L-Proline Catalyzed Michael Additions of Thiophenols to α,β-Unsaturated Compounds, Particularly α-Enones, in the Ionic Liquid [bmim]PF6
Abstract
:Introduction
Results and Discussion
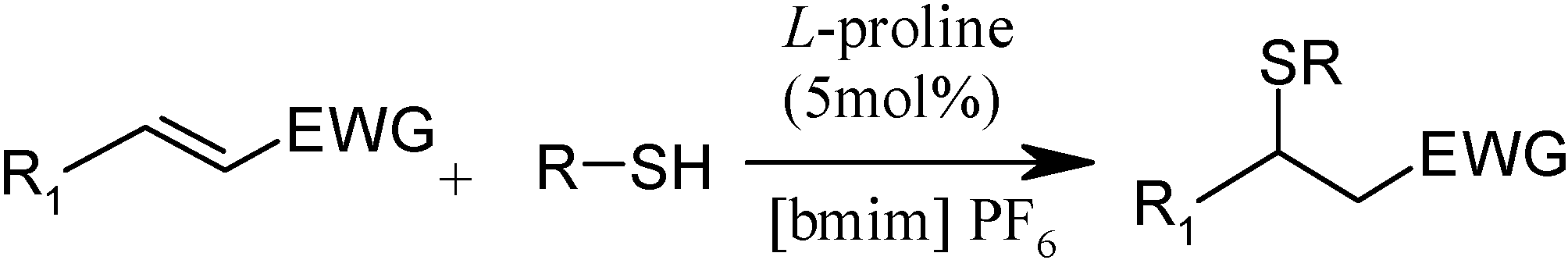
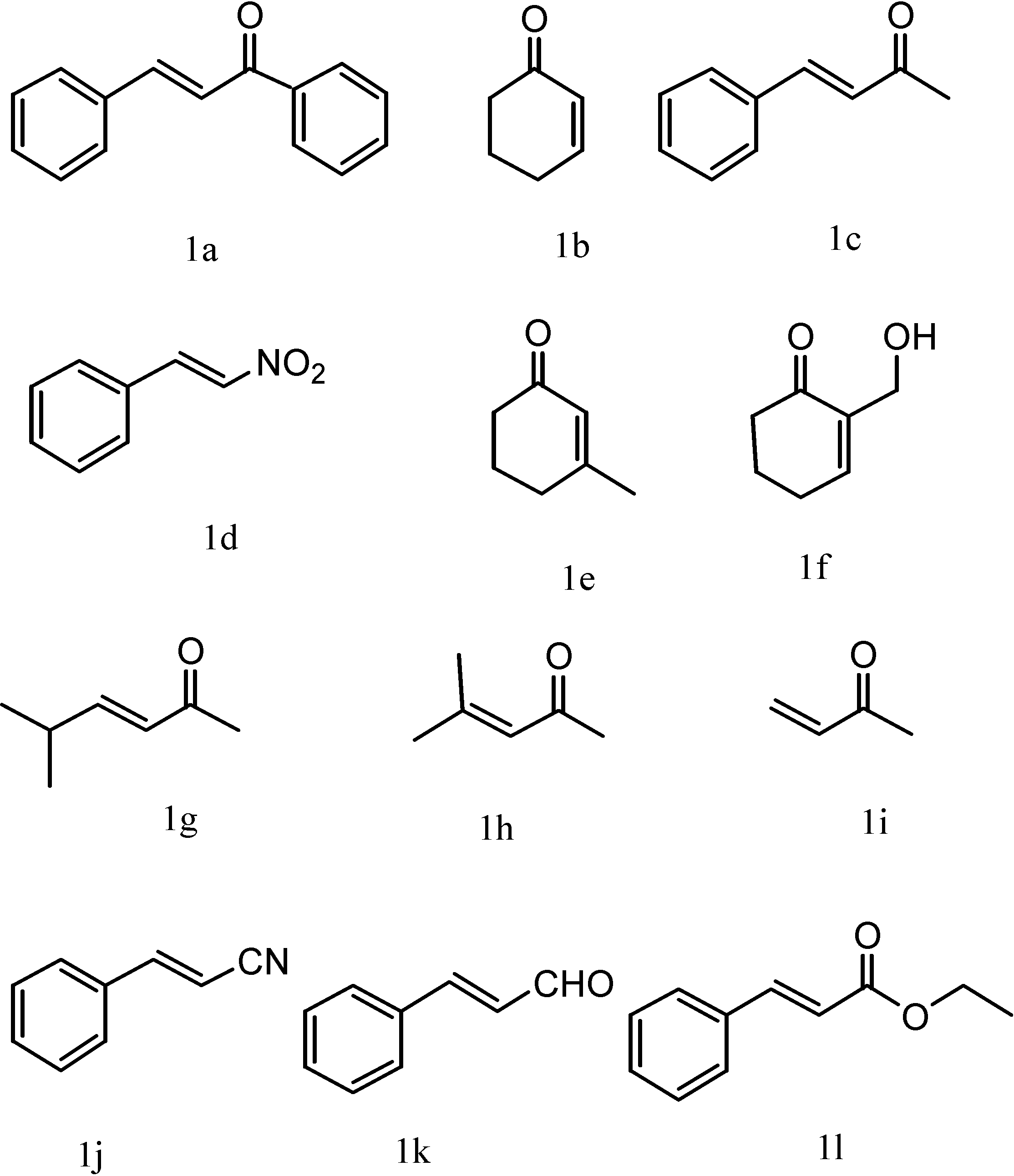
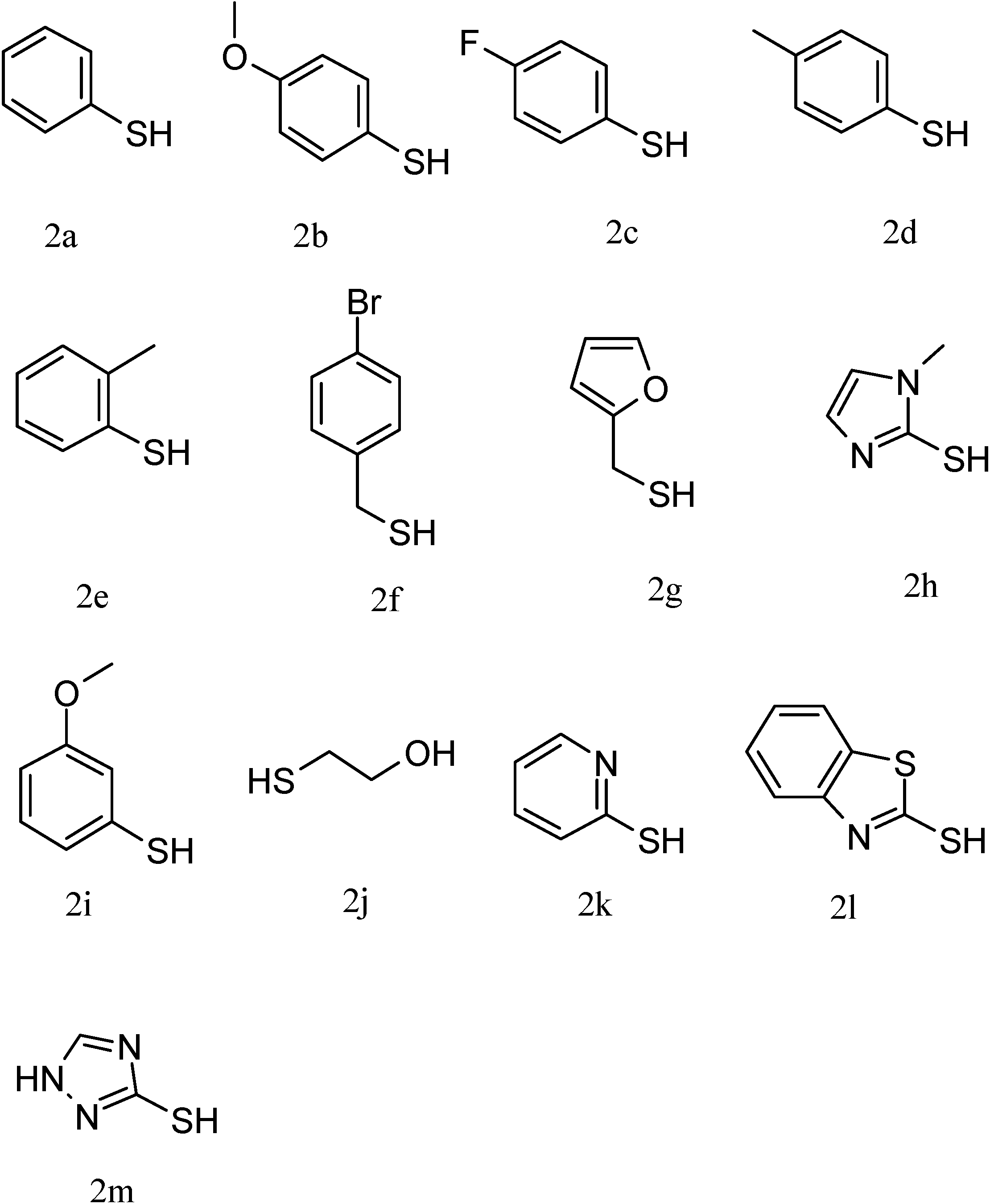

| Entry | Substrate | Reagent | Time (min) | Yield (%) | Product |
|---|---|---|---|---|---|
| 1 | 1a | 2a | 10 | 99 | P1 |
| 2 | 1a | 2b | 5 | 89.8 | P3 |
| 3 | 1a | 2c | 20 | 86 | P4 |
| 4 | 1a | 2d | 10 | 91.5 | P5 |
| 5 | 1a | 2e | 65 | 74 | P6 |
| 6 | 1a | 2f | 30 | 74 | P7 |
| 7 | 1a | 2g | 40 | 83 | P8 |
| 8 a | 1a | 2h | 480 | 27 | P9 |
| 9 | 1b | 2a | 30 | 98 | P2 |
| 10 | 1b | 2i | 30 | 94 | P12 |
| 11 | 1b | 2j | 30 | 96 | P13 |
| 12 | 1c | 2a | 120 | 93 | P10 |
| 13 | 1d | 2a | 30 | 89 | P11 |
| 14 | 1e | 2i | 60 | 19 | P14 |
| 15 b | 1f | 2a | 60 | 18 | P15 |
| 16 | 1g | 2a | 30 | 75 | P16 |
| 17 | 1h | 2a | 30 | 30 | P17 |
| 18 | 1i | 2a | 30 | 95 | P18 |
Conclusions
Acknowledgements
Experimental
General
General experimental procedure
Characterization of the products
References
- Kondo, T.; Mitsudo, T. Chem. Rev. 2000, 56, 3205.
- Garg, S.K.; Kumar, R.; Chakraborti, A.S. Synlett 2005, 1370.
- List, B.; Lerner, R.A.; Barbas, C.F., III. J. Am. Chem. Soc. 2000, 122, 2395.
- List, B. Synlett 2001, 1675.
- List, B. Tetrahedron 2002, 58, 5573.
- Grőger, H.; Wilken, J. Angew. Chem.Int. Ed. 2001, 40, 529. [CrossRef]
- Dalko, P.I.; Moisan, L. Angew. Chem. Int. Ed. 2001, 40, 3727. [CrossRef]
- Berkessel, A.; Grőger, A. (Eds.) Asymmetric Organocatalysis; Wiley-VCH: Weinheim, 2005.
- Helder, R.; Arends, R.; Bolt, W.; Hiemstra, H.; Wynberg, H. Tetrahedron Lett. 1977, 2185.
- Hiemstra, H.; Wynberg, H. J. Am. Chem. Soc. 1981, 103, 417.
- Agami, C.; Platzer, N.; Puchot, C.; Sevestre, H. Tetrahedron Lett. 1987, 53, 1091.
- Mukaiyana, T.; Ikegawa, A.; Suzuki, K. Chem. Lett. 1981, 165.
- Suzuki, K.; Ikegawa, A.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1982, 55, 3277. [CrossRef]
- Kobayashi, S.; Ogawa, C.; Kawamura, M.; Sugiura, M. Synlett 1981, 983.
- Holbrey, J.D.; Seddon, K.R. Clean Prod. Processes 1999, 1, 223.
- Olivier-Bourbigou, H.; Magma, L. J. Mol. Catal. A. Chem. 2002, 182–183, 419.
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Chem. Rev. 2002, 102, 3667.
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Catal. Today 2002, 74, 157.
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, 2003.
- Sheldon, R. Chem. Commun. 2001, 2399.
- Song, C.E. Chem. Commun. 2004, 1033.
- Wilkes, J.S. J. Mol. Catal. A. Chem. 2004, 214, 11–17. [CrossRef]
- Welton, T. Coord. Chem. Rev. 2004, 248, 2459–2477.
- Kotrusz, P.; Kmentová, I.; Gotov, B.; Toma, Š. Chem. Commun. 2002, 2510. [CrossRef]
- Loh, J.S.; Feng, l.C.; Yang, H.Y.; Yang, J.Y. Tetrahedron Lett. 2002, 43, 8741.
- Kotrusz, P.; Toma, S.; Schmalz, H.G.; Adler, A. Eur. J. Org. Chem. 2004, 1577.
- Rasalkar, M.S.; Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. J. Mol. Catal. A. Chem. 2005, 235, 267–270. [CrossRef]
- Hagiwara, H.; Okabe, T.; Hoshi, T.; Suzuki, T. J. Mol. Catal. A: Chem. 2004, 214, 167–174.
- Yadav, S.; Reddy, B.V.S.; Baishya, G. J. Org. Chem. 2003, 68, 7098.
- Ranu, B.C.; Dey, S.S. Tetrahedron 2004, 60, 4183.
- Dere, R.T.; Pal, R.R.; Patil, P.S.; Salunkhe, M.M. Tetrahedron Lett. 2003, 44, 5351.
- Narasaka, K.; Arai, N.; Okauchi, T. Bull. Chem. Soc. Jpn. 1993, 66, 2995. [CrossRef]
- da Silva, F.M.; Gomes, A.K.; Jones, J. Can. J. Chem. 1999, 77, 624.
- Skarzewski, J.; Zielinska-Blajet, M.; Turowska-Tyrk, I. Tetrahedron Asymmetry 2001, 12, 1923.
- Omote, M. Nippon Kagaku Kaishi 1972, 780, [Chem. Abstr.; 1972, 77, 33637f].
- Boldwell, J.R.; Patwardhan, B.H.; Dittmer, D.C. J. Org. Chem. 1984, 49, 4192.
- Cann, S. J. Chem. Soc. Perkin Trans. 2 1974, 817. [CrossRef]
- MacNicol, D.D.; McKendrick, J.J. J. Chem. Soc. Perkin Trans. 1 1974, 2493. [CrossRef]
- Wabnitz, T.C.; Spencer, J.B. Org. Lett. 2003, 5, 2141.
- Sample availability: Samples of the reaction products can be obtained from the author (P.K.)
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kotrusz, P.; Toma, S. L-Proline Catalyzed Michael Additions of Thiophenols to α,β-Unsaturated Compounds, Particularly α-Enones, in the Ionic Liquid [bmim]PF6. Molecules 2006, 11, 197-205. https://doi.org/10.3390/11020197
Kotrusz P, Toma S. L-Proline Catalyzed Michael Additions of Thiophenols to α,β-Unsaturated Compounds, Particularly α-Enones, in the Ionic Liquid [bmim]PF6. Molecules. 2006; 11(2):197-205. https://doi.org/10.3390/11020197
Chicago/Turabian StyleKotrusz, Peter, and Stefan Toma. 2006. "L-Proline Catalyzed Michael Additions of Thiophenols to α,β-Unsaturated Compounds, Particularly α-Enones, in the Ionic Liquid [bmim]PF6" Molecules 11, no. 2: 197-205. https://doi.org/10.3390/11020197




