Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide
Abstract
:Introduction
Results and Discussion
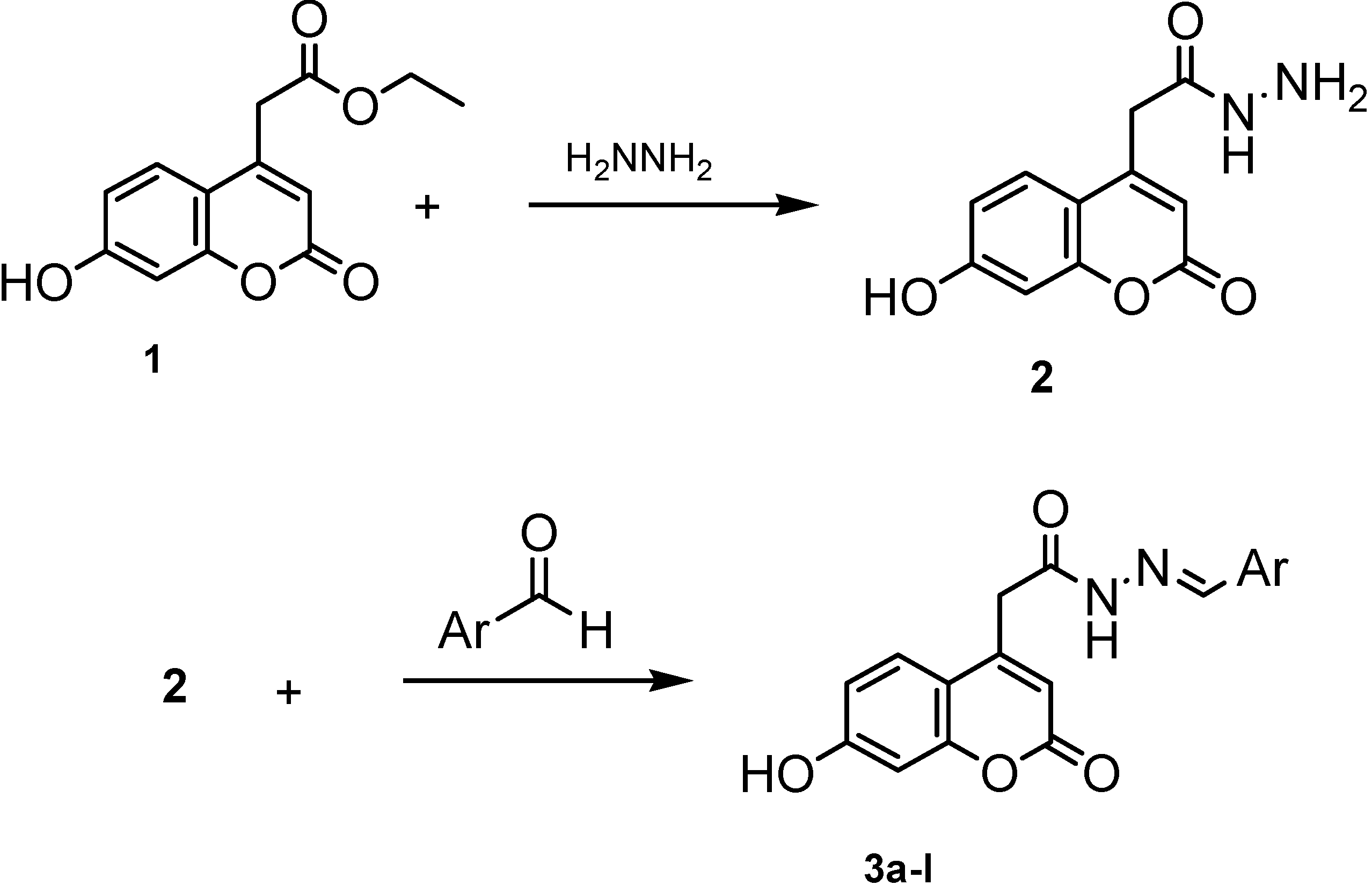
| Entry | Ar | Entry | Ar |
|---|---|---|---|
| a | phenyl | g | 2,5-dihydroxyphenyl |
| b | 2-hydroxyphenyl | h | 3-phenoxyphenyl |
| c | 2-chlorophenyl | i | 3-methoxy-4-hydroxyphenyl |
| d | 3-chlorophenyl | j | styryl |
| e | 2,3-dhydroxyphenyl | k | 4-N,N-dimethylaminophenyl |
| f | 2,4-dhydroxyphenyl | l | 2-hydroxy-5-nitrophenyl |
Antibacterial activity
Experimental Section
General
(7-Hydroxy-2-oxo-2H-chromen-4-yl) acetic acid hydrazide (2).
General procedure for the preparation of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid aryliden-hydrazides 3a-l.
N'-benzylidene -2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3a)
N'-(2-hydroxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3b)
N'-(2-chlorobenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3c)
N'-(3-chlorobenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3d)
N'-(2,3-dihydroxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3e)
N'-(2,4-dihydroxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3f)
N'-(2,5-dihydroxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3g)
N'-(3-phenoxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3h)
N'-(4-hydroxy-3-methoxybenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3i)
2-(7-hydroxy-2-oxo-2H-chromen-4-yl)-Nَ-(3-phenylallidene) acetohydrazide (3j)
N'-[4-(N,N-dimethylamino)benzylidene]-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3k)
N'-(2-hydroxy-5-nitrobenzylidene)-2-(7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (3l)
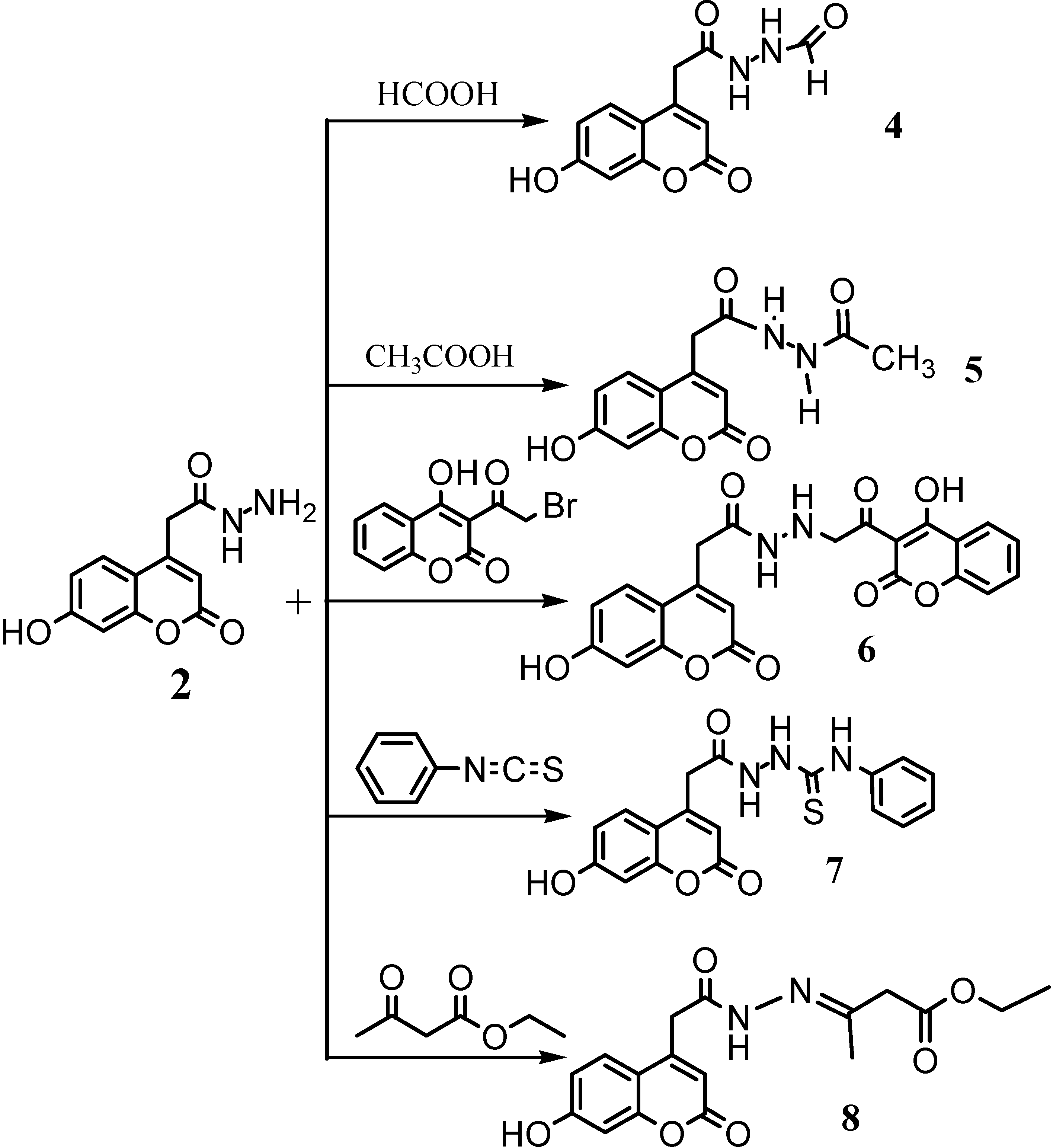
N'-[2-(7-hydroxy-2-oxo-2H-chromen-4-yl)-acetyl]-formic acid hydrazide (4)
N'-[2-(7-hydroxy-2-oxo-2H-chromen-4-yl)-acetyl] acetic acid hydrazide (5)
N'-[2-(4-hydroxy-2-oxo-2H-chromen-3-yl)-2-oxoethyl]-2-(-7-hydroxy-2-oxo-2H-chromen-4-yl) acetohydrazide (6)
4-Phenyl-1-(7-hydroxy-2-oxo-2H-chromen-4-acetyl-) thiosemicarbazide (7)
Ethyl 3-{2-[2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetyl] hydrazono}butanoate (8)
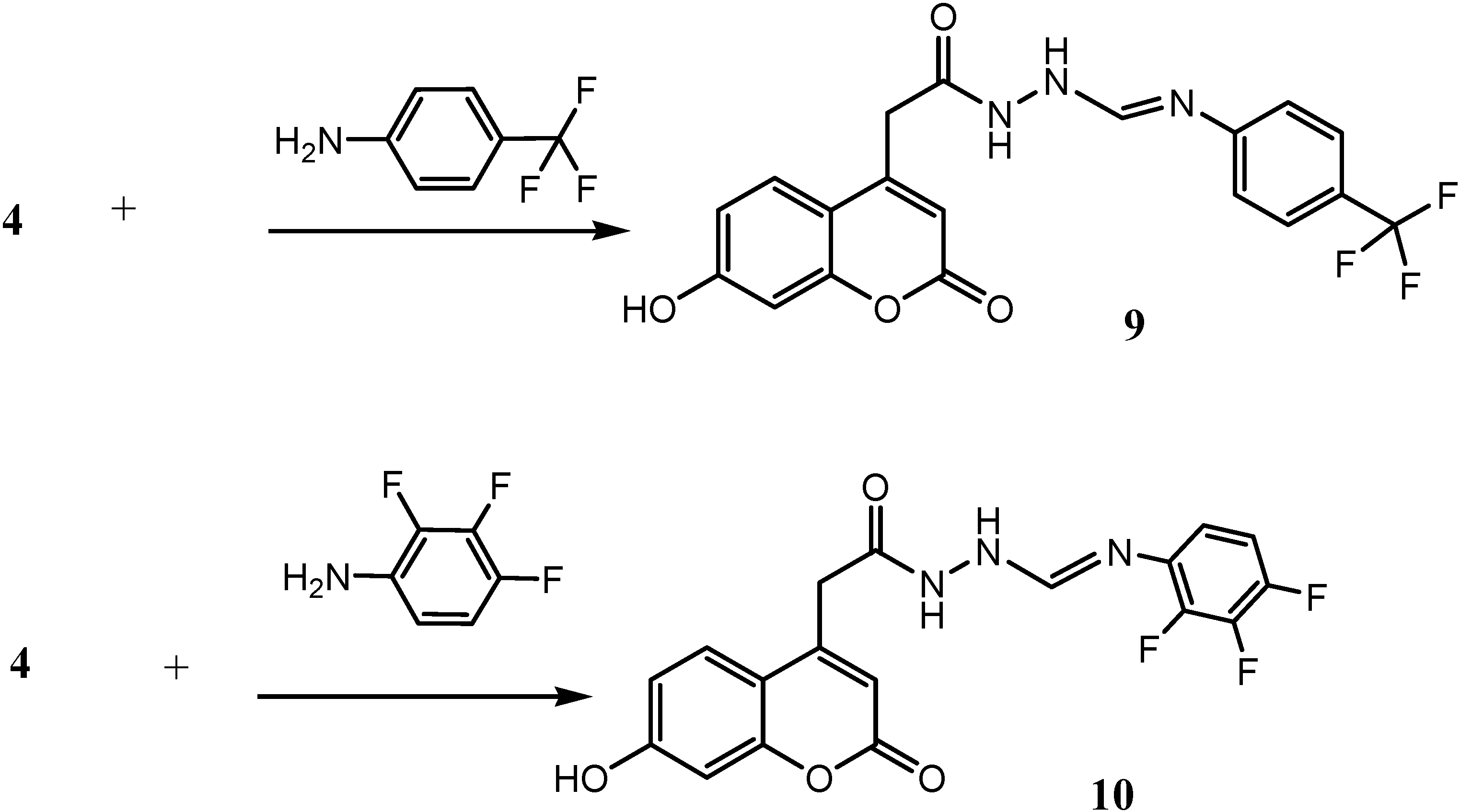
2-(7-Hydroxy-2-oxo-2H-chromen-4-yl)-N'-{[4-(trifluromethyl)phenylimino]methyl}acetohydrazide (9)
2-(7-Hydroxy-2-oxo-2H-chromen-4-yl)-N'-[(2,3,4-trifluorophenylimino)methyl] acetohydrazide (10)
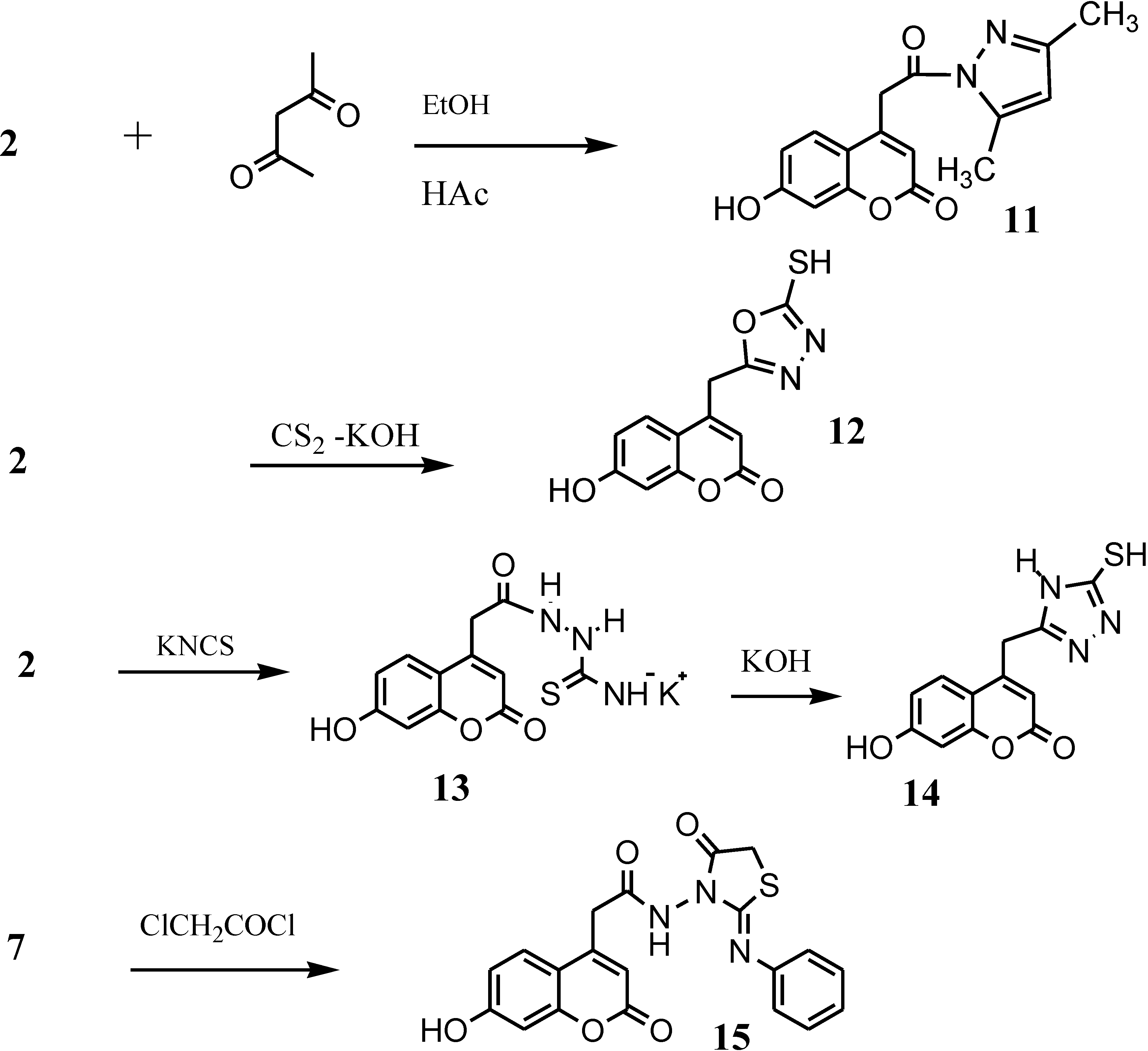
4-[2-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-oxoethyl]-7-hydroxy-2H-chromen-2-one (11)
7-Hydroxy-4-[(5-mercapto-1,3,4-oxadiazol-2-yl)methyl]-2H-chromen-2-one (12)
7-hydroxy-4-[(5-mercapto-4H-1,2,4-triazol-3-yl)methyl]-2H-chromen-2-one (14)
2-(7-hydroxy-2-oxo-2H-chromen-4-yl)-N-[4-oxo-2-(phenylimino)thiazolidin-3-yl] acetamide (15)
References and Notes
- Czerpack, R.; Skolska, S. Effect of selected synthetic regulators on Pseudomonas aeruginosa growth in liquid culture. Med. Dosw. Microbiol. 1982, 34, 37–50, [Chem. Abstr. 1983, 98, 50232]. [Google Scholar]
- Jund, L.; Corse, J.; King, A.S.; Bayne, H.; Mihrag, K. Antimicrobial properties of 6,7-dihydroxy‑, 7,8-dihydroxy-, 6-hydroxy- and 8-hydroxycoumarins. Phytochemistry 1971, 10, 2971–2974. [Google Scholar]
- El-Ansary, S.L.; Aly, E.I.; Halem, M.A. New coumarin derivatives as antibacterial agents. Egypt. J. Pharm. Sci. 1992, 33, 379–390. [Google Scholar]
- Reddy, Y.D.; Somayojulu, V.V. Synthesis, spectra and physiological activity of 7H-pyrano[3,2-c]benzoxazole-7-one. J. Ind. Chem. Soc. 1981, 58, 599–601. [Google Scholar]
- Abd Allah, O.A. Synthesis and biological studies of some benzopyrano[2,3-c]pyrazole derivatves. Il Farmaco 2000, 55, 641–649. [Google Scholar]
- Wkrner, W. Antitubercular Agents. Derivatives of pyridinecarboxylic acid hydrazides. J. Org. Chem. 1953, 18, 1333–1337. [Google Scholar]
- Parmer, S.S.; Kumar, R. Substituted quinazolinone hydrazides as possible antituberculous agents. J. Med. Chem. 1963, 11, 635–636. [Google Scholar]
- Bhamaria, R.P.; Bellare, R.A.; Deliwala, C.V. In intro effect of 1-acyl-4-alkyl-(or aryl)-thiosemicarbazides 1-(5-chlorosalicylidine)-4-alkyl-(or aryl)-thiosemicarbazones and some hydrazones of 5-chlorosalicylaldehyde against pathogenic bacteria including Mycobacterium tuberculosis (H37Rv). Indian J. Exp. Biol. 1968, 6, 62–63. [Google Scholar]
- Abdel-Al, E.H.; Al-Ashamawi, M.I.; Abd El-Fattah, B. Synthesis and antimicrobial testing of certain oxadiazoline and triazole derivatives. Die Pharmazie 1983, 38, 833–838. [Google Scholar]
- Gupta, A.K.S.; Garg, M.; Chandra, U. Synthesis of some new Mannich bases derived from substituted benzimidazole, benzoxazol-2-one, benzoxazol-2-thione, oxadiazol-2-thiones and their biological activities. J. Indian Chem. Soc. 1979, 56, 1230–1232. [Google Scholar]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Dutta, M.M.; Goswani, B.N.; Kataky, J.C.S. Studies on biologically active heterocycles. Part I. Synthesis and antifungal activity of some new aroyl hydrazones and 2,5-disubstituted-1,3,4-oxadiazoles. J. Heterocyclic Chem. 1986, 23, 793–795. [Google Scholar]
- Sharma, S.C. Synthesis of new fungicides. 2-(4'-arylthiazolyl-2'-imino)-3-aryl-4-thiazolidones. Bull. Chem. Soc. Japan 1967, 40, 2422–2424. [Google Scholar]
- Chaubey, V.N.; Singh, H. Synthesis of some new fungicides. Bull. Chem. Soc. Japan 1970, 43, 2233–2236. [Google Scholar]
- Foye, W.O.; Tovivich, P. N-Glucopyranosyl-5-aralkylidenerhodanines: Sinthesis and antibacterial and antiviral activities. J. Pharm. Sci. 1977, 66, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Akerblom, E.B. Synthesis and structure-activity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2-furylpropenylidene)thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J. Med. Chem. 1974, 17, 609–615. [Google Scholar] [CrossRef]
- Carter, D.S.; Vranken, D.L. Synthesis of homofascaplysinC and Indolo[2,3-a]carbazole from ditryptophans. J. Org. Chem. 1999, 64, 8537–8545. [Google Scholar] [CrossRef]
- Mazaleyrat, J.; Wakselman, M.; Formaggio, F.; Crisma, M.; Toniolo, C. Synthesis of terminally protected 9-amino-4,5-diazafluorene-9-carboxylic acid, the first rigid, transition-metal receptor, C-alpha, C-alpha-disubstituted glycine. Tetrahedron Lett. 1999, 40, 6245–6248. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, M.; Zhang, S. One-pot synthesis of cinnamoyl hydrazides. ARKIVOC 2000, (ix). 19–23. [Google Scholar]
- Patersson, J.; Ollmann, I.; Cravatt, B.; Boger, D.; Wong, C.; Lerner, R. Inhibition of oleamide hydrolase catalyzed hydrolysis of the endogenous sleep-inducing lipid cis-9-octadecenamide. J. Am. Chem. Soc. 1996, 118, 5938–5945. [Google Scholar] [CrossRef]
- Jansen, R.; Schummer, D.; Irschik, H.; Hoefle, G. Antibiotics from gliding bacteria, XLII. Chemical modification of SorangicinA and structure-activity relationship I: Carboxyl and hydroxyl group derivatives. Liebigs Ann. Chem. 1990, 10, 975–988. [Google Scholar]
- Miyasaka, T.; Hibino, S. Synthesis of novel streptonigrin 2-amide derivatives with 3,3'-(phenylphosphoryl)bis(1,3-thiazolidine-2-thione). J. Chem. Soc. Perkin Trans 1986, 1, 479–482. [Google Scholar] [CrossRef]
- Zhang, X.; Breslav, M.; Grimm, J.; Guan, K.; Huang, A.; Liu, F.; Maryanoff, C.A.; Palmer, D.; Patel, M.; Qian, Y.; Shaw, C.; Sorgi, K.; Stefancik, S.; Xu, D.A. New procedure for preparation of carboxylic acid hydrazides. J. Org. Chem. 2002, 67, 9471–9474. [Google Scholar] [CrossRef]
- Govori, S.; Rapic, V.; Leci, O.; Cacic, M.; Tabakovic, I. Synthesis and reactions of some 4-heteroaryl-3-nitrocoumarins. J. Heterocyclic. Chem. 1996, 33, 351–354. [Google Scholar] [CrossRef]
- Lacan, M.; Cacic, M.; Guslo, D. Thin-Layer Chromatography of some derivatives of 4,7-dihydroxycoumarin. Acta Pharm. Jugosl. 1981, 31, 47–51. [Google Scholar]
- Cacic, M.; Trkovnik, M.; Has-Schon, E. Synthesis of N1-Substituted Coumarino[4,3-c]pyrazoles. J. Heterocylic. Chem. 2002, 40, 833–836. [Google Scholar] [CrossRef]
- Dey, B.B.; Row, K.K. The reactivity of the methylene group in coumarin-4-acetic acids and their esters. Condensation with salicylaldehyde to 4:3'-Dicoumaryls. J. Indian Chem. Soc. 1924, 1, 107–123. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cacic, M.; Trkovnik, M.; Cacic, F.; Has-Schon, E. Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide. Molecules 2006, 11, 134-147. https://doi.org/10.3390/11010134
Cacic M, Trkovnik M, Cacic F, Has-Schon E. Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide. Molecules. 2006; 11(2):134-147. https://doi.org/10.3390/11010134
Chicago/Turabian StyleCacic, Milan, Mladen Trkovnik, Frane Cacic, and Elizabeth Has-Schon. 2006. "Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide" Molecules 11, no. 2: 134-147. https://doi.org/10.3390/11010134
APA StyleCacic, M., Trkovnik, M., Cacic, F., & Has-Schon, E. (2006). Synthesis and Antimicrobial Activity of Some Derivatives on the Basis (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic Acid Hydrazide. Molecules, 11(2), 134-147. https://doi.org/10.3390/11010134



