Preparation of compounds 3.
To a solution of the unsaturated C-phenyl glycopyranoside 1b (or 2b) (1 g, 4.8 mmol), imidazole (32 mg, 05 mmol) and triethylamine (1 mL, 6.7 mmol) in CH2Cl2 (8 mL) maintained at rt was added a solution of tert-BuMe2SiCl (990 mg, 6.6 mmol) in CH2Cl2 (10 mL). After being stirred at rt for 24 h, the solution was poured into cold water (10 mL), and the mixture was extracted with CH2Cl2. (3x10 mL). After evaporation of the solvent under reduced pressure, the residue was purified by column chromatography on silica using petroleum ether/diethyl ether as the eluent to gave compound 3a (or 3b).
(6-O-tert-Butyldimethylsilyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranosyl)benzene (3a). Yield 66%, oil, Rf 0.47 (petroleum ether-diethyl ether 2:1), [α]D20 -75 (c 1, CHCl3); 1H-NMR (CDCl3) δ 0.05 (s, 3H, SiMe), 0.07 (s, 3H, SiMe), 0.88 (s, 9H, CMe3), 3.01 (bs, 1H, OH), 3.46 (ddd, 1H, J = 7.7, 7.5, 5.5 Hz, H-5), 3.71 (dd, 1H, J = 9.6, 7.7 Hz, H-6), 3.83 (dd, 1H, J = 9.6, 5.5 Hz, H-6), 4.26 (d, 1H, J = 7.5 Hz, H-4), 5.23 (s, 1H, H-1), 6.05 (m, 2H, H-2, H-3), 7.35 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.5 (SiMe), -5.4 (SiMe), 18.2 (CMe3), 25.9 (CMe3), 65.7 (C-6), 67.0 (C-4), 71.4 (C-5), 73.9 (C-1), 128.0, 128.4, 128.5, 129.6 and 139.5 (C-2, C-3, Carom); Anal. calcd for C18H28O3Si: C, 67.46; H, 8.81. Found: C, 66.81; H, 8.81.
(6-O-tert-Butyldimethylsilyl-2,3-dideoxy-β-D-erythro-hex-2-enopyranosyl)benzene (3b). Yield 82%, oil, Rf 0.36 (petroleum ether-diethyl ether 2:1), [α]D20 +120 (c 0.9, CHCl3); 1H-NMR (CDCl3) δ 0.10 (s, 6H, SiMe), 0.90 (s, 9H, CMe3), 3.40 (bs, 1H, OH), 3.65 (ddd, 1H, J = 8.1, 5.1, 4.4 Hz, H-5), 3.77 (dd, 1H, J = 9.5, 8.1 Hz, H-6), 4.03 (dd, 1H, J = 9.5, 5.1 Hz, H-6), 4.40 (dd, 1H, J = 4.4, 1.5 Hz, H-4), 5.15 (s, 1H, H-1), 5.83 (dd, 1H, J = 10.3, 1.5 Hz, H-3), ), 5.90 (d, 1H, J = 10.3 Hz, H-3), 7.21 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.5 (SiMe), -5.6 (SiMe), 18.2 (CMe3), 25.9 (CMe3), 66.5 (C-6), 67.9 (C-4), 77.2, and 77.3 (C-1, C-5), 127.3, 128.2, 128.3, 130.4 and 140.5 (C-2, C-3, Carom); Anal. calcd for C18H28O3Si: C, 67.46; H, 8.81. Found: C, 67.40; H, 8.75.
General procedure for the epoxidation.
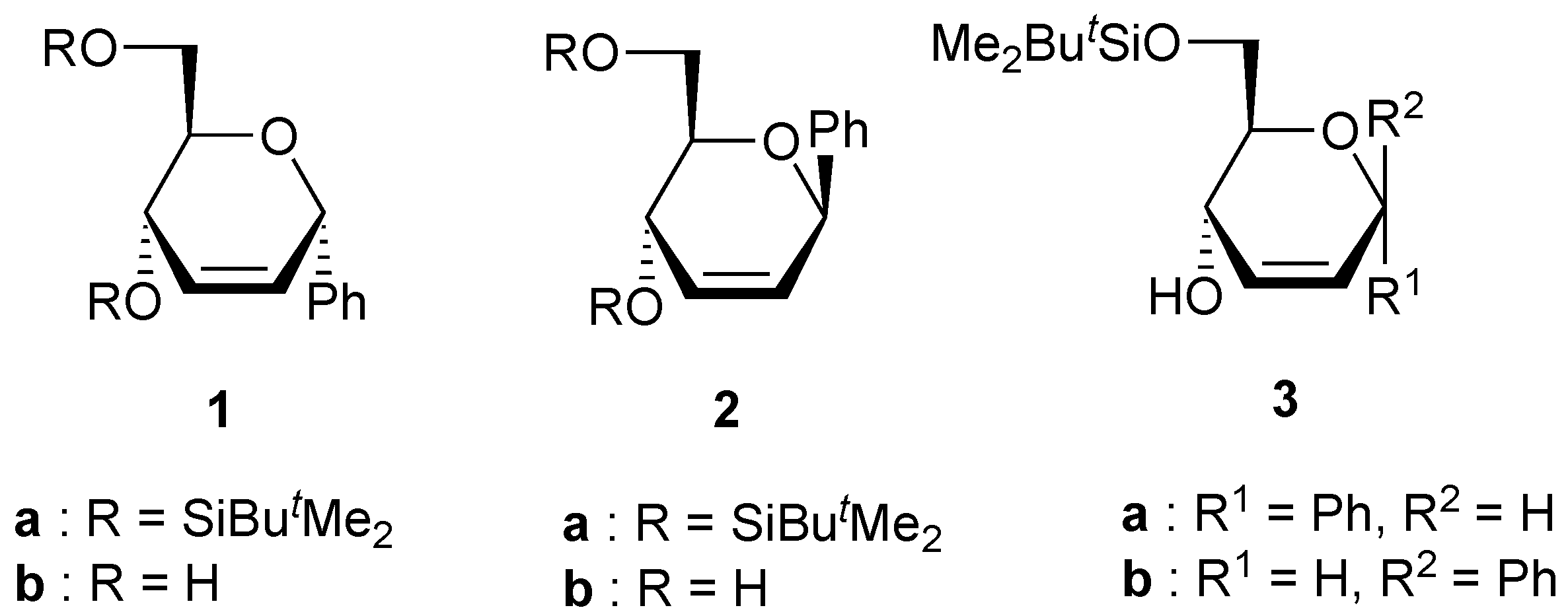
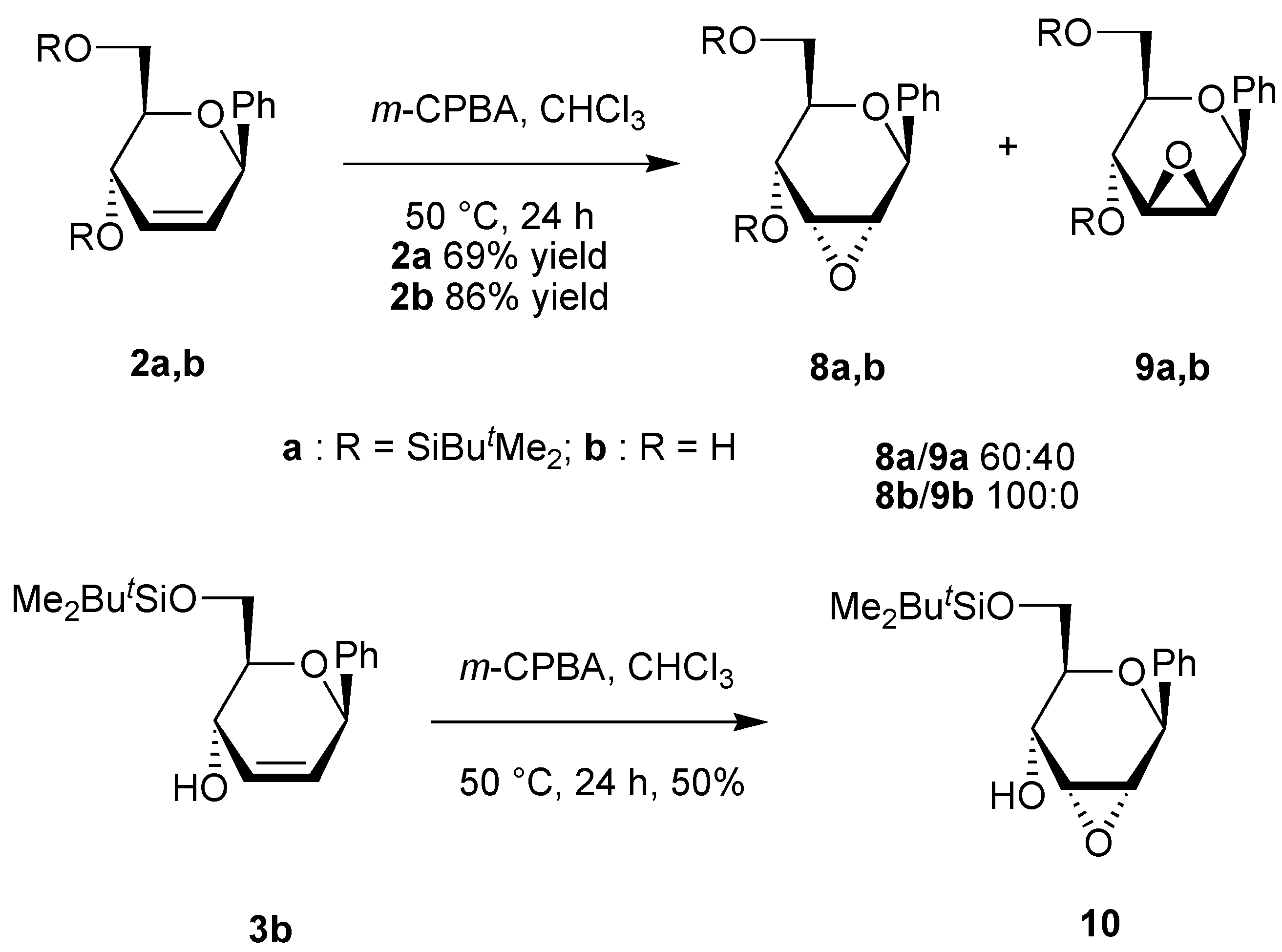
A solution of the unsaturated carbohydrate (0.23 mmol) and m-CPBA (1.17 g, 0.69 mmol) in CHCl3 (10 mL) was stirred at 50 °C for 24 h. The solution was neutralized with a saturated aqueous solution of NaHCO3, the organic phase was separated, and the aqueous phase was extracted with CHCl3 (2x10 mL). The organic phases were dried over Na2SO4. After evaporation of the solvent under reduced pressure, the residue was purified by column chromatography on silica using the appropriate eluent.
(4,6-Di-O-tert-butyldimethylsilyl-2,3-anhydro-α-D-allopyranosyl)benzene (4a). Yield 9%, oil, Rf 0.18 (petroleum ether-ethyl acetate-triethylamine 40:1:1), [α]D20 +18 (c 0.9, CH2Cl2); 1H-NMR (CDCl3) δ 0.13 (s, 6H, SiMe), 0.17 (s, 3H, SiMe), 0.20 (s, 3H, SiMe), 0.94 (s, 9H, CMe3), 0.98 (s, 9H, CMe3), 3.54 (dd, 1H, J = 4.2, 1.7 Hz, H-3), 3.48-3.57 (m, 1H, H-5), 3.75 (dd, 1H, J = 11.2, 6.1 Hz, H-6), 3.95 (dd, 1H, J = 11.2, 1.9 Hz, H-6), 3.98 (dd, 1H, J = 4.2, 3.6 Hz, H-2), 4.02 (dd, 1H, J = 9.1, 1.7 Hz, H-4), 5.26 (bd, 1H, J = 3.6 Hz, H-1), 7.31-7.61 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.2 (SiMe), -5.0 (SiMe), -4.7 (SiMe), -4.0 (SiMe), 18.1 (CMe3), 18.6 (CMe3), 25.8 (CMe3), 26.1 (CMe3), 55.8 and 57.5 (C-2, C-3), 63.4 (C-6), 66.9 (C-4), 71.1 (C-5), 72.4 (C-1), 126.5, 127.4, 128.5 and 139.1 (Carom); Anal. calcd for C24H42O4Si2: C, 63.95; H, 9.39. Found: C, 63.61; H, 9.38.
(4,6-Di-O-tert-butyldimethylsilyl-2,3-anhydro-α-D-mannopyranosyl)benzene (5a). Yield 70%, oil, Rf 0.20 (petroleum ether-ethyl acetate-triethylamine 40:1:1), [α]D20 +21 (c 0.9, CH2Cl2); 1H-NMR (CDCl3) δ 0.06 (s, 3H, SiMe), 0.08 (s, 3H, SiMe), 0.15 (s, 3H, SiMe), 0.22 (s, 3H, SiMe), 0.92 (s, 9H, CMe3), 0.95 (s, 9H, CMe3), 3.27 (ddd, 1H, J = 9.0, 6.7, 2.2 Hz, H-5), 3.36 (d, 1H, J = 3.7 Hz, H-2 or H-3), 3.60 (d, 1H, J = 3.7 Hz, H-2 or H-3), 3.65 (dd, 1H, J = 11.1, 6.7 Hz, H-6), 3.77 (d, 1H, J = 9.0 Hz, H-4), 3.83 (dd, 1H, J = 11.1, 2.2 Hz, H-6), 5.27 (s, 1H, H-1), 7.31-7.61 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.2 (SiMe), -5.1 (SiMe), -4.8 (SiMe), -4.3 (SiMe), 18.0 (CMe3), 18.5 (CMe3), 25.8 (CMe3), 26.0 (CMe3), 52.4 and 56.9 (C-2, C-3), 63.6 (C-4), 63.6 (C-6), 72.3 (C-5), 73.1 (C-1), 127.6, 128.0, 128.7 and 137.5 (Carom); Anal. calcd for C24H42O4Si2: C, 63.95; H, 9.39. Found: C, 63.72; H, 9.42.
(2,3-Anhydro-α-D-allopyranosyl)benzene (4b). Yield 20%, oil, Rf 0.24 (diethylether), [α]D20 –6.0 (c 0.7, CHCl3); 1H-NMR (CDCl3) δ 2.84 (bs, 2H, OH), 3.31 (ddd, 1H, J = 8.8, 4.5, 3.4 Hz, H-5), 3.50 (dd, 1H, J = 4.3, 1.7 Hz, H-3), 3.63 (dd, 1H, J = 11.8, 4.5 Hz, H-6), 3.69 (dd, 1H, J = 11.8, 3.4 Hz, H-6), 3.79 (dd, 1H, J = 4.3, 3.6 Hz, H-2), 3.93 (dd, 1H, J = 8.8, 1.7 Hz, H-4), 5.06 (d, 1H, J = 3.6 Hz, H-1), 7.16-7.34 (m, 5H, Harom); 13C-NMR (CDCl3) δ 55.6 and 58.2 (C-2, C-3), 62.8 (C-6), 66.2 (C-4), 71.4 (C-5), 72.4 (C-1), 126.9, 128.2, 129.9 and 138.8 (Carom); Anal. calcd for C12H14O4: C, 64.85; H, 6.35. Found: C, 64.65; H, 6.46.
(6-O-tert-Butyldimethylsilyl-2,3-anhydro-α-D-allopyranosyl)benzene (6). Yield 60%, oil, Rf 0.40 (petroleum ether-ethyl acetate 2:1), [α]D20 -22 (c 1.2, CHCl3); 1H-NMR (CDCl3) δ 0.00 (s, 6H, SiMe), 0.81 (s, 9H, CMe3), 2.48 (bs, 1H, OH), 3.51 (dt, 1H, J = 7.1, 5.3 Hz, H-5), 3.66 (dd, 1H, J = 4.1, 2.3 Hz, H-3), 3.86 (d, 1H, J = 5.3 Hz, H-6), 3.93 (dd, 1H, J = 4.1, 3.4 Hz, H-2), 4.10 (dd, 1H, J = 7.1, 2.3 Hz, H-4), 5.21 (d, 1H, J = 3.4 Hz, H-1), 7.30-7.54 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.1 (SiMe), 18.1 (CMe3), 18.7 (CMe3), 26.3 (CMe3), 55.2 and 57.7 (C-2, C-3), 65.0 (C-6), 67.6, 71.4, and 72.1 (C-1, C-4, C-5), 127.0, 128.1, 128.8 and 138.8 (Carom); Anal. calcd for C18H28O4Si (mixture 6 + 7): C, 64.25; H, 8.39. Found: C, 64.26; H, 8.59.
(6-O-tert-Butyldimethylsilyl-2,3-anhydro-α-D-mannopyranosyl)benzene (7). Yield 10%, oil, Rf 0.54 (petroleum ether-ethyl acetate 2:1), [α]D20 -21 (c 1.2, CHCl3); 1H-NMR (CDCl3) δ 0.00 (s, 3H, SiMe), 0.03 (s, 3H, SiMe), 0.83 (s, 9H, CMe3), 2.50 (bs, 1H, OH), 3.17 (ddd, 1H, J = 8.7, 5.3, 4.3 Hz, H-5), 3.44 (d, 1H, J = 3.6, Hz, H-2 or H-3), 3.55 (d, 1H, J = 3.6 Hz, H-2 or H-3), 3.58 (dd, 1H, J = 9.4, 4.3 Hz, H-6), 3.70 (dd, 1H, J = 9.4, 5.3 Hz, H-6), 3.87 (d, 1H, J = 8.7 Hz, H-4), 5.11 (s, 1H, H-1), 7.32-7.46 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.3 (SiMe), -5.2 (SiMe), 18.5 (CMe3), 26.2 (CMe3), 51.9 and 55.3 (C-2, C-3), 66.3 (C-6), 66.9, 69.4, and 72.9 (C-1, C-4, C-5), 128.6, 128.9, 129.1 and 136.8 (Carom).
(4,6-Di-O-tert-butyldimethylsilyl-2,3-anhydro-β-D-allopyranosyl)benzene (8a). Yield 32%, oil, Rf 0.22 (petroleum ether-ethyl acetate-triethylamine 50:1:1), [α]D20 +116 (c 1.2, CH2Cl2); 1H-NMR (CDCl3) δ 0.02 (s, 3H, SiMe), 0.03 (s, 3H, Me, 0.17 (s, 3H, SiMe), 0.19 (s, 3H, SiMe), 0.90 (s, 9H, CMe3), 0.95 (s, 9H, CMe3), 3.33-3.40 (m, 2H, H-2, H-3), 3.49 (ddd, 1H, J = 9.0, 2.9, 2.7 Hz, H-5), 3.77-3.88 (m, 2H, H-6), 4.27 (dd, 1H, J = 9.0, 1.5 Hz, H-4), 4.90 (s, 1H, H-1), 7.29-7.44 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.2 (SiMe), -5.1 (SiMe), -4.7 (SiMe), -4.2 (SiMe), 18.1 (CMe3), 18.4 (CMe3), 25.8 (CMe3), 26.0 (CMe3), 55.4 and 60.5 (C-2, C-3), 62.3 (C-6), 65.9 (C-4), 74.7 (C-5), 76.0 (C-1), 126.6, 127.9, 128.4 and 139.7 (Carom); Anal. calcd for C24H42O4Si2: C, 63.95; H, 9.39. Found: C, 63.93; H, 9.34.
(4,6-Di-O-tert-butyldimethylsilyl-2,3-anhydro-β-D-mannopyranosyl)benzene (9a). Yield 20%, oil, Rf 0.18 (petroleum ether-ethyl acetate-triethylamine 50:1:1), [α]D20 +89 (c 1.1, CH2Cl2); 1H-NMR (CDCl3) δ 0.01 (s, 3H, SiMe), 0.04 (s, 3H, SiMe), 0.15 (s, 3H, SiMe), 0.19 (s, 3H, SiMe), 0.88 (s, 9H, CMe3), 0.94 (s, 9H, CMe3), 3.22 (ddd, 1H, J = 9.1, 5.1, 2.0 Hz, H-5), 3.33-3.40 (s, 2H, H-2, H-3), 3.72 (dd, 1H, J = 11.5, 5.1 Hz, H-6), 3.83 (dd, 1H, J = 11.5, 2.0 Hz, H-6), 3.90 (d, 1H, J = 9.1 Hz, H-4), 4.83 (s, 1H, H-1), 7.30-7.48 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.1 (2xSiMe), -4.9 (SiMe), -4.4 (SiMe), 18.0 (CMe3), 18.5 (CMe3), 25.8 (CMe3), 26.0 (CMe3), 53.4 and 57.4 (C-2, C-3), 62.6 (C-4), 63.1 (C-6), 75.7 (C-5), 80.5 (C-1), 128.0, 128.3, 128.4 and 138.7 (Carom); Anal. calcd for C24H42O4Si2: C, 63.95; H, 9.39. Found: C, 63.95; H, 9.38.
(2,3-Anhydro-β-D-allopyranosyl)benzene (8b). Yield 80%, oil, Rf 0.26 (diethyl ether); 1H-NMR (CDCl3) δ 2.80 (bs, 2H, OH), 3.46 (d, 1H, J = 4.4 Hz, H-2), 3.50-3.58 (m, 2H, H-3, H-5), 3.78 (dd, 1H, J = 11.7, 5.5 Hz, H-6), 3.90 (dd, 1H, J = 11.7, 3.7 Hz, H-6), 4.10 (dd, 1H, J = 9.2, 1.8 Hz, H-4), 4.89 (s, 1H, H-1), 7.25-7.50 (m, 5H, Harom); 13C-NMR (CDCl3) δ 54.9 and 60.3 (C-2, C-3), 62.4 (C-6), 65.9 (C-4), 74.0 (C-5), 76.3 (C-1), 126.7, 128.4, 128.7 and 138.7 (Carom); Anal. calcd for C12H14O4: C, 64.85; H, 6.35. Found: C, 64.94; H, 6.28.
(6-O-tert-Butyldimethylsilyl-2,3-anhydro-β-D-allopyranosyl)benzene (10). Yield 50%, oil, Rf 0.32 (petroleum ether-diethyl ether 2:1), [α]D20 +100 (c 0.9, CHCl3); 1H-NMR (CDCl3) δ 0.10 (s, 6H, SiMe), 0.93 (s, 9H, CMe3), 3.32 (d, 1H, J = 3.9 Hz, OH), 3.44 (d, 1H, J = 4.3 Hz, H-2), 3.57 (dd, 1H, J = 4.3, 2.2 Hz, H-3), 3.60 (ddd, 1H, J = 9.0, 6.9, 4.9 Hz, H-5), 3.79 (dd, 1H, J = 10.1, 6.9 Hz, H-6), 3.96 (dd, 1H, J = 10.1, 4.9 Hz, H-6), 4.19 (ddd, 1H, J = 9.0, 3.9, 2.2 Hz, H-4), 4.91 (s, 1H, H-1), 7.38 (m, 5H, Harom); 13C-NMR (CDCl3) δ -1.3 (2xSiMe), 18.7 (CMe3), 26.3 (CMe3), 55.2 and 57.7 (C-2, C-3), 65.0 (C-6), 67.6 (C-4), 71.4 (C-5), 72.1 (C-1), 127.0, 128.1, 128.8 and 138.8 (Carom); Anal. calcd for C18H28O4Si: C, 64.25; H, 8.39. Found: C, 64.28; H, 8.79.
General procedure for the bis-hydroxylation.
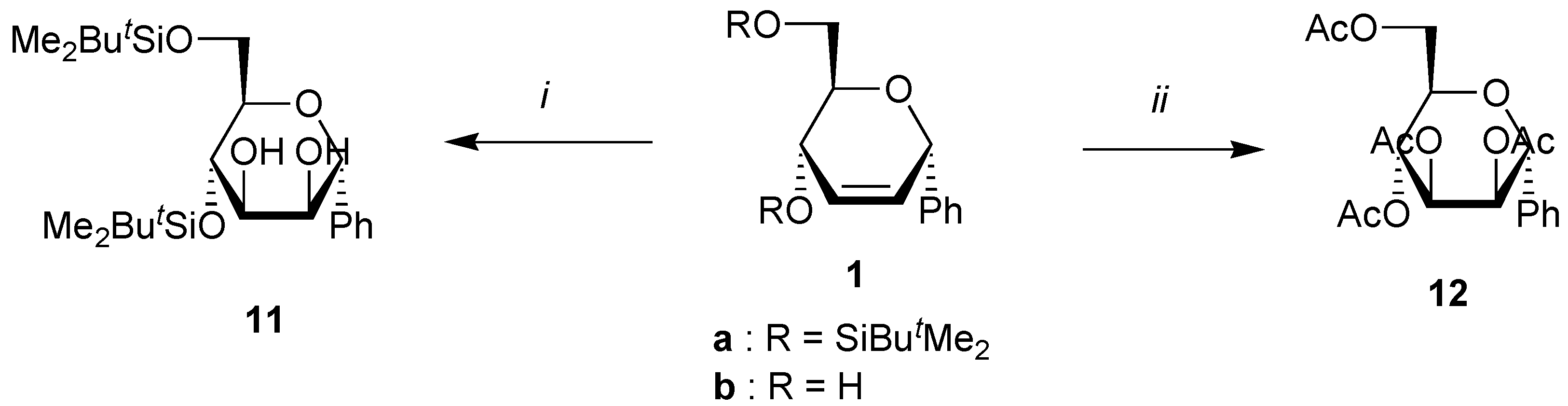
A solution of the unsaturated carbohydrate (1 mmol), N-methylmorpholine oxide (470 mg, 4 mmol), and OsO4 (5 mg,0.02 mmol, 2%) in acetone/water (4 mL/1 mL) was stirred at rt until all the starting carbohydrate has disappeared as shown by TLC. Na2SO3 (500 mg) was then added, and the solution was stirred at rt for 0.5 h. After addition of brine (10 mL), the mixture was extracted with ethyl acetate (3x10 mL). Evaporation of the solvent under reduced pressure gave a residue that was purified by column chromatography on silica using the corresponding eluent for the silylated products. When starting from dihydroxy compounds 2a and 2b, the crude mixture was directly acetylated using a standard procedure, and the tetraacetates were purified by chromatography.
(4,6-Di-O-tert-butyldimethylsilyl-α-D-mannopyranosyl)benzene (11). Yield 70%, oil, Rf 0.40 (petroleum ether-ethyl acetate 4:1), [α]D20 +17 (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 0.04 (s, 3H, SiMe), 0.06 (s, 3H, SiMe), 0.08 (s, 3H, SiMe), 0.10 (s, 3H, SiMe), 0.85 (s, 9H, CMe3), 0.95 (s, 9H, CMe3), 2.25 (bs, 1H, OH), 2.25 (d, 1H, J = 7.4 Hz, OH), 3.60 (ddd, 1H, J = 5.9, 5.0, 3.7 Hz, H-5), 3.74 (ddd, 1H, J = 7.4, 6.6, 2.9 Hz, H-3), 3.80 (dd, 1H, J = 11.0, 5.0 Hz, H-6), 3.96 (dd, 1H, J = 6.6, 5.9 Hz, H-4), 4.03 (dd, 1H, J = 11.0, 3.7 Hz, H-6), 4.27 (bdd, 1H, J = 5.9, 2.9 Hz, H-2), 4.90 (d, 1H, J = 5.9 Hz, H-1), 7.40 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.0 (SiMe), -4.9 (SiMe), -4.5 (SiMe), -4.0 (SiMe), 18.5 (CMe3), 18.7 (CMe3), 26.2 (2xCMe3), 64.4 (C-6), 70.8, 71.2 and 72.2 (C-1, C-4, C-5), 77.6, and 77.7 (C-2, C-3), 127.3, 128.3, 129.0 and 138.7 (Carom); Anal. calcd for C24H44O5Si2: C, 61.50; H, 9.47. Found: C, 61.50; H, 9.77.
(2,3,4,6-Tetra-O-acetyl-α-D-mannopyranosyl)benzene (
12). Yield 43%, white solid, m.p. 136 °C,
Rf 0.60 (petroleum ether-ethyl acetate 1:1), [α]
D20 +46 (
c 1.0, CHCl
3);
1H-NMR (CDCl
3) δ 2.01 (s, 3H, Me), 2.06 (s, 3H, Me), 2.13 (s, 3H, Me), 2.17 (s, 3H, Me), 3.77 (ddd, 1H,
J = 8.8, 6.1, 2.6 Hz, H-5), 4.14 (dd, 1H,
J = 12.0, 6.1 Hz, H-6), 4.38 (dd, 1H,
J = 12.0, 2.6 Hz, H-6), 5.12 (d, 1H,
J = 2.9 Hz, H-1), 5.17 (dd, 1H,
J = 9.1, 3.1 Hz, H-3), 5.35 (dd, 1H,
J = 9.1, 8.8 Hz, H-4), 6.20 (dd, 1H,
J = 3.1, 2.9 Hz, H-2), 7.35-7.55 (m, 5H, H
arom);
13C-NMR (CDCl
3) δ 21.1 (2xMe), 21.2 (Me), 21.4 (Me), 62.8 (C-6), 67.2 (C-4), 69.6 (C-2), 70.1 (C-3), 71.5 (C-5), 76.2 (C-1), 126.8, 128.9, 129.5 and 135.6 (C
arom), 170.0 (CO), 170.3 (CO), 170.7 (CO), 171.1 (CO). All the data are in agreement with the literature [
21].
(4,6-Di-O-tert-butyldimethylsilyl-β-D-mannopyranosyl)benzene (13) (as a mixture with 14). Yield 56%, oil, Rf 0.45 (petroleum ether-ethyl acetate 4:1); 1H-NMR (CDCl3) δ 0.10 (s, 3H, SiMe), 0.14 (s, 3H, SiMe), 0.15 (s, 3H, SiMe), 0.19 (s, 3H, SiMe), 0.93 (s, 9H, CMe3), 0.94 (s, 9H, CMe3), 1.70 (bs, 1H, OH), 2.40 (bs, 1H, OH), 3.31 (ddd, 1H, J = 9.2, 5.5, 2.9 Hz, H-5), 3.66 (dd, 1H, J = 8.8, 3.3 Hz, H-3), 3.88 (dd, 1H, J = 9.2, 8.8 Hz, H-4), 3.93 (m, 2H, H-6), 4.06 (d, 1H, J = 3.3 Hz, H-2), 4.60 (s, 1H, H-1), 7.28 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.1 (CH3), -5.0 (CH3), -4.8 (CH3), -4.0 (CH3), 18.4 (CMe3), 18.5 (CMe3), 26.0 (CMe3), 26.1 (CMe3), 62.3 (C-6), 69.1, 73.1, and 76.3 (C-1, C-4, C-5), 79.4 and 81.5 (C-2, C-3), 126.0, 127.7, 128.4 and 138.0 (Carom); Anal. calcd for C24H44O5Si2 (mixture 13 + 14): C, 61.50; H, 9.47. Found: C, 61.86; H, 9.31.
(4,6-Di-O-tert-butyldimethylsilyl-β-D-allopyranosyl)benzene (14) (as a mixture with 13). Yield 14%, oil, Rf 0.45 (petroleum ether-ethyl acetate 4:1); 1H-NMR (CDCl3) δ 0.00 (s, 3H, SiMe), 0.03 (s, 3H, SiMe), 0.07 (s, 3H, SiMe), 0.16 (s, 3H, SiMe), 0.90 (s, 9H, CMe3), 0.93 (s, 9H, CMe3), 1.50 (bs, 1H, OH), 2.60 (bs, 1H, OH), 3.50 (dd, 1H, J = 9.9, 2.9 Hz, H-2), 3.61 (ddd, 1H, J = 9.5, 2.9, 1.5 Hz, H-5), 3.80 (dd, 1H, J = 11.4, 2.9 Hz, H-6), 3.87 (dd, 1H, J = 11.4, 1.5 Hz, H-6), 4.01 (dd, 1H, J = 9.5, 2.9 Hz, H-4), 4.20 (dd, 1H, J = 2.9, 2.9 Hz, H-3), 4.50 (d, 1H, J = 9.9 Hz, H-1), 7.40 (m, 5H, Harom); 13C-NMR (CDCl3) δ -5.2 (CH3), -5.0 (CH3), -4.8 (CH3), -4.5 (CH3), 18.0 (CMe3), 18.2 (CMe3), 25.8 (CMe3), 26.0 (CMe3), 62.2 (C-6), 67.8, 71.5 and 73.1 (C-1, C-4, C-5), 76.1 and 77.6 (C-2, C-3), 127.4, 128.0, 128.2 and 137.0 (Carom).
(2,3,4,6-Tetra-O-acetyl-β-D-mannopyranosyl)benzene (
15) (as a mixture with
16). Yield 19%, oil,
Rf 0.30 (petroleum ether-ethyl acetate 1:1);
1H-NMR (CDCl
3) δ 2.00 (s, 3H, Me), 2.03 (s, 3H, Me), 2.11 (s, 3H, Me), 2.20 (s, 3H, Me), 3.83 (ddd, 1H,
J = 9.5, 5.9, 2.6 Hz, H-5), 4.21-4.30 (m, 1H, H-6), 4.35 (dd, 1H,
J = 12.5, 5.9 Hz, H-6), 4.79 (d, 1H,
J = 1.1 Hz, H-1), 5.25 (dd, 1H,
J = 10.2, 3.3 Hz, H-3), 5.33 (dd, 1H,
J = 10.2, 9.5 Hz, H-4), 5.55 (dd, 1H,
J = 3.3, 1.1 Hz, H-2), 7.30 (m, 5H, H
arom);
13C-NMR (CDCl
3) δ 20.2 (Me), 20.3 (Me), 20.8 (Me), 21.0 (Me), 60.4 (C-6), 66.6 (C-4), 73.1 (C-2), 77.3 (C-3), 77.4 (C-5), 81.8 (C-1), 127.2, 128.7, 129.2, and 136.3 (C
arom), 168.7 (CO), 168.9 (CO), 169.2 (CO), 170.6 (CO). All data are in agreement with the literature [
21].
(2,3,4,6-Tetra-O-acetyl-β-D-allopyranosyl)benzene (16) (as a mixture with 15). Yield 56%, oil, Rf 0.30 (petroleum ether-ethyl acetate 1:1); 1H-NMR (CDCl3) δ 1.80 (s, 3H, Me), 2.03 (s, 3H, Me), 2.08 (s, 3H, Me), 2.23 (s, 3H, Me), 4.15-4.27 (m, 3H, H-5, H-6), 4.71 (d, 1H, J = 10.3 Hz, H-1), 5.03 (dd, 1H, J = 10.3, 2.9 Hz, H-2), 5.12 (dd, 1H, J = 9.9, 2.6 Hz, H-4), 5.73 (dd, 1H, J = 2.9, 2.6 Hz, H-3), 7.35-7.43 (m, 5H, Harom); 13C-NMR (CDCl3) δ 20.4 (Me), 20.6 (Me), 20.8 (Me), 21.4 (Me), 62.8 (C-6), 66.6 (C-4), 68.5 (C-2), 70.6 (C-3), 71.4 (C-5), 76.2 (C-1), 126.9, 127.2, 128.4 and 136.9 (Carom), 168.8 (CO), 169.2 (CO), 170.0 (CO), 170.8 (CO).