obtained for the Schiff bases, although in some cases the addition of ytterbium triflate was found to improve the yield of product, it acting as a Lewis acid catalyst.
Experimental
General
Bromoacetophenones, chloroacetophenones and fluoroacetophenones were obtained commercially from Lancaster Research Chemicals and 2-formylphenoxyactic acid from the Aldrich Chemical Company. 1H-NMR and 13C-NMR spectra were recorded on a Bruker DPX-400 instrument at 400 and 100 MHz, respectively. Chemical shifts are reported in ppm referenced to the residual solvent signal. IR spectra were recorded on a Perkin Elmer Paragon 1000 spectrometer. Mass spectra were recorded on a Jeol SX-102 instrument using FAB ionization. Melting points were recorded on a Stuart Scientific-SMP3 apparatus and are uncorrected.
Synthesis of 2-amino-4-(2’-bromophenyl)-thiazole (1a)
Method A: The title compound was prepared by addition of resublimed iodine (2.54 g, 0.01 mol) to 2’‑bromoacetophenone (1.99 g, 0.01 mol) and thiourea (1.52 g, 0.02 mol), followed by heating of the mixture overnight in an oil bath at 100 °C. After cooling, the reaction mixture was triturated with diethyl ether (ca. 50 mL) to remove any unreacted iodine and bromoacetophenone. The solid residue was put in cold distilled water (200 mL) and treated with 25% aqueous ammonium hydroxide (to pH 9-10). The precipitated thiazole was collected and purified by crystallization from hot ethanol. The yield was 85% and m.p. 123 °C; FABMS: m/z 255 (MH+), in agreement with the molecular formula C9H7BrN2S; IR; IR (υmax, KBr, cm-1): 3320 (d of NH2); 1510, 1460, 1045 (characteristic of the thiazole nucleus); 1H-NMR (MeOH-d4): δ 6.87 (1H, s, thiazole H-5), 7.34 (1H, td, J 11.6, 1.6 Hz, ArH), 7.45 (1H, td. J 7.6,1.6 Hz, ArH), 7.58 (1H, dd, J 7.2, 1.2 Hz, ArH), 7.72 (1H, dd, J 8.0,1.2 Hz, ArH); 13C-NMR (MeOH-d4): δ 171.2, 144.6, 134.9, 133.1, 131.7, 130.0, 128.8, 123.4, 107.7; Anal. Calcd. for C9H7BrN2S: C, 42.37; H, 2.77; N, 10.98; Found: C, 42.39; H, 2.76; N, 10.96.
Method B: 2-Amino-4-(2’-bromophenyl)-thiazole was also prepared following the reported procedure [14]. The spectroscopic data of the compound 1a thus prepared were identical to those given above.
The following compounds were prepared by Method A, as described above:
2-amino-4-(4’-bromophenyl)thiazole (1b)
Yield: 80%; m.p. 178 ºC; FABMS: m/z 255 (MH+), in agreement with the molecular formula C9H7BrN2S; IR (υmax, KBr, cm-1): 3320 (d of NH2); 1515, 1455, 1050 (characteristic of the thiazole nucleus); 1H-NMR (DMSO-d6): δ 8.7-7.8 (2H, bs, NH2), 7.67 (4H, s, ArH), 7.07 (1H, s, thiazole H-5); 13C-NMR (DMSO-d6): δ 169.7, 140.7, 131.8, 129.5, 127.8, 122.0, 103.5; Anal. Calcd. for C9H7BrN2S: C, 42.37; H, 2.77; N, 10.98; Found: C, 42.35; H, 2.75; N, 10.97.
2-amino-4-(2’-fluorophenyl)thiazole (1c)
Yield: 97%; FABMS: m/z 195 (MH+), in agreement with the molecular formula C9H7FN2S; IR (υmax, KBr, cm-1): 3320 (d of NH2); 1510, 1455, 1050 (characteristic of the thiazole nucleus); 1H-NMR (MeOH-d4): δ 6.93 (1H, s, thiazole H-5), 7.13-7.36 (3H, m, ArH), 7.93 (1H, td, J 7.9, 1.7 Hz, ArH); 13C-NMR (MeOH-d4): δ 170.7, 145.1, 133.5, 133.4, 132.4, 131.4, 130.8, 128.2 and 107.9. Anal. Calcd. for C9H7FN2S: C, 55.66; H, 3.63; N, 14.42; Found: C, 55.62; H, 3.59; N, 14.41.
2-amino-4-(3’-fluorophenyl)thiazole (1d)
Yield: 86%; FABMS: m/z 194 (MH+), in agreement with the molecular formula C9H7FN2S; IR (υmax, KBr, cm-1): 3320 (d of NH2); 1510, 1460, 1045 (characteristic of the thiazole nucleus); 1H-NMR (MeOH-d4): δ 7.01 (s, thiazole H-5), 7.09-7.56 (4H, m, ArH); 13C-NMR (MeOH‑d4): δ 171.8, 165.7, 163.3, 135.9, 131.7, 122.8, 116.2, 113.8 and 104.5. Anal. Calcd. for C9H7FN2S: C,55.66; H, 3.63, N, 14.42; Found: C, 55.60; H, 3.58, N, 14.39.
2-amino-4-(4’-fluorophenyl)thiazole (1e)
Yield: 87%; FABMS: m/z 195 (MH+), in agreement with the molecular formula C9H7FN2S; IR (υmax, KBr, cm-1): 3320 (d of NH2); 1510, 1460,1045 (characteristic of the thiazole nucleus); 1H-NMR (Py-d5): δ 7.31 (2H, brs, NH2), 8.10 (1H, s, thiazole H-5), 8.18-9.20 (4H, m, ArH); 13C (Py-d5): δ 171.4, 165.2, 162.7, 133.9, 129.9, 129.8, 117.3, 117.1 and 103.5; Anal. Calcd. for C9H7N2FS: C, 55.66; H, 3.63; N, 14.42; Found: C, 55.65; H, 3.61; N, 14.40.
Preparation of (2-{[4-(2-bromophenyl)thiazol-2-ylimino]methyl}phenoxy)acetic acid (2a):
2-Formylphenoxyacetic acid (4.0 mmol, 0.72 g) was added to 2-amino-4-(2’-bromophenyl)-thiazole (4.0 mmol, 1.02 g) in absolute EtOH (20 mL) in addition to molecular sieves (4Å, ca. 5 g) and Na2SO4 (anhydr. ca. 5 g) and refluxed (oil bath at 90 ºC) for 3 days under N2 (g). After filtration, evaporation and recrystallisation from EtOH the yield of the title Schiff base was found to be 60%; m.p. 180 ºC; HRMS (FAB, MH+) calcd. for C18H13N2O3BrS: 416.9909, found: 416.9904; IR (υmax, KBr, cm-1): 3030, 1635, 1550, 1240 cm-1; 1H-NMR, (MeOH-d4): δ 5.77 (2H, s, CH2), 6.91 (1H, d, J 9.2 Hz, ArH), 7.46-7.73 (7H, m, ArH), 7.92 (1H, s CH=N); 13C-NMR (MeOH-d4): δ 67.1, 113.4, 122.1, 122.3, 128.4, 129.5, 130.0, 130.8, 131.1, 130.3, 133.2, 133.8, 136.4, 144.9, 156.3, 1661.5,168.9, 172.0; Anal. Calcd. for C18H13BrN2O3S: C, 51.81; H, 3.14; N, 6.71. Found: C, 51.79, H, 3.12; N, 6.69;
Preparation of (2-{[4-(4-bromophenyl)-thiazole-2-ylimino]methyl}phenoxy)acetic acid (2b)
2-Formylphenoxyacetic acid (4.0 mmol, 0.72 g) was added to a solution of 2-amino-4-(4’-bromophenyl)-thiazole (4.0 mmol, 1.02 g) in dioxane (40 mL) in addition to molecular sieves (4Å, ca. 5 g) and Na2SO4 (anhydr. ca. 5 g) and refluxed above 100 ºC for 2 days under N2 (g). The product was purified by crystallization from EtOH and the yield of the Schiff base was found to be 67%; m.p. 185-187 ºC; HRMS (FAB, MH+) calcd. for C18H13BrO3N2S: 416.9909, found: 416.9904; IR (υmax, KBr, cm-1): 3030, 1650, 1550, 1240. Anal. Calcd. for C18H13BrO3N2S: C, 51.81; H, 3.14; N, 6.71. Found: C, 51.80, H, 3.10; N, 6.70; 1H-NMR, (MeOH-d4): δ 6.17 (2H, s, CH2), 6.77 (1H, d, J 8.0 Hz, ArH) 7.10-7.63 (7H, m, ArH), 7.99 (1H, s, CH=N); 13C-NMR (MeOH-d4): δ 65.9,113.4, 122.8, 122.9, 123.9, 124.4, 129.4, 129.8, 130.7, 131.0, 131.1, 131.2, 132.8, 132.9, 140.1, 156.3, 160.9, 172.0
Preparation of (2-{[4-(2’-fluorophenyl)-thiazole-2-ylimino]methyl}phenoxy)acetic acid (2c)
2-Formylphenoxyacetic acid (1.0 mmol, 0.18 g) was added to a solution of 2-amino-4-(2’-fluorophenyl)-thiazole (1.0 mmol, 0.19 g) in absolute EtOH (10 mL) in addition to 10% mmol of Yb(OTf)3 as Lewis catalyst and refluxed for 10 hours under N2 (g). The reaction mixture was filtered through a column of silica gel, charcoal and Celite® to remove the catalyst. After evaporation of the ethanol, the product was purified by recrystallisation from CHCl3/MeOH (a few drops) to give the Schiff base in 70% yield; m.p. 167 ºC; HRMS (FAB, MH+) calcd. for C18H13FN2O3S: 357.0709, found: 357.0712; IR (υmax, KBr, cm-1): 3030, 1640, 1550, 1240; 1H-NMR, (MeOH-d4): δ 5.64 (2H, s, CH2), 6.79-7.68 (8H, m, ArH), 7.99 (1H, s, CH=N); 13C-NMR (MeOH-d4): δ 66.3, 116.8, 122.2, 122.5, 125.1, 126.3, 128.1, 128.4, 129.3, 130.3, 130.9, 132.9, 138.2, 140.1, 157.2, 160.1, 169.5, 172.8; Anal. calcd. for C18H13FN2O3S: C, 51.81; H, 3.14; N, 6.71. Found: C, 51.60, H, 3.08; N, 6.64;
Preparation of (2-{[4-(3’-fluorophenyl)thiazole-2-ylimino]methyl}phenoxy)acetic acid (2d)
Compound 2d was synthesized by the method described above. The product was purified by crystallization from chloroform and the yield of the title Schiff base was 70%; m.p. 178 ºC (decomp.); HRMS (FAB, MH+) calcd. for C18H13FN2O3S: 357.0709; found: 357.0712; IR (υmax, KBr, cm-1): 3030, 1635, 1550, 1240; 1H-NMR (MeOH-d4): δ 6.15 (2H, s, CH2), 6.93-7.37 (8H, m, ArH), 7.91 (1H, s, CH=N); 13C-NMR (MeOH-d4): δ 66.3, 113.4, 116.4, 122.3, 124.6, 124.9, 125.3, 129.4, 130.4, 131.2, 131.3, 131.5, 131.6, 138.9, 140.9, 157.9, 162.4, 169.7; Anal. Calcd. for C18H13FN2O3S: C, 51.81; H, 3.14; N, 6.71. Found: C, 51.64, H, 3.10; N, 6.56.
Preparation of (2-{[4-(4’-fluorophenyl)-thiazole-2-ylimino]methyl}phenoxy)acetic acid (2e)
Compound 2e was synthesized by the method described above. The product was purified by crystallization from chloroform and the yield of the Schiff base was 70%; m.p. 158-160 ºC; HRMS (FAB, MH+) calcd. for C18H13FN2O3S: 357.0709, found: 357.0713; IR (υmax, KBr, cm-1): 3030, 1630, 1550, 1240; 1H-NMR (MeOH-d4): δ 6.09 (2H, s, CH2), 6.90-7.48 (8H, m, ArH), 7.91 (1H, s, CH=N); 13C-NMR (MeOH-d4): δ 66.0,113.4, 116.6, 116.8, 122.9, 123.6, 128.0, 129.4, 130.5, 131.0, 131.7, 131.8, 138.9, 140.6, 156.4,163.2, 165.7, 171.6; Anal. Calcd. for C18H13FN2O3S: C, 51.81; H, 3.14; N, 6.71. Found: C, 51.59, H, 3.40; N, 6.49;
Acknowledgements
We greatly acknowledge the Higher Education Commission of Pakistan, Islamabad, as well as University of the Punjab, Lahore and Loughborough University for financial support. We thank Mr John C. Kershaw for running mass spectra and Mr J. Alastair Daley for elemental analysis.
References
- Karia, F. D.; Parsania, P.H. Synthesis, biological and thermal properties of Schiff bases of bisphenol-C. Asian J. Chem. 1999, 11, 991–995. [Google Scholar]
- More, P. G.; Bhalvankar, R. B.; Pattar, S. C. Synthesis and biological activities of Schiff bases of aminothiazoles. J. Indian Chem. Soc. 2001, 78, 474–475. [Google Scholar]
- El-Masry, A. H.; Fahmy, H. H.; Abdelwahed, S. H. A. Synthesis and antimicrobial activity of some new benzimidazole derivatives. Molecules 2000, 5, 1429–1438. [Google Scholar]
- Baseer, M. A.; Jadhav, V. D.; Phule, R. M.; Archana, Y. V.; Vibhute, Y. B. Synthesis and antimicrobial activity of some new Schiff bases. Orient. J. Chem. 2000, 16, 553–556. [Google Scholar]
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. IL Farmaco 1999, 54, 624–628. [Google Scholar]
- Singh, W. M.; Dash, B. C. Synthesis of some new Schiff bases containing thiazole and oxazole nuclei and their fungicidal activity. Pesticides 1988, 22, 33–37. [Google Scholar]
- Hodnett, E. M.; Dunn, W. J. Structure-antitumour activity correlation of some Schiff bases. J. Med. Chem. 1970, 13, 768–770. [Google Scholar]
- Desai, S. B.; Desai, P. B.; Desai, K.R. Synthesis of some Schiff bases, thiazolidones, and azetidinones derived from 2,6-diaminobenzo[1,2-d:4,5-d’]bisthiazole and their anticancer activities. Heterocycl. Commun. 2001, 7, 83–90. [Google Scholar]
- Pathak, P.; Jolly, V. S.; Sharma, K.P. Synthesis and biological activities of some new substituted arylazo Schiff bases. Oriental. J. Chem. 2000, 16, 161–162. [Google Scholar]
- Samadhiya, S.; Halve, A. Synthetic utility of Schiff bases as potential herbicidal agents. Orient. J. Chem. 2001, 17, 119–122. [Google Scholar]
- Aydogan, F.; Öcal, N.; Turgut, Z.; Yolacan, C. Transformations of aldimines derived from pyrrole-2-carboxaldehyde. Synthesis of thiazolidino-fused compounds. Bull. Korean Chem. Soc. 2001, 22, 476–480. [Google Scholar]
- Taggi, A. E.; Hafez, A. M.; Wack, H.; Young, B.; Ferraris, D.; Lectka, T. The development of the first catalysed reaction of ketenes and imines: catalytic asymmetric synthesis of β-lactams. J. Am. Chem. Soc. 2002, 124, 6626–6635. [Google Scholar]
- King, L.C.; Hlavacek, R.J. Reaction of ketones with iodine and thiourea. J. Am. Chem. Soc. 1950, 72, 3722. [Google Scholar]
- Zav’Yalov, S.I.; Dorofeeva, O.V.; Rumyantseva, E.E.; Kulikova, L.B.; Ezhova, G.I.; Kravchenko, N.E.; Zavozin, A.G. Synthesis of 2-aminothiazole derivatives. Pharm. Chem. J. 2001, 35, 96–98. [Google Scholar]
- Sample availability: Contact the authors
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.