A Simple Synthetic Route to Methyl 3-Fluoropyridine-4-carboxylate by Nucleophilic Aromatic Substitution
Abstract
:1. Introduction
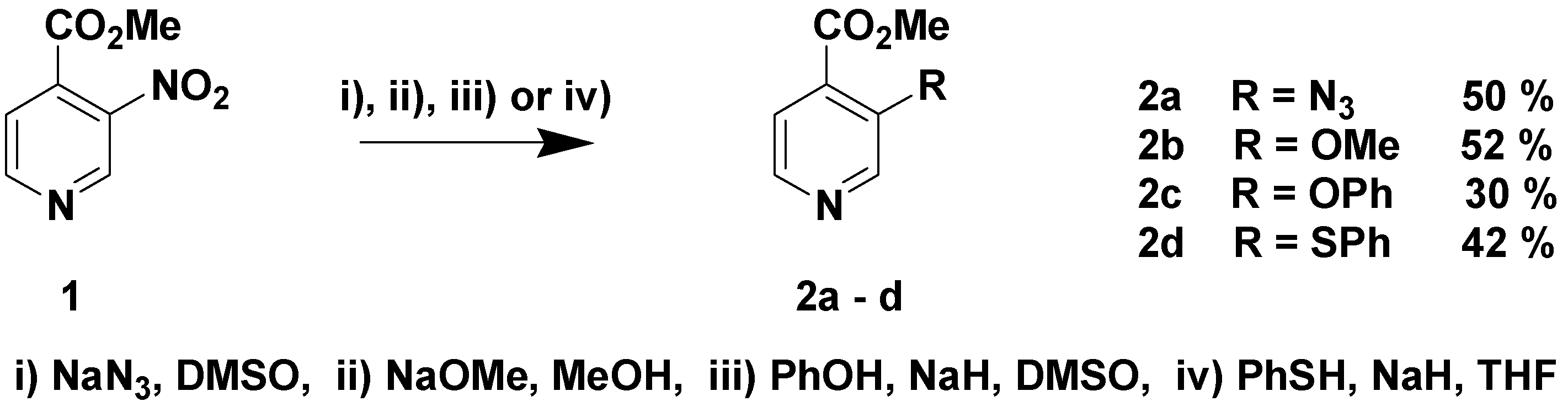
2. Results and Discussion
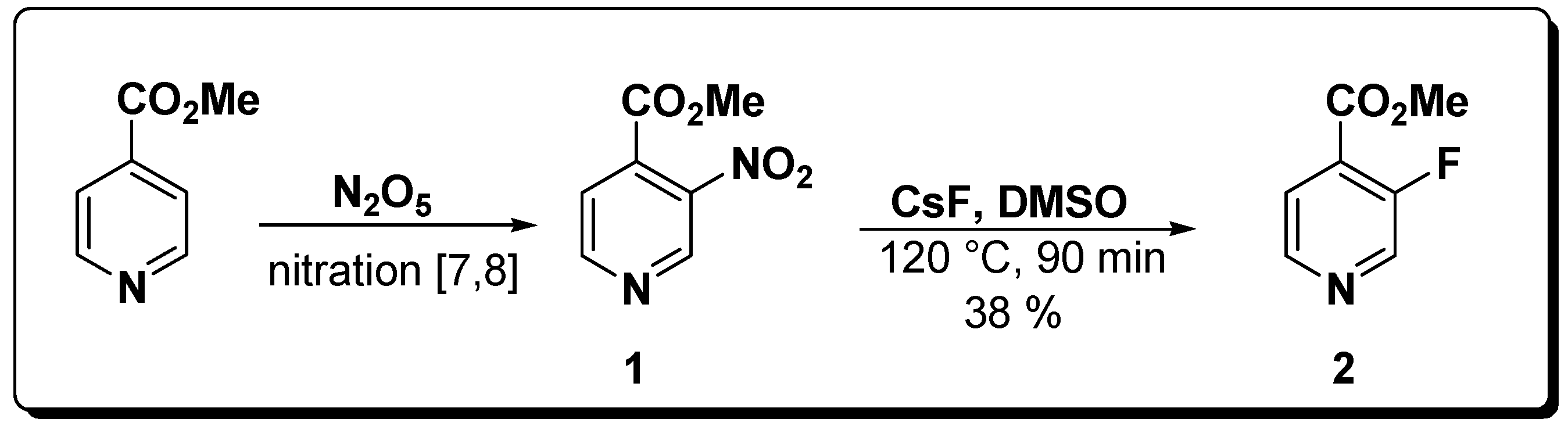
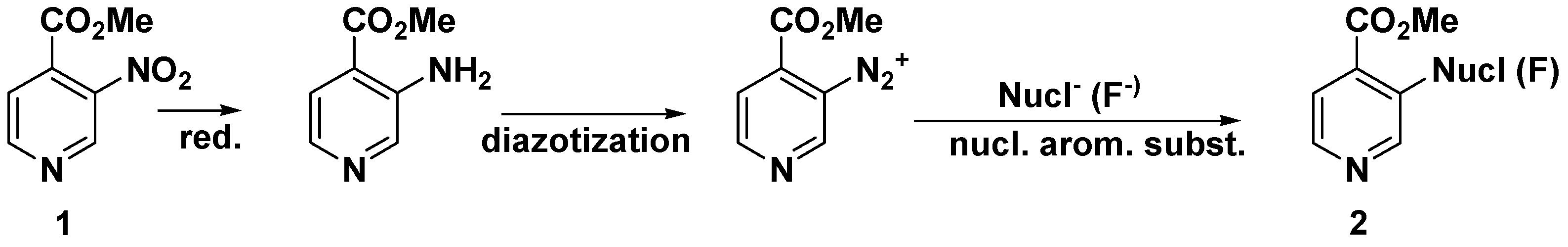
3. Conclusions
4. Experimental
General
Methyl 3-fluoropyridine-4-carboxylate (2).
References
- Wakefield, B. Fluorinated Pharmaceuticals. Innov. Pharmaceut. Tech. 2000, 74–78. [Google Scholar]
- Chambers, R. D. Fluorine in Organic Chemistry; Blackwell: Oxford, 2004. [Google Scholar]
- Effenberger, F.; Koch, M.; Streicher, W. Nucleophilic substitution of nitrite in nitrobenzenes, nitrobiphenyls and nitronaphthalenes. Chem. Ber. 1991, 124, 163–173. [Google Scholar] [CrossRef]
- Beck, J. R. Nucleophilic displacement of aromatic nitro groups. Tetrahedron 1978, 34, 2057–2068. [Google Scholar] [CrossRef]
- Kuduk, S. D.; DiPardo, R. M.; Bock, M. G. Tetrabutylammonium Salt Induced Denitration of Nitropyridines: Synthesis of Fluoro-, Hydroxy-, and Methoxypyridines. Org. Lett. 2005, 7, 577–579. [Google Scholar] [CrossRef]
- Holt, J.; Tjosås, F.; Bakke, J.; Fiksdahl, A. Nucleophilic aromatic substitution of methyl 3-nitropyridine-4-carboxylate. J. Heterocyclic Chem. 2004, 41, 987–989. [Google Scholar]
- Bakke, J. M.; Hegbom, I.; Øvreeide, K.; Aaby, K. Nitration of aromatic and heteroaromatic compounds by dinitrogen pentaoxide. Acta Chem. Scand. 1994, 48, 1001–1006. [Google Scholar]
- Bakke, J. M.; Ranes, E. A new efficient synthesis of 3-nitropyridine and substituted derivatives. Synthesis 1997, 281–283. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tjosaas, F.; Fiksdahl, A. A Simple Synthetic Route to Methyl 3-Fluoropyridine-4-carboxylate by Nucleophilic Aromatic Substitution. Molecules 2006, 11, 130-133. https://doi.org/10.3390/11020130
Tjosaas F, Fiksdahl A. A Simple Synthetic Route to Methyl 3-Fluoropyridine-4-carboxylate by Nucleophilic Aromatic Substitution. Molecules. 2006; 11(2):130-133. https://doi.org/10.3390/11020130
Chicago/Turabian StyleTjosaas, Freddy, and Anne Fiksdahl. 2006. "A Simple Synthetic Route to Methyl 3-Fluoropyridine-4-carboxylate by Nucleophilic Aromatic Substitution" Molecules 11, no. 2: 130-133. https://doi.org/10.3390/11020130



