Dechlorophyllation by Electrocoagulation
Abstract
:Introduction
Results and Discussion
| Plant | Absorbance | |||
|---|---|---|---|---|
| Solution 1 | Solution 2 | |||
| 665 – 666 nm | 408 – 410 nm | 665 – 666 nm | 408 – 410 nm | |
| Solanum laciniatum Andrographis paniculata Stevia rebaudiana Centella asiatica Cassia siamea | 0.35 0.30 0.27 0.65 0.30 | 5.40 1.85 -* 3.50 -* | 1.18 0.70 1.50 1.60 1.75 | 9.03 5.70 -* 6.50 -* |
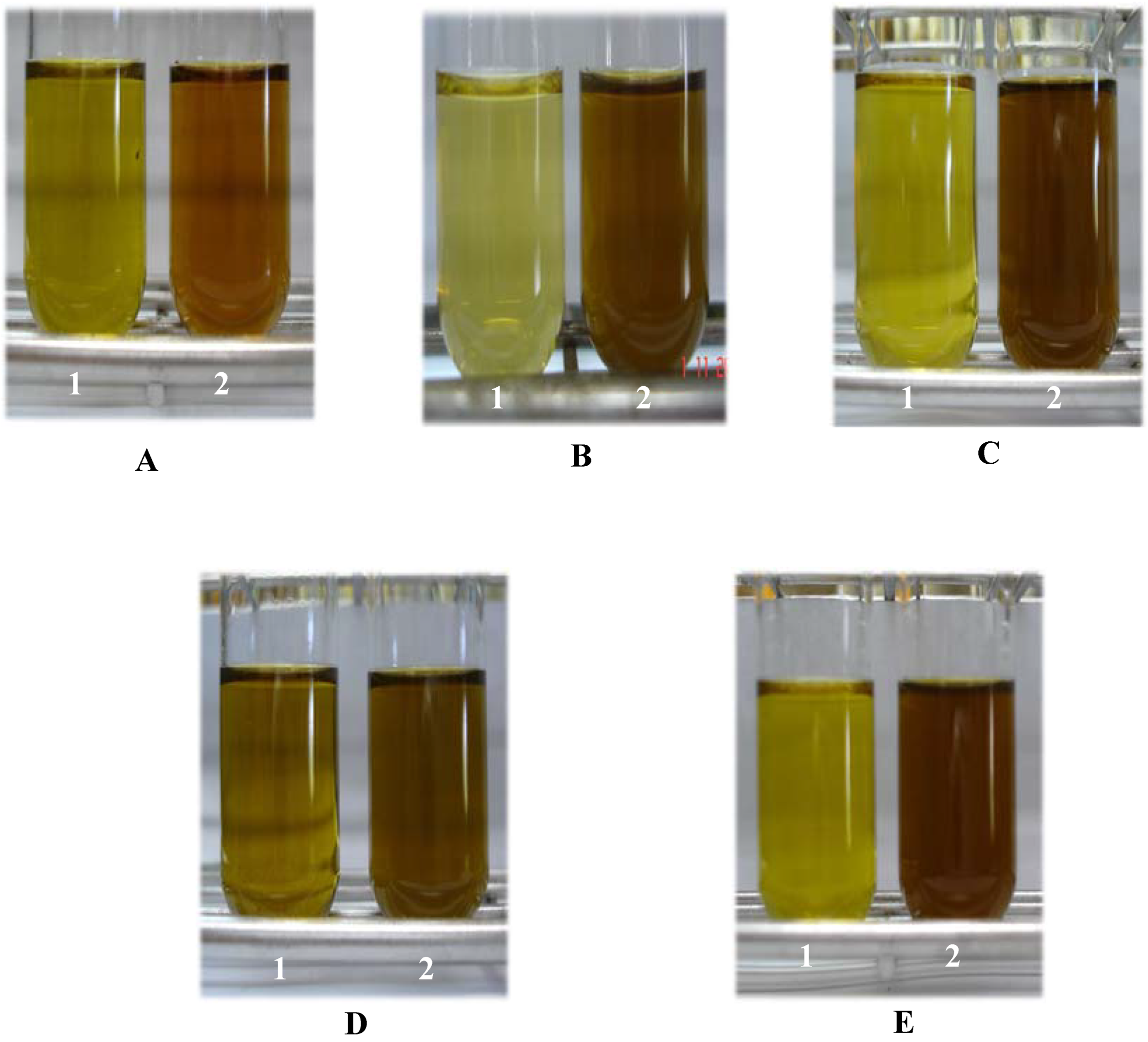
Conclusions
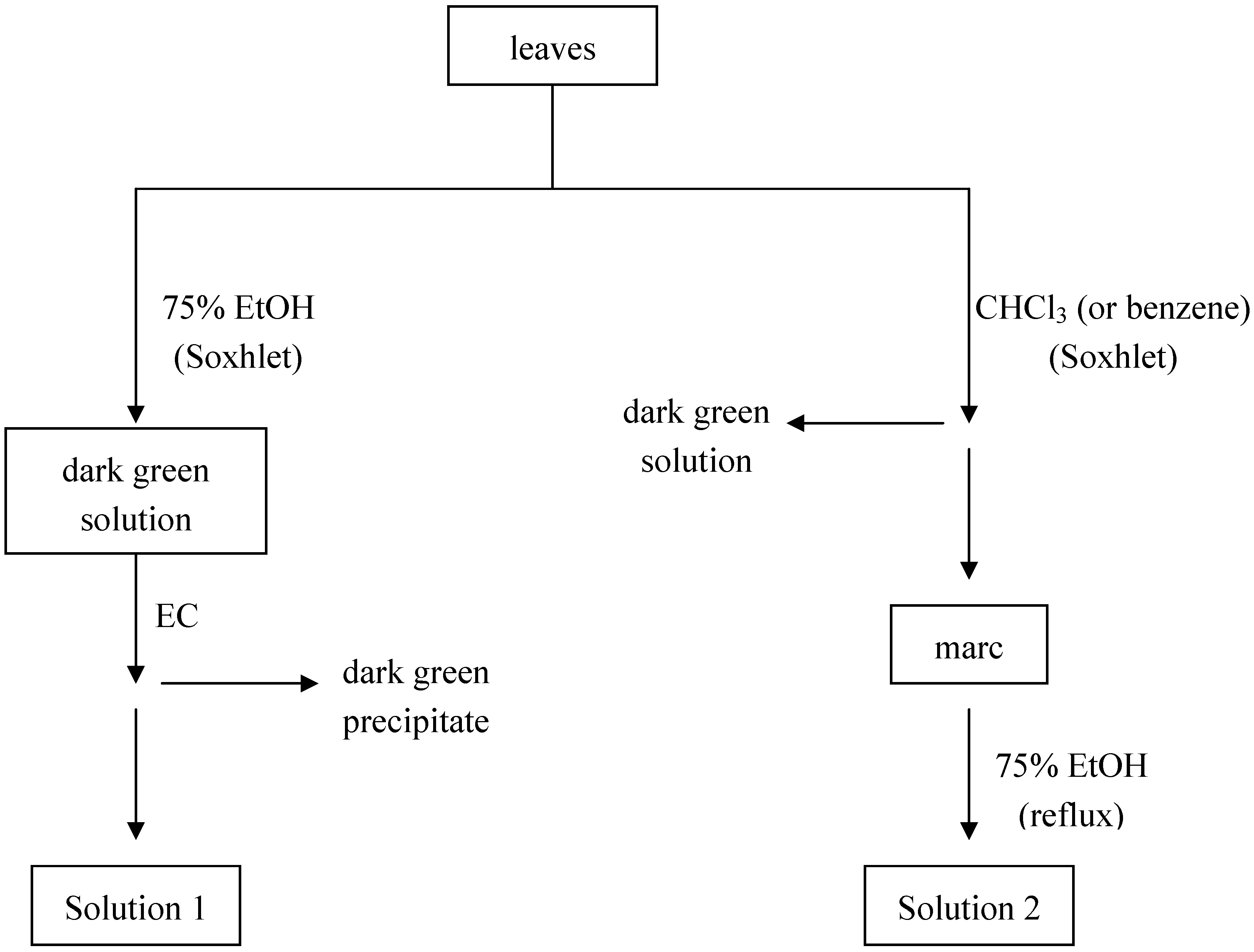
Experimental
Acknowledgements
References
- Miwa, K. Extraction and purification of plant components. Jpn. Kokai Tokkyo Koho. 78105500, 1978. [Google Scholar]
- Beck, E. C.; Giannini, A. P.; Ramirez, E. R. Electrocoagulation clarifies food wastewater. Food Tech. 1974, 28, 18–19. [Google Scholar]
- Dobolyi, E. Experiments aimed at the removal of phosphate by electrochemical methods. Water Res. 1978, 12, 1113–1116. [Google Scholar]
- Mrozowski, J.; Zielinski, J. Studies of zinc and lead removal from industrial wastes by electrocoagulation. Environ. Protect. Eng. 1983, 9, 77–85. [Google Scholar]
- Jenke, D. R.; Diebold, F. E. Electroprecipitation treatment of acid mine wastewater. Water Res. 1984, 18, 855–859. [Google Scholar] [CrossRef]
- Cenkin, V. E.; Belevstev, A. N. Electrochemical treatment of industrial wastewater. Effl. Water Treatment J. 1985, 25, 243–244. [Google Scholar]
- Groeterud, O.; Smoczynski, L. Phosphorus removal from water by means of electrolysis. Water Res. 1986, 20, 667–669. [Google Scholar]
- Renk, R. R. Electrocoagulation of tar sand and oil shale wastewaters. Energy Prog. 1988, 8, 205–208. [Google Scholar]
- Do, J. S.; Chen, M. L. Decolourization of dye-containing solutions by electrocoagulation. J. Appl. Electrochem. 1994, 24, 785–790. [Google Scholar]
- Kongsricharoern, N.; Polprasert, C. Electrochemical precipitating of chromium (Cr6+) from an electroplating wastewater. Water Sci. Tech. 1995, 31, 109–117. [Google Scholar] [CrossRef]
- Pouet, M. F.; Grasmick, A. Urban wastewater treatment by electrocoagulation and flotation. Water Sci. Tech. 1995, 31, 275–283. [Google Scholar] [CrossRef]
- Matteson, M. J.; Dobson, R. L.; Glenn Jr, R. W.; Kukunoor, N. S.; Waits III, W.H.; Clayfield, E. J. Electrocoagulation and separation of aqueous suspension of ultrafine particles. Colloids Surf. Physicochem. Eng. Aspects 1995, 104, 101–109. [Google Scholar]
- Kongsricharoern, N.; Polprasert, C. Chromium removal by a bipolar electrochemical precipitation process. Water Sci. Tech. 1996, 34, 109–116. [Google Scholar]
- Tsai, C. T.; Lin, S. T.; Shue, Y. C.; Su, P. L. Electrolysis of soluble organic matter in leachate from landfills. Water Res. 1997, 31, 3070–3081. [Google Scholar]
- Abuzaid, N. S.; Bukhari, A. A.; Al-Hamouz, Z. M. Removal of bentonite causing turbidity by electrocoagulation. J. Environ. Sci. Health. 1998, A33(7), 1341–1358. [Google Scholar]
- Belongia, B. M.; Hawarth, P. D.; Baygents, J. C.; Raghavan, S. Treatment of alumina and silica chemical mechanical polishing waste by electrodecantation and electrocoagulation. J. Electrochem. Soc. 2005, 146, 4124–4130. [Google Scholar]
- Buso, A.; Balbo, L.; Giomo, M.; Farnia, G.; Sandona, G. Electrochemical removal of tannins from aqueous solutions. Ind. Eng. Chem. Res. 2000, 39, 494–499. [Google Scholar]
- Pinisakul, A.; Polprasert, C.; Parkpian, P.; Satayavivad, J. Arsenic removal efficiency and mechanisms by electro-chemical precipitation process. Water Sci. Tech. 2002, 46, 247–254. [Google Scholar]
- Thaveemaitree, Y.; Polprasert, C.; Seung-hwan, L. Application of electrochemical process for landfill leachate treatment with emphasis on heavy metals organic removal. Environ. Tech. 2003, 24, 1135–1145. [Google Scholar] [CrossRef]
- Vik, E. A.; Carlson, D. A.; Eikum, A. S.; Gjessing, E. T. Electrocoagulation of potable water. Water Res. 1984, 18, 1355–1360. [Google Scholar]
- Mameri, N.; Yeddou, A. R.; Lounici, H.; Belhocine, D.; Grib, H.; Bariou, B. Defluoridation of septentrional Sahara water of North Africa by electrocoagulation process using bipolar aluminium electrodes. Water Res. 1998, 32, 1604–1612. [Google Scholar]
- Phutdhawong, W.; Chowwanapoonpohn, S.; Buddhasukh, D. Simple isolation and purification of D-Pinitol from Cassia siamea Lamk by electrolytic decolourization. ACGC Chem. Res. Comm. 2000, 10, 61–62. [Google Scholar]
- Phutdhawong, W. Ph.D. Thesis, Chiang Mai University, Thailand, 2002.
- Chowwanapoonpohn, S. Ph.D. Thesis, Chiang Mai University, Thailand, 2003.
- Jumpatong, K.; Phutdhawong, W.; Chowwanapoonpohn, S.; Garson, M. J.; Pyne, S. G.; Buddhasukh, D. Trends in Electrochemistry Research; Nova Publishers: New York. (in press)
- Adduci, J.; Buddhasukh, D.; Ternai, B. Improved isolation and purification of stevioside. J. Sci. Soc. Thailand. 1987, 13, 179–183. [Google Scholar]
- Saleh, A. A. Steroid alkaloid and sapogenin constituents of Solanum laciniatum Aiton grown in Egypt. Pharmazie 1974, 29, H.5. 346–347. [Google Scholar]
- Fayez, M. B. E.; Saleh, A. A. The steroidal alkaloids of Solanum Wrightii Benth. Phytochem. 1967, 6, 433–436. [Google Scholar] [CrossRef]
- Jewvachdamrongkul, Y.; Chokechaijaroenporn, O.; Chavalittumrong, P.; Dechatiwongse, T. Chemical quality evaluation of Fah Talai Joan. Bull Dept. Med. Sci. 1987, 29, 231–237. [Google Scholar]
- Dechatiwongse, T.; Dechwisissakul, P.; Techadamrongsin, Y.; Bansiddhi, J.; Chuthaputti, A. Standard of Thai Herbal Medicine: Andrographis paniculata(Burm. F.) Nees; The War Veterans Organization Press: Bangkok, 2005; pp. 46–47. [Google Scholar]
- Kim, K.; Seo, S. K.; Park, J. K.; Lee, S. Y.; Hwang, B. R. Water-soluble extract of asiaticoside and madecassoside from Centella asiatica. Eur. Pat. Appl. EP 97-114291, 1998. [Google Scholar]
- Das, A; Mallick, R. Correlation between genomic diversity and asiaticoside content in Centella asiatica (L.) Urban. Bot. Bull. Acad. Sinica 1991, 32, 1–8. [Google Scholar]
- Sample Availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jumpatong, K.; Phutdhawong, W.; Buddhasukh, D. Dechlorophyllation by Electrocoagulation. Molecules 2006, 11, 156-162. https://doi.org/10.3390/11020156
Jumpatong K, Phutdhawong W, Buddhasukh D. Dechlorophyllation by Electrocoagulation. Molecules. 2006; 11(2):156-162. https://doi.org/10.3390/11020156
Chicago/Turabian StyleJumpatong, Kanlaya, Weerachai Phutdhawong, and Duang Buddhasukh. 2006. "Dechlorophyllation by Electrocoagulation" Molecules 11, no. 2: 156-162. https://doi.org/10.3390/11020156
APA StyleJumpatong, K., Phutdhawong, W., & Buddhasukh, D. (2006). Dechlorophyllation by Electrocoagulation. Molecules, 11(2), 156-162. https://doi.org/10.3390/11020156




