CuCl2-catalyzed One-pot Formation of Tetrahydroquinolines from N-Methyl-N-alkylanilines and Vinyl Ethers in the Presence of t-Butylhydroperoxide
Abstract
:Introduction
Results and Discussion
 |
 |
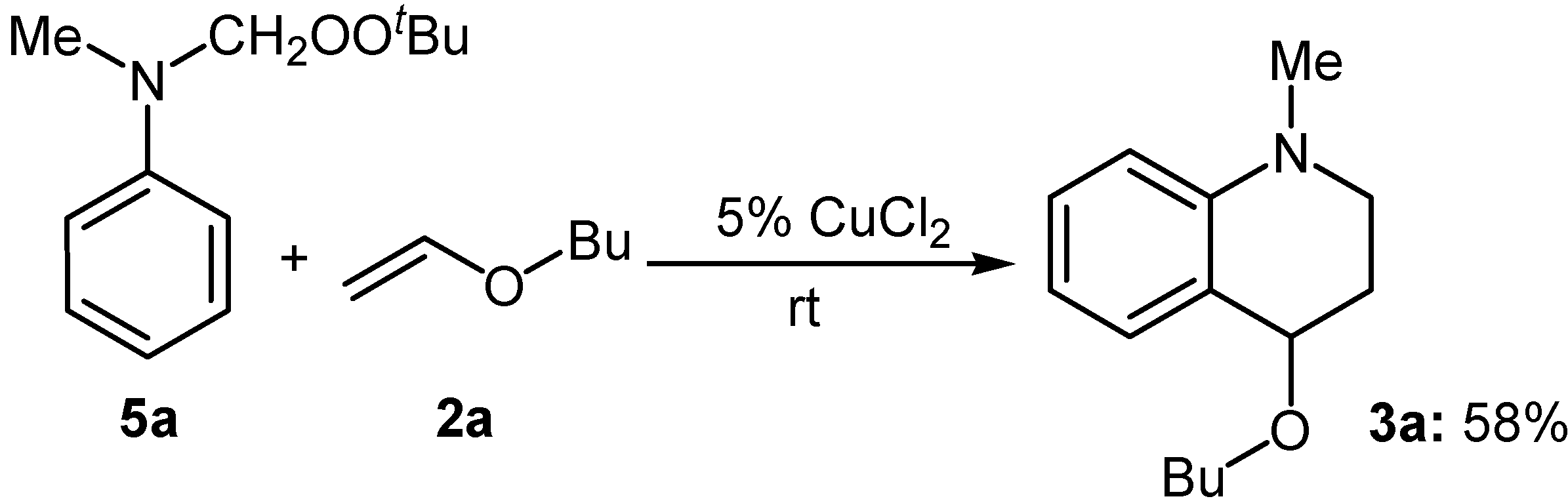
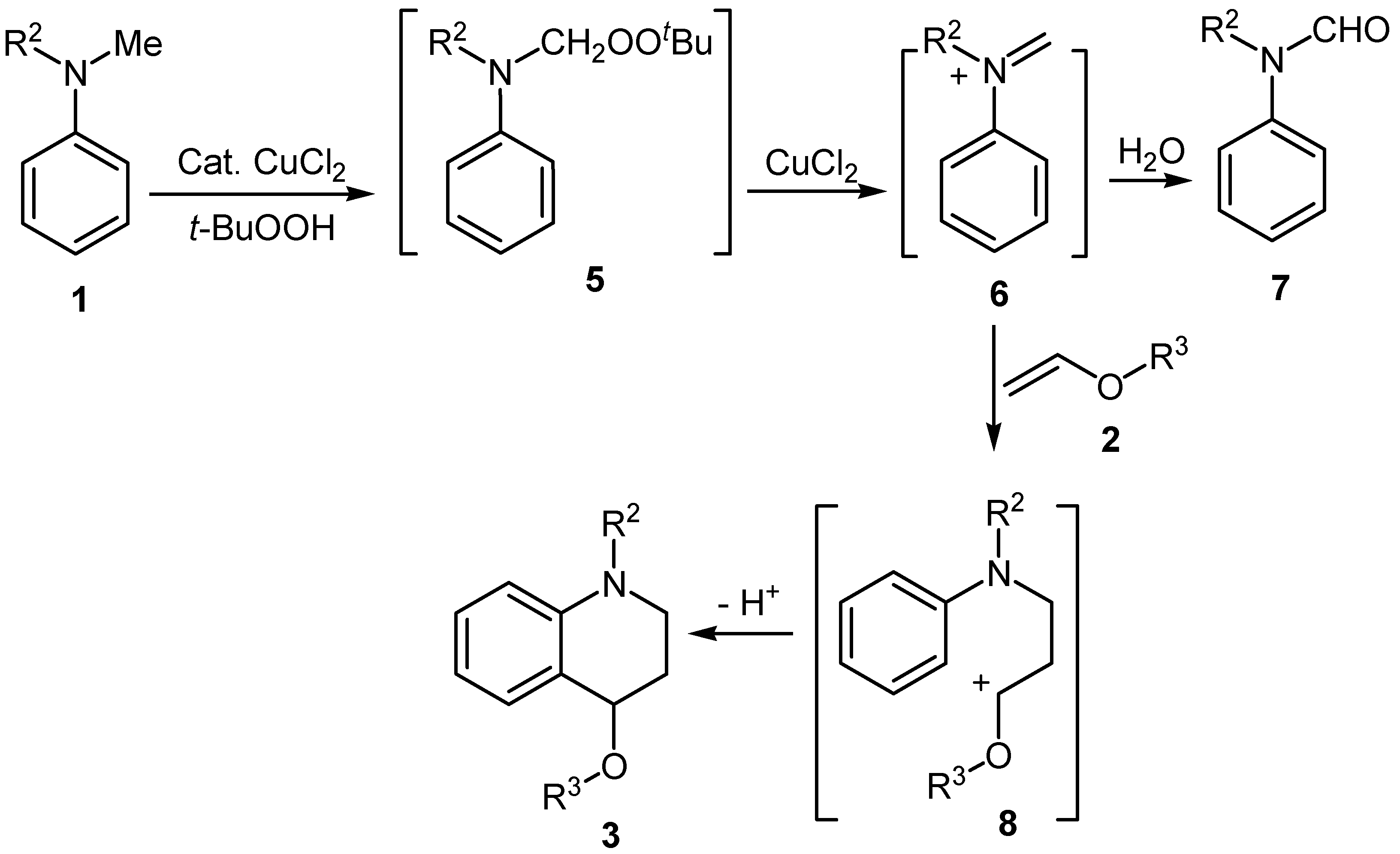
Conclusions
Experimental
General
Representative procedure for the CuCl2-catalyzed one-pot formation of tetrahydroquinolines from N‑methyl-N-alkylanilines and vinyl ethers in the presence of t-butyl hydroperoxide: reaction of N,N-dimethylaniline (1a) and n-butyl vinyl ether (2a)









Acknowledgements
References and Notes
- Perry, N. B.; Blunt, J. W.; McCombs, J. D.; Munro, M. H. G. Discorhabdin C, A Highly Cytotoxic Pigment From A Sponge of the Genus Latrunculia. J. Org. Chem. 1986, 51, 5476–5478. [Google Scholar] Williamson, N. M.; March, P. R.; Ward, A. D. An Improved Synthesis of 2,2-Disubstituted-1,2-dihydroquinolines and Their Conversion to 3-Chloro-2,2-disubstituted-tetra-hydroquinolines. Tetrahedron Lett. 1995, 36, 7721–7724. [Google Scholar] Romesh, M.; Moham, P. S.; Shanmugam, P. A Convenient Synthesis of Flindersine, Atanine and Their Analogues. Tetrahedron 1984, 40, 4041–4049. [Google Scholar] Mertes, M. P.; Lin, A. J. Cofactor inhibitors of Thymidylate Synthetase. Piperidine and Tetrahydroquinoline Analogs of Tetrahydrofolic Acid. J. Med. Chem. 1970, 13, 276–279. [Google Scholar] Padwa, A.; Brodney, M. A.; Liu, B.; Satake, K.; Wu, T. A Cycloaddition Approach Toward the Synthesis of Substituted Indolines and Tetrahydroquinolines. J. Org. Chem. 1999, 64, 3595–3607. [Google Scholar] De Kempe, N.; Keppens, M. Novel Routes to Indoles, Indolines, Quinolines And Tetrahydroquinolines From N-(Cyclohexylidene)amines. Tetrahedron 1996, 52, 3705–3718. [Google Scholar] Hiessbock, R.; Wolf, C.; Richter, E.; Hitzler, M.; Chiba, P.; Kratzel, M.; Ecker, G. Synthesis and in Vitro Multidrug Resistance Modulating Activity of a Series of Dihydrobenzopyrans and Tetrahydroquinolines. J. Med. Chem. 1999, 42, 1921–1926. [Google Scholar]
- For leading references see: Nishio, T.; Tabata, M.; Koyama, H.; Sakamoto, M. Photochemistry of N-(2-Acylphenyl)-2-methylprop-2-enamides: Competition Between Photocyclization and Long-Range Hydrogen Abstraction. Helv. Chim. Acta. 2005, 88, 78–86. [Google Scholar] Katritzky, A. R.; Rachwal, S.; Rachwal, B. Recent Progress in the Synthesis of 1,2,3,4,-Tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] Isabelle, G.-D.; Gastaud, P.; RajanBabu, T. V. Asymmetric Synthesis of Functionalized 1,2,3,4-Tetrahydroquinolines. Org, Lett. 2001, 3, 2053–2056. [Google Scholar]
- Lautens, M.; Tayama, E.; Herse, C. Palladium-Catalyzed Intramolecular Coupling Between Aryl Iodides and Allyl Moieties via Thermal and Microwave-Assisted Conditions. J. Am. Chem. Soc. 2005, 127, 72–73. [Google Scholar] Yi, C. S.; Yun, S. Y. Ruthenium-catalyzed Intermolecular Coupling Reactions of Arylamines with Ethylene and 1,3-Dienes: Mechanistic Insight on Hydroamination vs ortho-C-H Bond Activation. Org, Lett. 2005, 7, 2181–2183. [Google Scholar] Zhang, J.; Li, C.-J. InCl3-Catalyzed Domino Reaction of Aromatic Amines with Cyclic Enol Ethers in Water: A Highly Efficient Synthesis of New 1,2,3,4-Tetrahydroquinoline Derivatives. J. Org. Chem. 2002, 67, 3969–3971. [Google Scholar] Chen, R.; Qian, C. One-pot Synthesis of Tetrahydroquinolines Catalyzed by Dy(OTf)3 in Aqueous Solution. Synthetic Commun. 2002, 32, 2543–2548. [Google Scholar] Luo, Y.; Li, Z.; Li, C.-J. A Silver-Catalyzed Domino Route toward 1,2-Dihydroquinoline Derivatives from Simple Anilines and Alkynes. Org, Lett. 2005, 7, 2675–2678. [Google Scholar] Sundararajan, G.; Prabagaran, N.; Varghese, B. First Asymmetric Synthesis of Quinoline Derivatives by Inverse Electron Demand (IED) Diels-Alder Reaction Using Chiral Ti(IV) Complex. Org, Lett. 2001, 3, 1973–1976. [Google Scholar] Talukdar, S.; Chen, C.-T.; Fang, J.-M. A Stereoselective Route to Polysubstituted Tetrahydro-quinolines by Benzotriazole-Promoted Condensation of Aliphatic Aldehydes and Aromatic Amines. J. Org. Chem. 2000, 65, 3148–3153. [Google Scholar]
- Shono, T.; Matsumura, Y.; Inoue, K.; Ohmizu, H.; Kashimura, S. Electroorganic Chemistry. 62. Reaction of Iminium Ion with Nucleophile: A Versatile Synthesis of Tetrahydroquinolines and Julolidines. J. Am. Chem. Soc. 1982, 104, 5753–5757. [Google Scholar] Murahashi, S.-I.; Naota, T.; Nakato, T. Convenient Method for the Construction of Quinoline Skeletons from N-Methylarylamines via N-(t-Butyldioxymethyl)arylamines. Synlett. 1992, 835–836. [Google Scholar] Murahashi, S.-I.; Naota, T.; Yonemura, K. Ruthenium-catalyzed Cytochrome P-450 Type Oxidation of Tertiary Amines with Alkylhydroperoxides. J. Am. Chem. Soc. 1988, 110, 8256–8258. [Google Scholar] Murata, S.; Miura, M.; Nomura, M. Oxidation of 3- or 4-Substituted N,N-Dimethylanilines with Molecular Oxygen in the Presence of Either Ferric Chloride or [Fe(salen)]OAc. J. Org. Chem. 1989, 54, 4700–4702. [Google Scholar] Murata, S.; Miura, M.; Nomura, M. Iron-catalyzed Oxidation of N,N-Dimethylaniline with Molecular Oxygen. J. Chem. Soc. Chem. Commun. 1989, 116–118. [Google Scholar]
- Krause, N. Modern Organocopper Chemistry; Wiley-VCH Verlag GmbH: Weinheim, 2002. [Google Scholar] Hathaway, B. J. Comprehensive Coordination Chemistry; Wilkinson, G., Ed.; Pergamon: Oxford, 1987; Vol. 5, pp. 533–774. [Google Scholar]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] Kwong, F. Y.; Klapars, A.; Buchwald, S. L. Copper-Catalyzed Coupling of Alkylamines and Aryl Iodides: An Efficient System Even in an Air Atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] Rispens, T.; Engberts, Jan B. F. N. Efficient Catalysis of a Diels-Alder Reaction by Metallo-Vesicles in Aqueous Solution. Org. Lett. 2001, 3, 941–943. [Google Scholar] Smrčina, M.; Vyakočil, Š.; Máca, B.; Polášek, M.; Claxton, T. A.; Abbott, A. P.; Kočovský, P. Selective Cross-coupling of 2-Naphthol and 2-Naphthylamine Derivatives. A Facile Synthesis of 2,2',3-Trisubstituted and 2,2',3,3'-Tetrasubstituted 1,1'-Binaphthyls. J. Org. Chem. 1994, 59, 2156–2163. [Google Scholar] Smrčina, M.; Lorenc, M.; Hanuš, V.; Sedmera, P.; Kočovsky, P. Synthesis of Enantiomerically Pure 2,2'-Dihydroxy-1,1'-binaphthyl, 2,2'-diamino-1,1'-binaphthyl and 2-amino-2'-hydroxy-1,1'-binaphthyl. Comparison of Processes Operating as Diastereoselective Crystallization and as Second Order Asymmetric Transformation. J. Org. Chem. 1992, 57, 1917–1920. [Google Scholar]
- Billman, J. H.; Radike, A.; Mundy, B. W. Alkylation of Amines. I. J. Am. Chem. Soc. 1942, 64, 2977–2978. [Google Scholar] Thomas, D. G.; Billman, J. H.; Davis, C. E. Alkylation of Amines. II. N,N-Dialkylation of Nuclear Substituted Anilines. J. Am. Chem. Soc. 1946, 68, 895–896. [Google Scholar]
- Sample Availability: Contact the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yang, X.; Xi, C.; Jiang, Y. CuCl2-catalyzed One-pot Formation of Tetrahydroquinolines from N-Methyl-N-alkylanilines and Vinyl Ethers in the Presence of t-Butylhydroperoxide. Molecules 2006, 11, 978-987. https://doi.org/10.3390/11120978
Yang X, Xi C, Jiang Y. CuCl2-catalyzed One-pot Formation of Tetrahydroquinolines from N-Methyl-N-alkylanilines and Vinyl Ethers in the Presence of t-Butylhydroperoxide. Molecules. 2006; 11(12):978-987. https://doi.org/10.3390/11120978
Chicago/Turabian StyleYang, Xianghua, Chanjuan Xi, and Yanfeng Jiang. 2006. "CuCl2-catalyzed One-pot Formation of Tetrahydroquinolines from N-Methyl-N-alkylanilines and Vinyl Ethers in the Presence of t-Butylhydroperoxide" Molecules 11, no. 12: 978-987. https://doi.org/10.3390/11120978





