Hyperbranched Molecular Structures with Potential Antiviral Activity: Derivatives of 5,6-Dihydroxyindole-2-Carboxylic Acid
Abstract
:Introduction
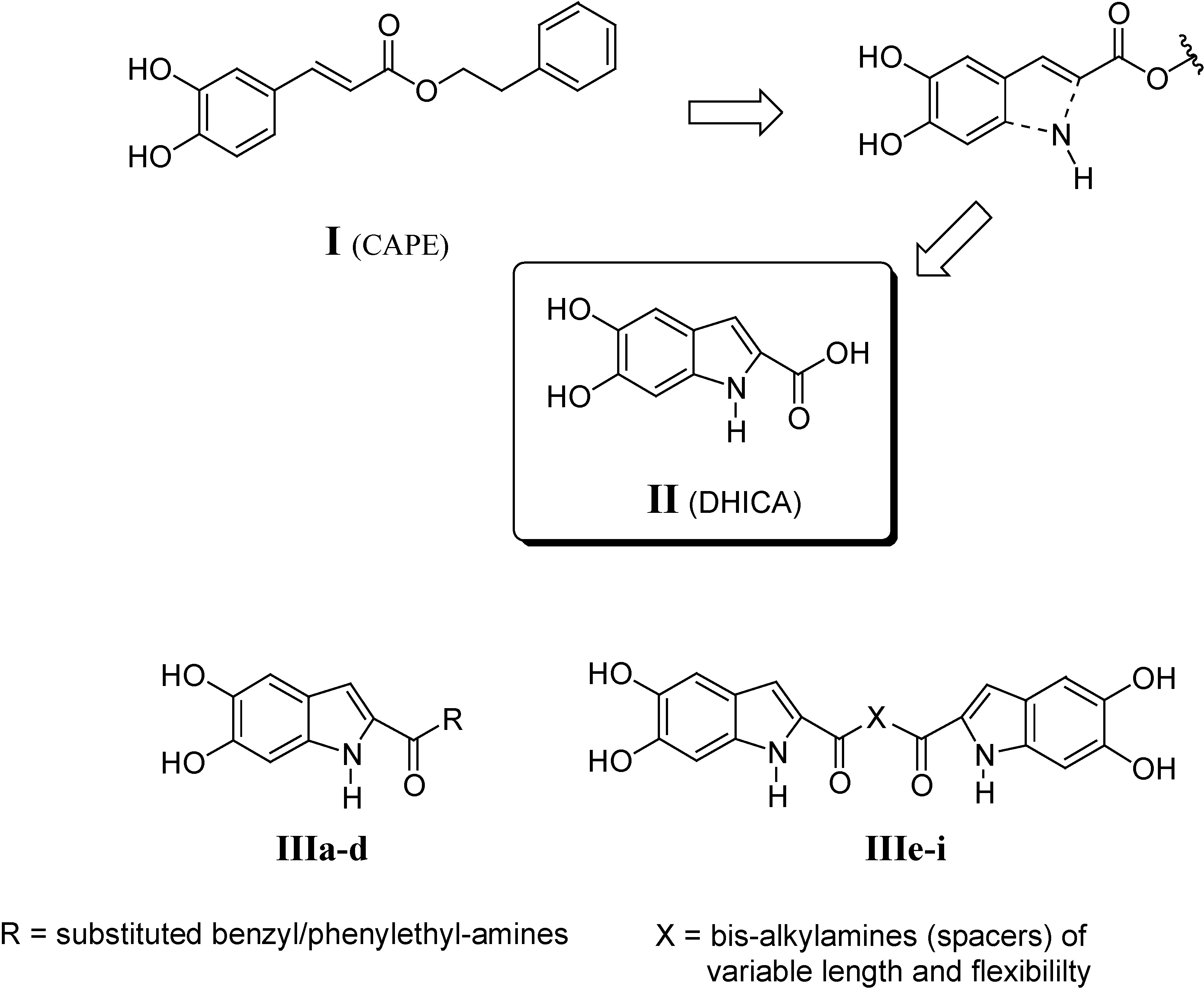
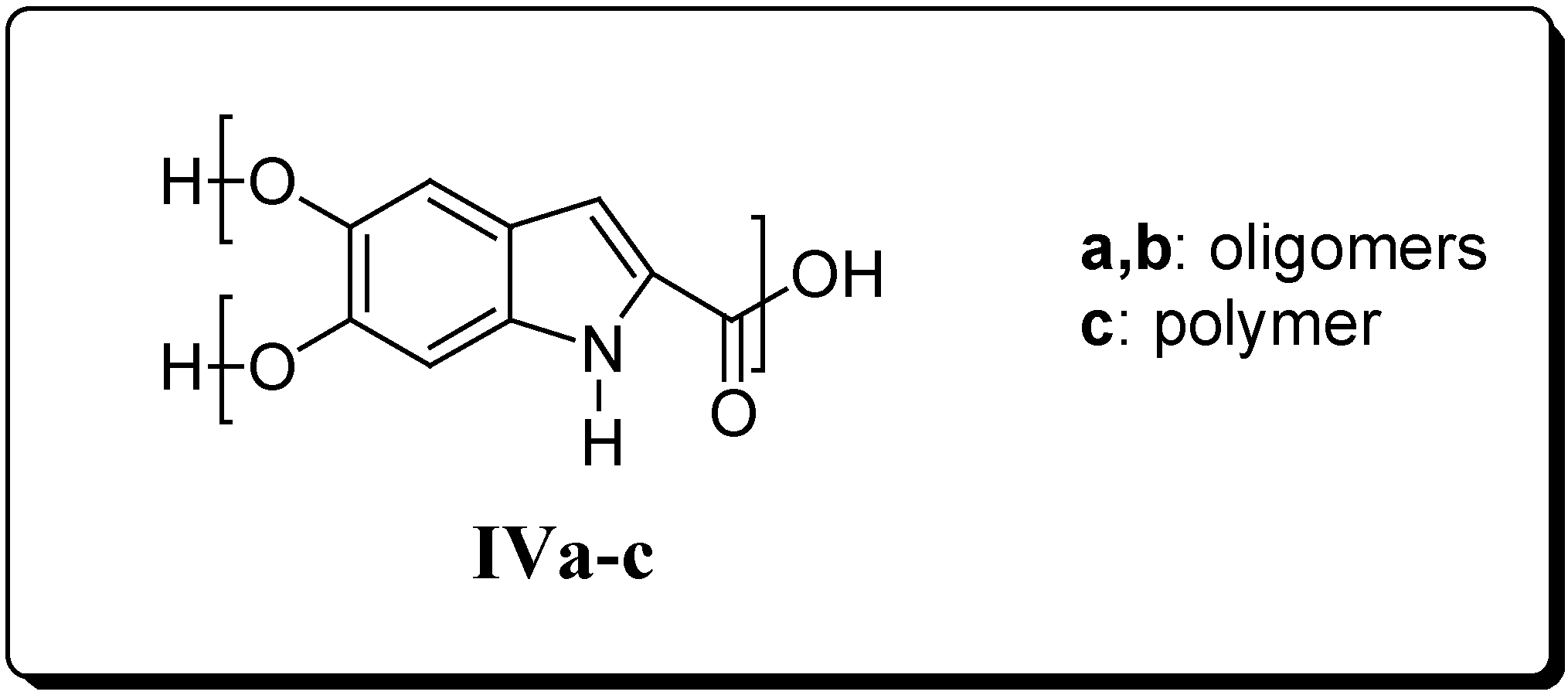
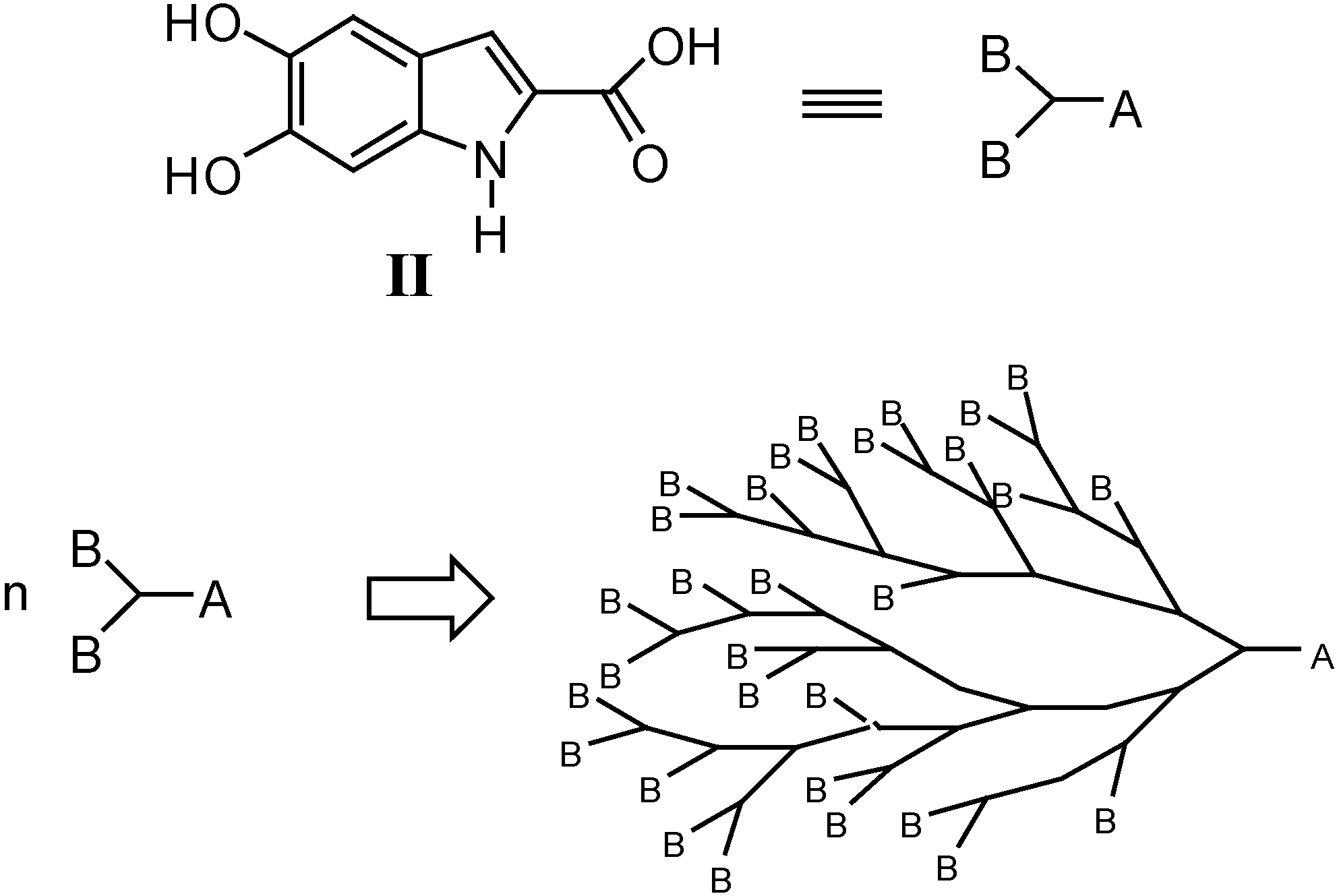
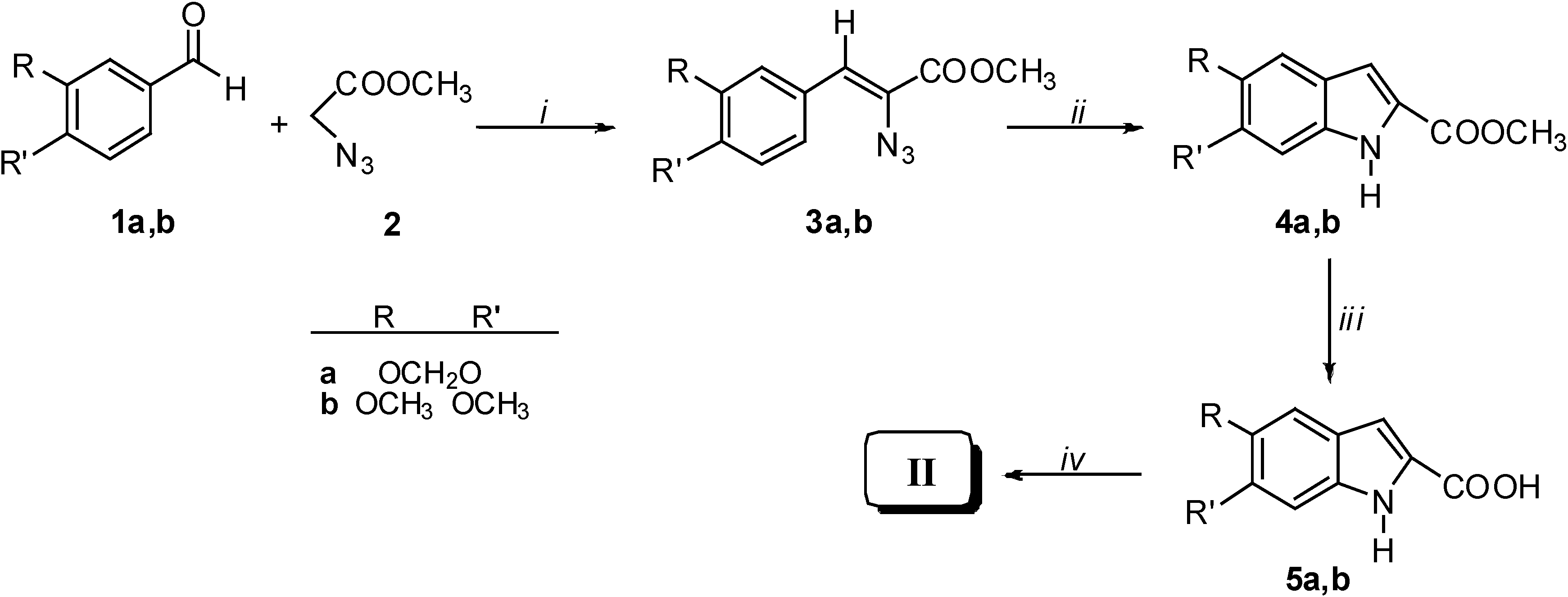
Results and Discussion
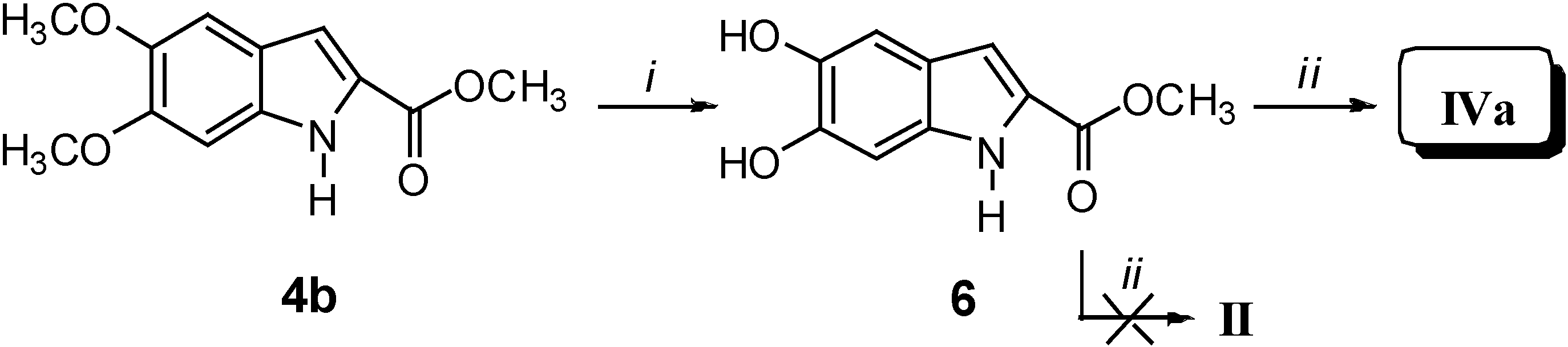
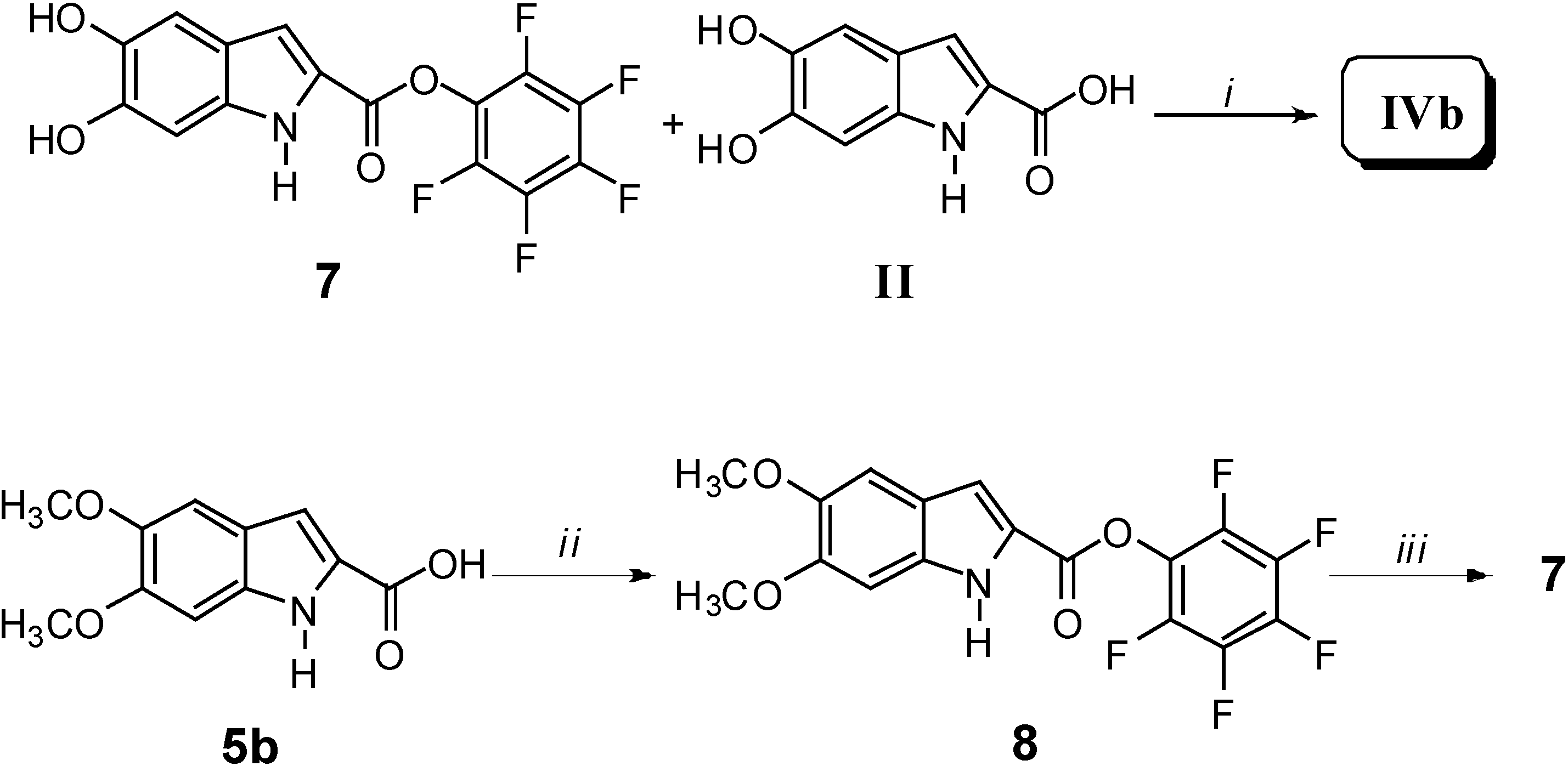
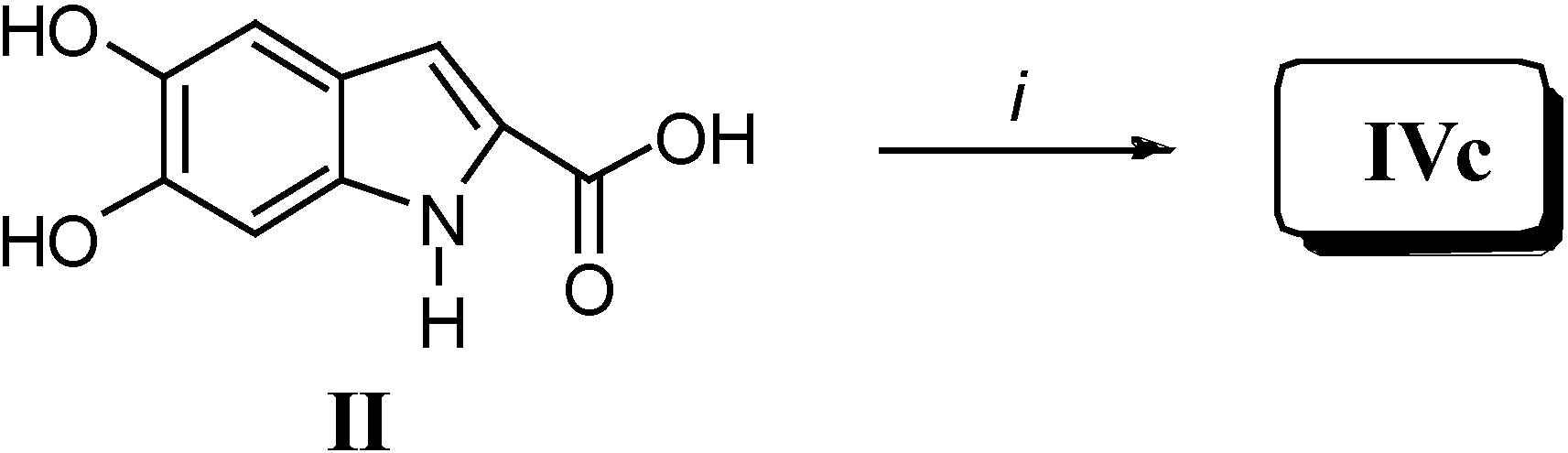
Biological Activity
| Cpd | aCC50 | bEC50 | cTI |
|---|---|---|---|
| II | 0.9 | >0.9 | - |
| IVa | 5 | 0.5 | 10 |
| IVb | 15 | 1.5 | 10 |
| IVc | >100 | >100 | - |
| dL-731,988 | 15.6 | 0.43 | 36 |
| eEFV | 11 | 0.0031 | >1000 |
Conclusions
Experimental
General
Differential Scanning Calorimetry (DSC) measurements
Acknowledgements
References and Notes
- Sechi, M.; Angotzi, G.; Dallocchio, R.; Dessì, A.; Carta, F.L.; Sannia, Mariani A.; Fiori, S.; Sanchez, T.; Movssessian, L.; Plasencia, C.; Neamati, N. Design and synthesis of novel dihydroxyindole-2-carboxylic acids as HIV-1 integrase inhibitors. Antivir. Chem. & Chemother. 2004, 15, 67–82. [Google Scholar]b)d’Ischia, N.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and Indole-5,6-diones. In Advances in Heterocyclic Chemistry; Academic Press: New York, 2005; Vol. 89, Chapter 1; pp. 1–65. [Google Scholar]
- Jikei, M.; Kakimoto, M. Hyperbranched polymers: a promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–85. [Google Scholar] [CrossRef]
- Monticelli, O.; Mendichi, R.; Bissano, S.; Mariani, A.; Russo, S. Synthesis, characterization and properties of a hyperbranched aromatic polyamide: poly(ABZAIA). Macromol. Chem. Phys. 2000, 201, 2123–27. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Taylor, A.M.; Goddard, W.A., III. Starburst dendrimers: Control of size, shape, surface, chemistry, and topology. Angew. Chem., Int. Ed. Engl. 1990, 102, 119–157. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–36. [Google Scholar] [CrossRef] [PubMed]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic Polymers in Biomedical Applications: From Potential to Clinical Use in Diagnostics and Therapy. Angew. Chem., Int. Ed. 2002, 41, 1329–34. [Google Scholar]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–8. [Google Scholar]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Cloninger, M.J. Biological applications of dendrimers. Curr. Opin. Chem. Biol. 2002, 6, 742–8. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.R.; Holan, G. US Patent No. 6,190,650, 2001.
- Bourne, N.; Stanberry, L.R.; Kern, E.R.; Holan, G.; Matthews, B.; Bernstein, D.I. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 2000, 44, 2471–4. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Matthews, B.; Cheung, D.; Tam, T.; Gadawski, I.; Leung, D.; Holan, G.; Raff, J.; Sacks, S. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antiviral Res. 2002, 55, 319–29. [Google Scholar] [CrossRef] [PubMed]
- Luscher-Mattli, M. Polyanions - a lost chance in the fight against HIV and other virus diseases? Antiviral Chem. Chemother. 2000, 11, 249–59. [Google Scholar]
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G.; Pannecouque, C. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 2000, 58, 1100–8. [Google Scholar]
- Turpin, J.A. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Invest. Drugs 2002, 11, 1077–97. [Google Scholar] [CrossRef]
- D'Cruz, O.J.; Uckun, F.M. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 2004, 10, 315–36. [Google Scholar]
- Dezzutti, C.S.; James, V.N.; Ramos, A.; Sullivan, S.T.; Siddig, A.; Bush, T.J.; Grohskopf, L.A.; Paxton, L.; Subbarao, S.; Hart, C.E. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 2004, 48, 3834–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Emau, P.; Cairns, J.S.; Flanary, L.; Morton, W.R.; McCarthy, T.D.; Tsai, C.C. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 2005, 21, 207–13. [Google Scholar]
- Gong, E.; Matthews, B.; McCarthy, T.; Chu, J.; Holan, G.; Raff, J.; Sacks, S. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 2005, 68, 139–46. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R.J. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar]
- Barbaro, G.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr. Pharm. Des. 2005, 11, 1850–1843. [Google Scholar] [CrossRef]
- Hazuda, D.J.; Felock, P.; Witmer, M.; Wolfe, A.; Stillmock, K.; Grobler, J.A.; Espeseth, A.; Gabryelski, L.; Schleif, W.; Blau, C.; Miller, M.D. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000, 287, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Wyler, H.; Dreiding, A.S. Darstellung und Abbauprodukte des Betanidins. 3. Über die Konstitution des Randenfarbstoffes Betanin. Helv. Chim. Acta 1959, 42, 1699–702. [Google Scholar]
- Sample Availability: Samples of the compounds I-IV and 1-8 are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Sechi, M.; Casu, F.; Campesi, I.; Fiori, S.; Mariani, A. Hyperbranched Molecular Structures with Potential Antiviral Activity: Derivatives of 5,6-Dihydroxyindole-2-Carboxylic Acid. Molecules 2006, 11, 968-977. https://doi.org/10.3390/11120968
Sechi M, Casu F, Campesi I, Fiori S, Mariani A. Hyperbranched Molecular Structures with Potential Antiviral Activity: Derivatives of 5,6-Dihydroxyindole-2-Carboxylic Acid. Molecules. 2006; 11(12):968-977. https://doi.org/10.3390/11120968
Chicago/Turabian StyleSechi, Mario, Fabio Casu, Ilaria Campesi, Stefano Fiori, and Alberto Mariani. 2006. "Hyperbranched Molecular Structures with Potential Antiviral Activity: Derivatives of 5,6-Dihydroxyindole-2-Carboxylic Acid" Molecules 11, no. 12: 968-977. https://doi.org/10.3390/11120968





