3. Experimental Section
Chemistry
GLC analyses were conducted on a Shimadzu GC-17A instrument equipped with a flame-ionization detector, using a DB-1 (30 m x 0.25 mm) glass column. Column chromatography was performed on silica gel 60 (70-230 mesh ASTM Merck). Radial thin-layer chromatography was carried out on a Chromatotron model 8924 (silica gel 60PF274 Merck). Melting points were determined on a Microquímica MQWAPF-301 apparatus and are uncorrected. Infrared spectra were recorded with a Bomen Hartman & Braun MB-Series spectrometer. 1H- and 13C- NMR spectra were recorded at 200 or 50 MHz, respectively, either on a Bruker ARX-200 or a Bruker DRX-400 spectrometer in CDCl3 with TMS as internal standard. The mass spectra were recorded on a Micromass mass spectrometer Quattro LC, coupled with a chemical ionization source (reagent MeOH) under atmospheric pressure (APCI). Microanalyses were performed on a Fisons EA 1108 CHNS-O analyzer, at the Chemistry Department, Universidade Federal de São Carlos. Solvents were purified prior to use: dichloromethane and hexane were refluxed over P2O5, distilled and stored over molecular sieves; pyridine was stirred and refluxed over KOH, distilled and stored over KOH; acetic anhydride was stirred over P2O5 and K2CO3, distilled and stored over molecular sieves.
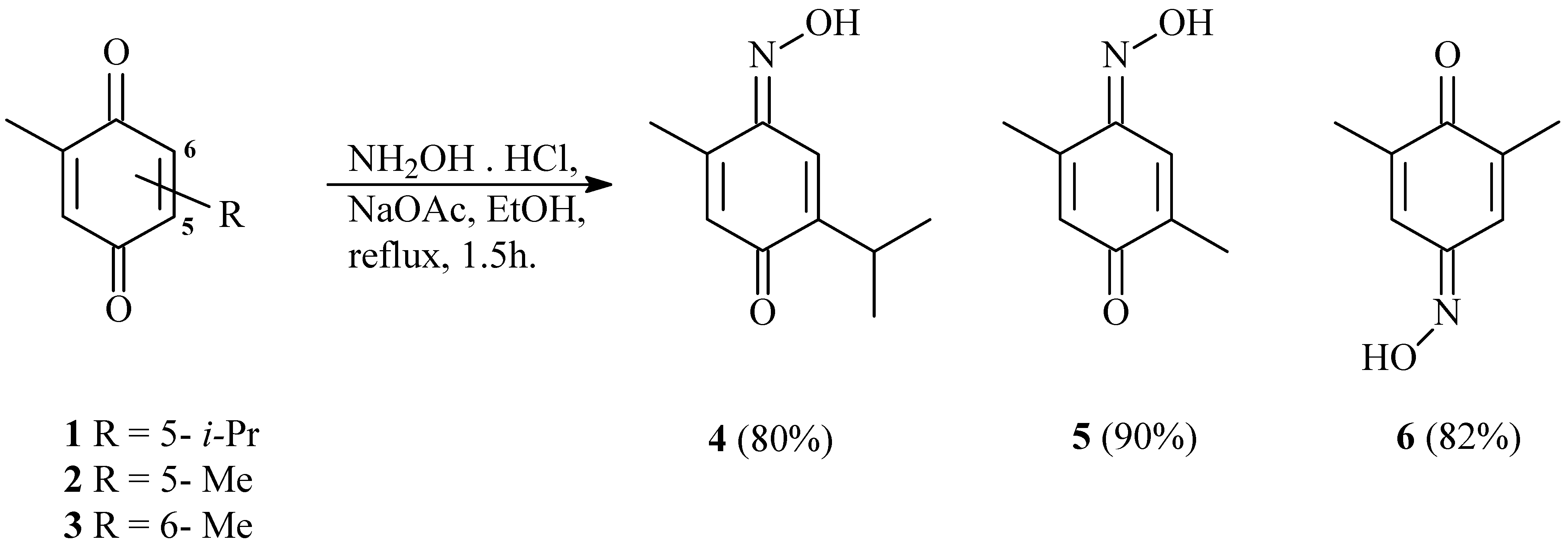
General procedure for the syntheses of the oximes 4, 5 and 6:
A solution of the hydroxylamine hydrochloride (0.221 g, 3.18 mmol) and sodium acetate (0.169 g, 2.08 mmol) were added to para-benzoquinone (1.22 mmol) in EtOH (11.70 ml), and the resulting mixture was stirred and refluxed for 1.5 hours. After the solvent was evaporated under reduced pressure, the solid residue was diluted in water (50 mL) and extracted with a mixture of ethyl acetate and ethyl ether (1:1, 3 × 20 mL). The organic layer was washed with aqueous NaHCO3 (3 × 20 mL) and NaCl (3 × 20 mL), dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The solid residue was washed with hexane (4 × 20 mL) to eliminate soluble impurities.
(4E)-2-isopropyl-5-methylbenzo-1,4-quinone 4-oxime (4)
Yield: 80%; Mp 142.6-143.2 °C; lit. 140 °C [
13], IR
(νmax., KBr, cm
–1): 3180, 2964, 1639, 1605, 1439, 1241, 1056;
1H-NMR δ: 1.13 (6H, d,
J = 6.8 Hz), 2.20 (3H, d,
J = 1.3 Hz), 3.07 (1H, d hept,
J = 1.1 and 6.8 Hz), 6.27 (1H, q,
J = 1.3 Hz), 7.56 (1H, d,
J = 1.1 Hz);
13C-NMR δ: 16.8, 21.5, 26.3, 118.2, 128.4, 146.0, 147.9, 150.1, 186.8; MS m/z (%): 180 [M+1]
+ (100), 163 (4), 109 (28); Anal. Calcd. for C
10H
13NO
2: 7.82 (N), 67.02 (C), 7.31 (H); Found: 7.56 (N), 67.11 (C), 7.55 (H).
(1E)-2,5-dimethylbenzo-1,4-quinone oxime (5)
Yield: 90%; Mp 126.5-127.4 °C; IR (νmax., KBr, cm–1): 3232, 2889, 1642, 1611, 1353, 1187, 1025; 1H-NMR δ: 2.03 (3H, d, J = 1.4 Hz), 2.19 (3H, d, J = 1.3 Hz), 6.31 (1H, q, J = 1.3 Hz), 7.63 (1H, q, J = 1.4 Hz); 13C-NMR δ: 15.8, 16.8, 121.5, 128.2, 138.9, 146.9, 150.2, 187.9; MS (m/z (%): 152 [M+1]+ (100), 124 (5), 109 (7); Anal. Calcd. for C8H9NO2: 9.27 (N), 63.56 (C), 6.00 (H). Found: 9.13 (N), 63.13 (C), 5.88 (H).
(4E)-2,6-dimethylbenzo-1,4-quinone oxime (6)
Yield: 82%; Mp 138.4-139.0 °C; IR (νmax., KBr, cm–1): 3216, 2853, 1632, 1604, 1423, 1199, 1058; 1H-NMR δ: 2.01 (3H, d, J = 1.1 Hz), 2.04 (3H, d, J = 1.2 Hz), 7.02 (1H, q, J = 1.1 Hz), 7.58 (1H, q, J = 1.2 Hz); 13C-NMR δ: 15.7, 16.3, 120.5, 133.8, 136.4, 139.0, 149.2, 187.8; MS m/z (%): 152 [M+1]+ (100), 138 (5), 122 (14); Anal. Calcd. for C8H9NO2: 9.27 (N), 63.56 (C), 6.00 (H). Found: 9.28 (N), 63.23 (C), 5.98 (H).
(4E)-2-isopropyl-5-methylbenzo-1,4-quinone 4-(O-methyloxime) (7)
A solution of 4 (0.100 g; 0.56 mmol) in dichloromethane (2.5 mL) and THF (0.1 mL), was added to sodium hydride (60%, 0.020 g, 0.83 mmol, previously washed several times with dry hexane) and the resulting mixture was stirred for 1 hour at room temperature. Methyl iodide (0.16 mL, 2.57 mmol) was added and the reaction was allowed to stand at room temperature for 18 hours. The mixture was diluted with water (20 mL) and the layers were separated. The aqueous layer was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with aqueous NH4Cl (3 × 20 mL), dried over anhydrous Na2SO4 and the solvents were evaporated under reduced pressure. The oily residue was purified by radial thin-layer chromatography (9:1 hexane-ethyl acetate as eluent) to provide a pure sample of 7: (0.091 g, 0.470 mmol, 84% yield). IR (νmax., KBr, cm–1): 2972, 1637, 1613, 1518, 1372, 1240; 1H-NMR δ: 1.12 (6H, d, J = 6.8 Hz), 2.16 (3H, d, J = 0.8 Hz), 3.06 (1H, d hept, J = 6.8, 0.8 Hz), 3.46 (3H, s), 6.26 (1H, d, J = 0.8 Hz), 7.38 (1H, d, J = 0.8 Hz); 13C-NMR δ: 16.8, 22.1, 26.6, 63.7, 118.4, 129.1, 145.6, 149.0, 149.6, 186.7; MS m/z (%): 194 [M+1]+ (100), 179 (26), 164 (30), 152 (5); Anal. Calcd. for C11H15NO2: 7.25 (N), 68.37 (C), 7.82 (H). Found: 6.98 (N), 67.99 (C), 7.72 (H).
(4E)-2-isopropyl-5-methylbenzo-1,4-quinone 4-(O-acetyloxime) (8)
Pyridine (0.34 mL) was added to a mixture of 4 (0.100 g, 0.56 mmol), DMAP (catalytic amount) and acetic anhydride (0.13 mL, 1.36 mmol). The resulting mixture was stirred for 20 hours at room temperature. The mixture was diluted with ethyl ether (20 mL) and washed successively with 10% aqueous CuSO4 (3 × 20 mL), 5% aqueous NaHCO3 (2 × 20 mL) and distilled water (20 mL), dried over Na2SO4 and the solvent was evaporated under reduced pressure. The solid residue was purified by radial thin-layer chromatography (9:1 hexane-ethyl acetate as eluent) to provide a pure sample of 8: (0.088 g, 0.40 mmol, 71% yield). Mp 68.4 – 70.4 °C; IR (νmax., KBr, cm–1): 2965, 1785, 1650, 1630, 1601, 1522, 1249, 1150; 1H-NMR δ: 1.13 (6H, d, J = 6.9 Hz), 2.26 (3H, d, J = 1.4 Hz), 2,37 (3H, s), 3.09 (1H, d hept, J1 = 1.1 Hz and J2 = 6.9 Hz), 6.39 (1H, d, J = 1.4 Hz), 7.39 (1H, d, J = 1.1 Hz); 13C-NMR δ: 17.3, 19.8, 21.7, 34.1, 118.4, 131.1, 145.5, 151.9, 154.1, 168.9, 186.7; MS m/z (%): 222 [M+1]+ (45), 180 (100), 164 (24), 152 (18), 138 (30), 110 (90); Anal. Calcd. for C12H15NO3: 6.33 (N), 65.14 (C), 6.79 (H). Found: 6.00 (N), 64.89 (C), 6.56 (H).
(4E)-2-isopropyl-5-methylbenzo-1,4-quinone 4-[O-(2-bromoethyl)-oxime] (9)
A solution of 4 (0.118 g, 0.66 mmol) in dichloromethane (2.5 mL) and THF (0.1 mL) was added to sodium hydride (60%, 0.020 g, 0.83 mmol, previously washed several times with dry hexane) and the resulting mixture was stirred for 1 hour at room temperature. Dibromoethane (0.11 mL, 1.32 mmol) was added and the reaction was allowed to stand at room temperature for 6 hours. The mixture was diluted with water (20 mL) and the layers were separated. The aqueous layer was extracted with ethyl acetate (3 × 20 mL). The organic layers were combined, washed with aqueous NH4Cl (3 × 20 mL), dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The oily residue was purified by radial thin-layer chromatography (9:1 hexane-ethyl acetate as eluent) to provide a pure sample of 9: (0.132 g, 0.46 mmol, 70% yield). IR (νmax., KBr, cm–1): 2963, 1630, 1623, 1424, 1239, 1077, 1010; 1H-NMR δ: 1.11 (6H, d, J = 6.7 Hz), 2.14 (3H, s), 3.05 (1H, m), 3.62 (2H, t, J = 6.3 Hz), 4.59 (2H, t, J = 6.3 Hz), 7.39 and 6.28 (2H,s); 13C-NMR δ: 16.8, 21.6, 26.7, 28.9, 75.0, 118.5, 129.5, 133.3, 145.4, 149.6, 186.7; MS m/z (%): 287 [M+1]+ (100), 268 (10), 235 (15), 179 (45), 163 (18); Anal. Calcd. for C12H16NO2Br: 4.89 (N), 50.37 (C), 5.64 (H). Found: 4.76 (N), 50.24 (C), 5.54 (H).
(4E)-2-isopropyl-5-methylbenzo-1,4-quinone-(O-[2-(diethylamino)-ethyl]-oxime) (10)
A solution of (9) (0.40 g; 1.39 mmol) in EtOH (15 ml) was added to diethylamine (0.43 ml, 0.307 g; 4.2 mmol) and the resulting mixture was stirred for 6 hours at room temperature. The solvent and excess diethylamine were evaporated under reduced pressure. The oily residue was purified by radial thin-layer chromatography (9:1 hexane-ethyl acetate as eluent) to provide a pure sample of 10: (0.192 g, 0.69 mmol, 49% yield). IR (νmax., KBr, cm–1): 2966, 1640, 1626, 1466, 1306, 1240, 1044; 1H-NMR δ: 1.10 (6H, t, J = 7.2 Hz), 1.15 (6H, d, J = 6.9 Hz), 2.15 (3H, d, J = 1.3 Hz), 2.7 (4H, q, J = 7.2 Hz), 2.95 (2H, t, J = 5.9 Hz), 3.08 (1H, d hept, J1 = 1.1, J2 = 6.9 Hz), 4.5 (2H, t, J = 5.9 Hz), 6.28 (1H, q, J = 1.3 Hz), 7.39 (1H, d, J = 1.1 Hz); 13C-NMR δ: 11.2, 16.8, 21.7, 26.7, 47.5, 50.8, 74.2, 118.6, 129.2, 145.5, 149.3, 149.9, 186.7; MS m/z (%): 279 [M+1]+ (100), 164 (13), 116 (11); Anal. Calcd. for C16H24N2O2: 10.06 (N), 69.03 (C), 9.41 (H). Found: 9.80 (N), 68.65 (C), 9.12 (H).
Acute toxicity
The toxicity study was performed with different doses of compounds to groups of mice (n = 10) administered intraperitoneally (i.p.), and mortality was recorded for 48 h [
14].
Pharmacology
The forced swimming (behavioural despair) test was employed on 6-8 week old male Swiss albino mice (weighing 28-36 g). The animals were maintained at constant room temperature (23 ± 1°C) and on a 12/12 hour light-dark cycle (light from 07:00 to 19:00 hours) with free access to food and water. They were acclimatized for a minimum of 7 days.
The synthesized compounds (10 mg/kg) and imipramine (15 mg/kg) were dissolved in 5% Tween 80 and saline (0.9%), respectively. They were injected i.p. into mice (n = 6) in dose/volume of 1 mL/100 g, one hour prior to testing. Saline was used as control. One hour after injection the mice were placed one at a time into a plexi-glass cylinder (22 cm height, 10 cm diameter) containing 8 cm height of water at 24 °C and the immobility time of each mouse was measured between 2 and 6 min [
15].
Statistical analysis
Analysis of variance and Student’s “t” test were made and the results were considered significant when p < 0.05.