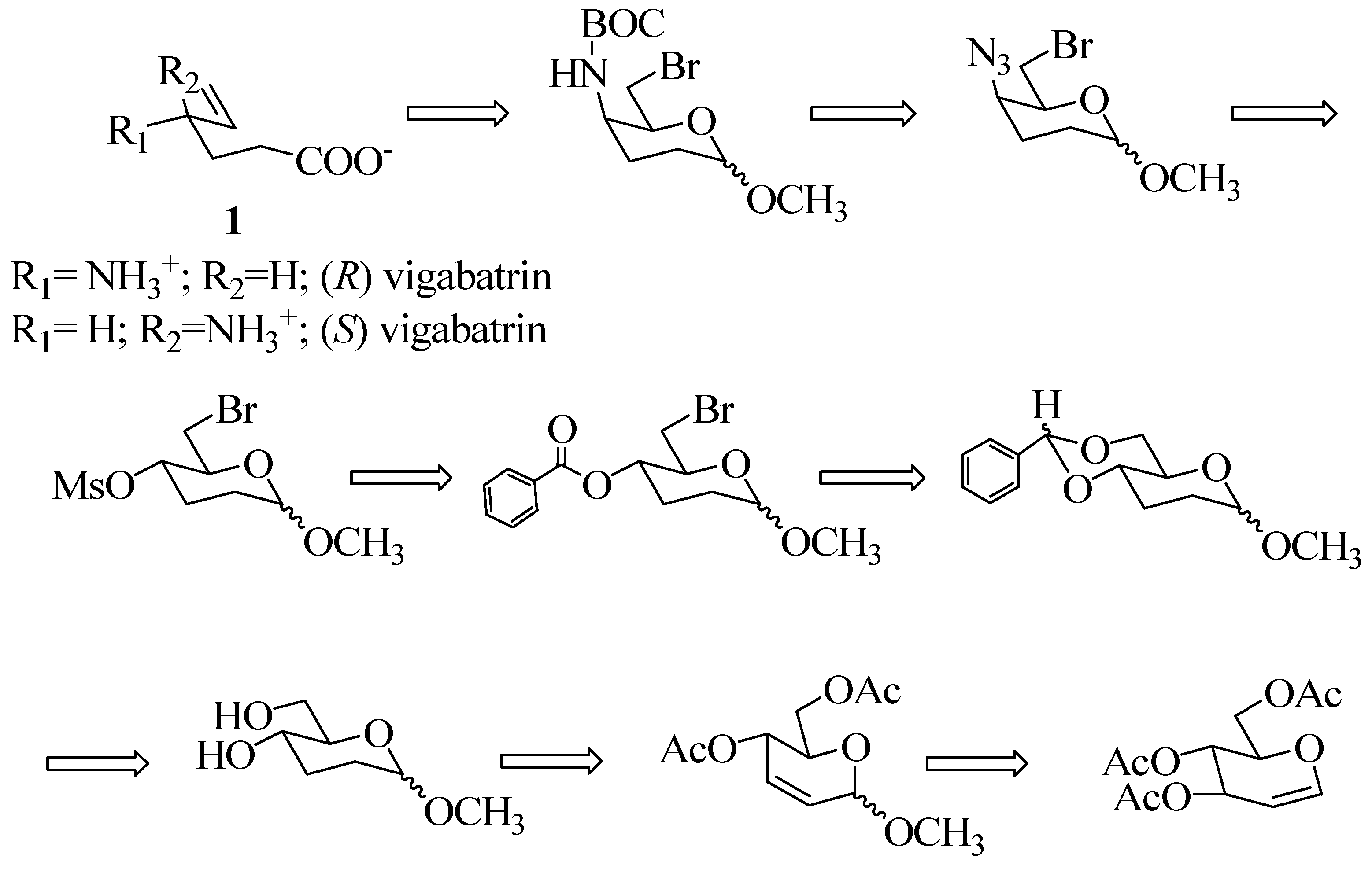
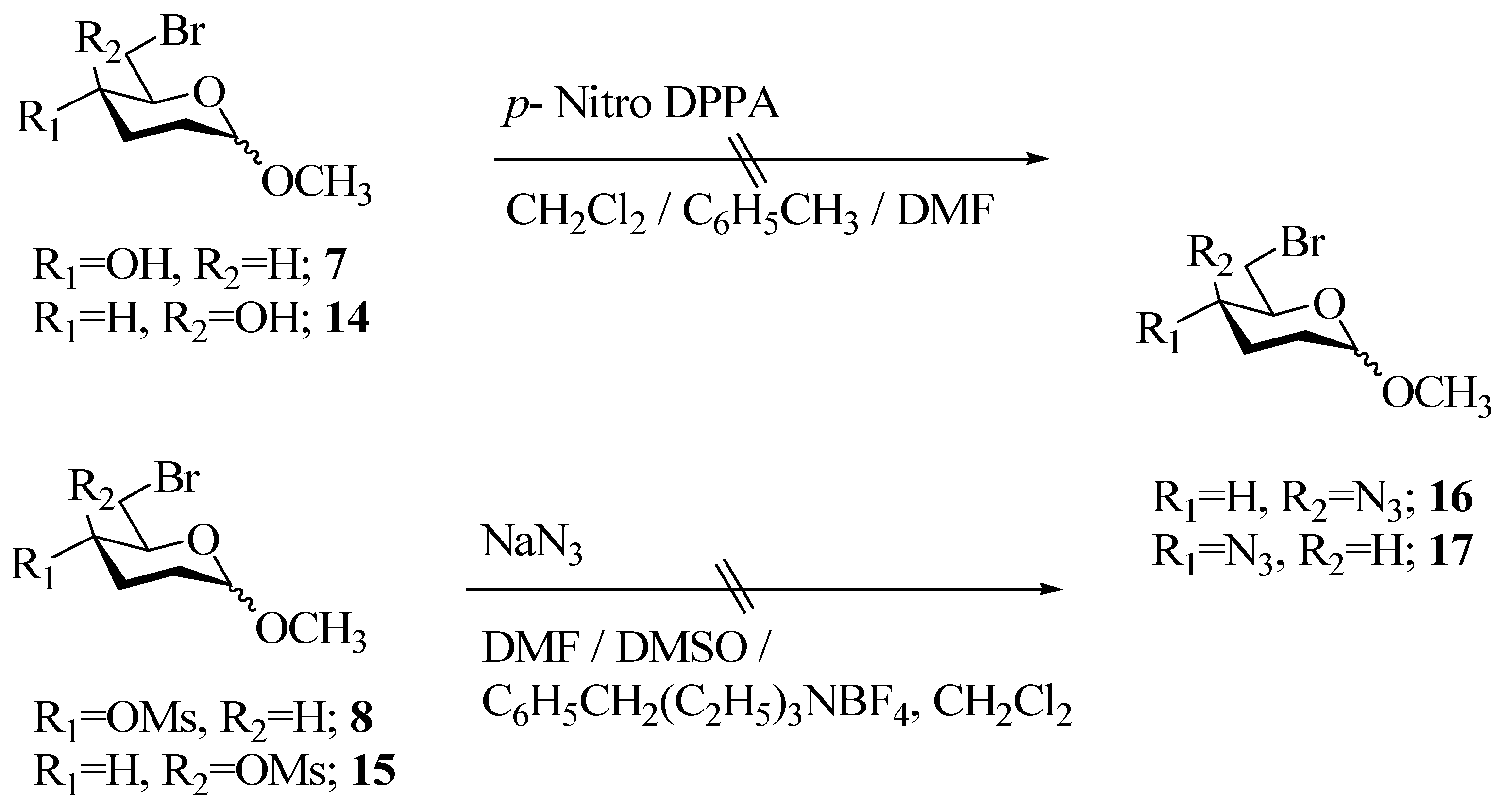
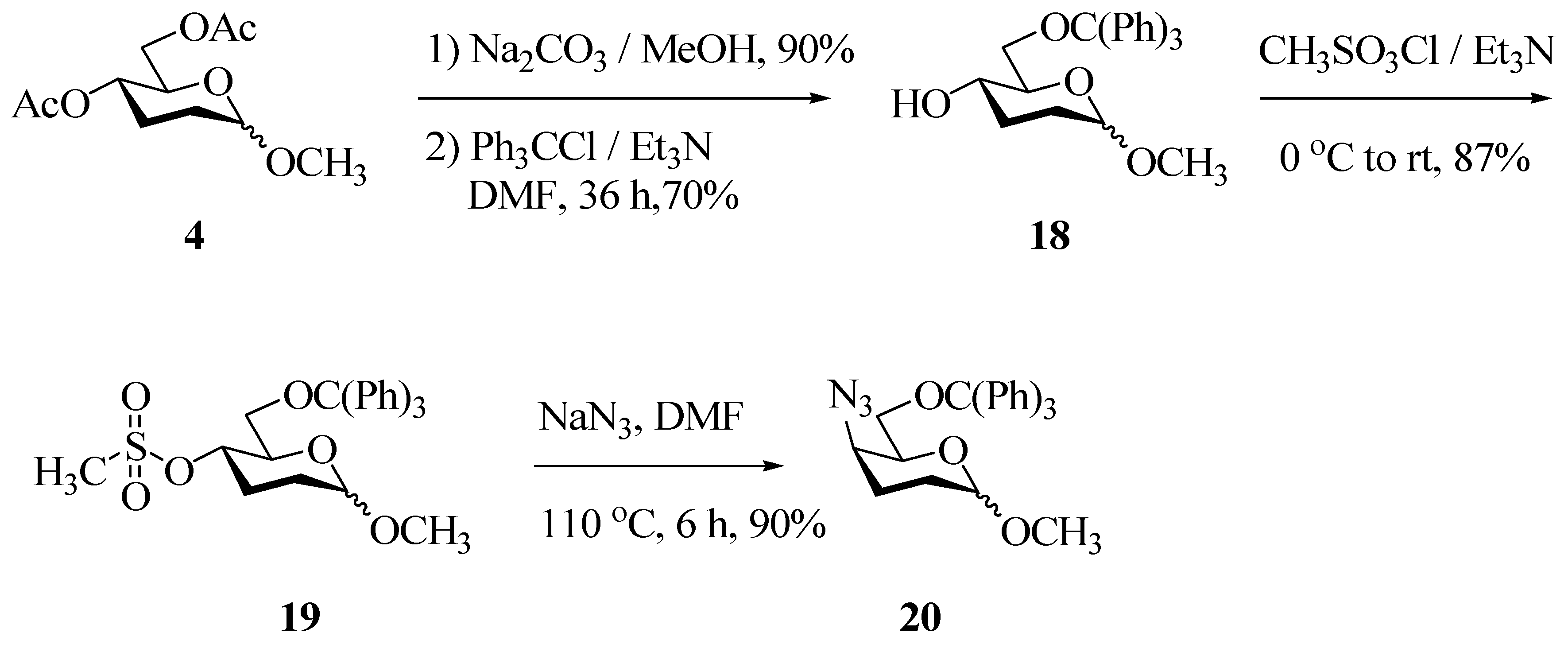
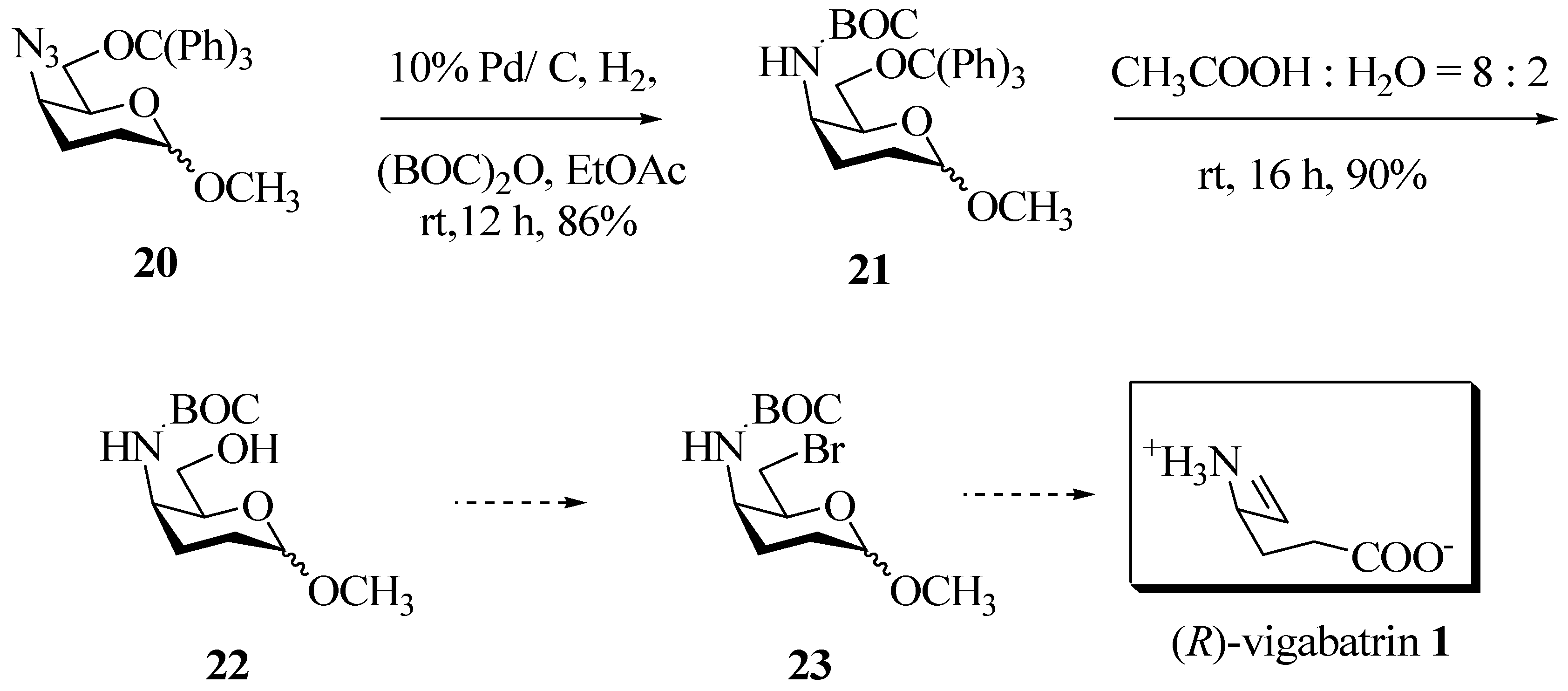
Experimental
General
1H- and 13C-NMR spectra were obtained using a Varian Gemini 200 NMR and were recorded at 200 and 50 MHz respectively. All reagents and chemicals were obtained from Aldrich Chemical Company (USA) and were used as received unless otherwise noted.
General procedure for the hydrogenation of methyl-4,6-di-O-acetyl-2,3-dideoxy-D-erythro-hex-2- enopyranosid (3) and methyl-4,6-di-O-acetyl-2,3-dideoxy-D-threo-hex-2-enopyranoside(10).
Compounds 3 or 10 (1 mmol) was placed in a 25 mL round bottomed flask. The substrate was dissolved in methanol (5 mL) and a catalytic amount of 10% Pd/C catalyst was added. Hydrogen gas was passed through the solution using a balloon. When there was no more uptake of hydrogen (5 h to 6 h), the reaction was stopped and the catalyst filtered. The solvent was evaporated in a rotary evaporator under reduced pressure to afford methyl-4,6-di-O-acetyl-2,3-dideoxy-D-erythro-hexopyranoside (4) and methyl-4,6-di-O-acetyl-2,3-dideoxy-D-threo-hexopyranoside (11).
Methyl-4,6-di-O-acetyl-2,3-dideoxy-D-erythro-hexopyranoside (4). Semi-solid; TLC: Rf: 0.7 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2960, 2848, 1734, 1452, 1360, 1270, 1126, 1084, 1062, 995, 969, 956, 899, 857; 1H-NMR δ (ppm): 1.44-1.99 (m, 4H, H-2a, H-2e, H-3a, H-3e), 1.96 (s, 3H, OCOCH3), 1.98 (s, 3H, -OCOCH3), 3.26 (s, 3H, OCH3), 3.92-4.06 (m, 3H, H-5, H-6a, H-6b), β), 4.65-4.81 (m, 1H, H-4), 5.20 (bs, 1H, H-1); 13C-NMR δ (ppm): 20.60 (q, -OCOCH3), 20.77 (q, -OCOCH3), 22.51 (t, C-3), 24.16 (t, C-2), 54.70 (q, -OCH3), 63.61 (t, C-6), 66.67 (d, C-5), 66.96 (d, C-4), 97.88 (d, C-1), 170.48 (s, -OCOCH3), 170.65 (s, -OCOCH3).
Methyl-4,6-di-O-acetyl-2,3-dideoxy-D-threo-hexopyranoside (11). Semi solid; TLC: Rf: 0.7 (hexane- EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2962, 2848, 1735, 1458, 1368, 1272, 1124, 1088, 1060, h992, 966; 1H-NMR δ (ppm): 1.50-2.00 (m, 4H, H-2a, H-2e, H-3a, H-3e), 2.06 (s, 3H, OCOCH3), 2.11 (s, 3H, -OCOCH3), 3.38 (s, 3H, OCH3), 4.00-4.25 (m, 3H, H-5, H-6a, H-6b), 4.78 (apparent d, J = 1.18Hz, H-4), 4.93 (bs, 1H, H-1); 13C-NMR δ (ppm): 20.53 (q, -OCOCH3), 20.82 (q, -OCOCH3), 22.24 (t, C-3), 23.89 (t, C-2), 54.46 (q, -OCH3), 63.37 (t, C-6), 66.42 (d, C-5), 66.69 (d, C-4), 97.67 (d, C-1), 170.23 (s, -OCOCH3), 170.41 (s, -OCOCH3).
General procedure for the hydrolysis and subsequent benzylidene protection of methyl-4,6-di-O- acetyl-2,3-dideoxy-D-erythro-hexopyranoside (4) and methyl-4,6-di-O-acetyl-2,3-dideoxy-D-threo- hexopyranoside (11) to obtain the corresponding benzylidene acetals
Compounds 4 or 11 (1 mmol) was dissolved in absolute methanol (50 mL) and stirred for 3h at room temperature with anhydrous sodium carbonate (3 mol). The solids were removed by filtration and the filtrate was evaporated under reduced pressure to give the crude product, which can be used as such for benzylidene protection with out any purification. Diols were dissolved in dry DMF (5 mL) in a two necked round bottomed flask. To this was added benzaldehyde dimethylacetal (1.2 mmol), a catalytic amount of p-toluene-sulfonic acid (15-25 mg) and the contents stirred at room temperature (for 3 h in the case of gluco-4, 14 h in the case of galacto-11). The reaction mixture was poured into cold water (5 mL), stirred for an additional 10 min and the product extracted with hexane (3 x 25 mL), dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain methyl 4,6-O-benzylidene-2,3-dideoxy-D-erythro-hexopyranoside (5) and methyl-4,6-O-benzylidene-2,3-dideoxy-D-threo-hexopyranoside (12).
Methyl-4,6-O-benzylidene-2,3-dideoxy-D-erythro-hexopyranoside (5). Solid (m.p. = 63 °C); TLC: Rf: 0.7 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2944, 2864, 1452, 1369, 1315, 1289, 1126, 1097, 1052, 995, 976, 915; 1H-NMR δ (ppm): 1.64-2.12 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.36 (s, 3H, OCH3), 3.44-3.70 (m, 3H, H-5, H-6a, H-6b), 4.19-4.22 (m, 1H, H-4), 4.67 (d, J1,2a = 2.55Hz, 1H, H-1), 5.55 (s, 1H, ArCHO2), 7.23-7.49 (m, 3H, Ar-H), 7.61-7.88 (m, 2H, Ar-H); 13C-NMR δ (ppm): 23.97 (t, C-3), 29.45 (t, C-2), 55.30 (q, -OCH3), 64.00 (t, C-6), 67.00 (d, C-5), 67.63 (d, C-4), 96.92 (d, C-1), 101.66 (d, -CH-Ar), 124.96 (d, Ar-CH), 127.01 (d, Ar-CH), 127.36 (d, Ar-CH), 137.71 (s, Ar-C).
Methyl-4,6-O-benzylidene-2,3-dideoxy-D-threo-hexopyranoside (12). Semi-solid; TLC: Rf: 0.7 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2948, 2866, 1458, 1366, 1310, 1288, 1128, 1086, 1057, 998; 1H-NMR δ (ppm): 1.62-2.15 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.39 (s, 3H, OCH3), 3.31-3.59 (m, 2H, H-6a, H-6b), 3.83-4.24 (m, 2H, H-4, H-5), 4.83 (bs, 1H, H-1), 5.54 (s, 1H, PCHO2), 7.24-7.62 (m, 4H, Ar-H), 7.85 (d, J = 7.27Hz, 1H, Ar-H); 13C-NMR δ (ppm): 22.18 (t, C-3), 24.69 (t, C-2), 55.30 (q, -OCH3), 64.00 (t, C-6), 67.63 (d, C-5), 67.92 (d, C-4), 98.25 (d, C-1), 101.52 (d, -O2CHAr), 126.25 (d, Ar-CH), 127.66 (d, Ar-CH), 128.92 (d, Ar-CH), 138.30 (s, Ar-C).
General procedure for the opening of benzylidene acetals of methyl-4,6-O-benzylidene-2,3-dideoxy-D- erythro-hexopyranoside (5) and methyl-4,6-O-benzylidene-2,3-dideoxy-D-threo-hexopyranoside (12) using N-bromosuccinimide
Compound 5 or 12 (1 mmol) was dissolved in dry CCl4 (5 mL) in a two necked round bottomed flask. To this solution, N-bromosuccinimide (1.2 mmol) and barium carbonate (0.55 mol) were added. The reaction mixture is heated to reflux for the required period (4 h in the case of 5, 2.5 h in the case of 12). During the initial period of heating, a reddish orange color developed but faded before completion of the reaction. The yellowish gummy residue was washed with CCl4 (2 x 25 mL), and the filtrate and washings were evaporated under reduced pressure. The residue was dissolved in ether (50 mL) and the solution washed with water (3 x 25 mL), dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain methyl-4-O-benzoyl-6- bromo-2,3-dideoxy-D-erythro-hexopyranoside (6) and methyl-4-O-benzoyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (13).
Methyl-4-O-benzoyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (6). Viscous liquid; TLC: Rf: 0.8 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2944, 1718, 1596, 1446, 1360, 1312, 1267, 1126, 1113, 1062, 1027, 998, 944 ; 1H-NMR δ (ppm): 1.69-2.13 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.39 (s, 3H, OCH3), 3.42-3.62 (m, 2H, H-6a, H-6b), 4.03-4.09 (m, 1H, H-5), 4.76 (bs, 1H, H-1), 4.86-4.94 (m, 1H, H-4), 7.39-7.57 (m, 3H, Ar-H), 7.99 (d, J = 7.22Hz, 2H, Ar-H); 13C-NMR δ (ppm): 24.01 (t, C-3), 28.76 (t, C-2), 32.95 (t, C-6), 54.80 (q, -OCH3), 70.07 (d, C-5), 70.98 (d, C-4), 97.63 (d, C-1), 128.43 (d, Ar-CH), 129.60 (d, Ar-CH), 129.72 (d, Ar-CH), 133.25 (s, Ar-C), 165.37 (s, -OCO-Ar).
Methyl-4-O-benzoyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (13). Viscous liquid; TLC: Rf: 0.8 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2948, 1712, 1603, 1444, 1366, 1312, 1276, 1171, 1126, 1120, 1072, 1024, 979, 951, 905; 1H-NMR δ (ppm): 1.57-2.16 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.35 (s, 3H, OCH3), 3.32-3.60 (m, 2H, H-6a, H-6b), 4.14 (t, J5,6a = J5,6b = 6.81Hz, 1H, H-5), 4.84 (bs, 1H, H-1), 5.25 (bs, 1H, H-4), 7.24-7.58 (m, 3H, Ar-H), 8.04 (d, J = 7.38Hz, 2H, Ar-H); 13C-NMR δ (ppm): 21.81 (t, C-3), 24.15 (t, C-2), 31.28 (t, C-6), 55.44 (q, -OCH3), 69.26 (d, C-5), 70.18 (d, C-4), 97.99 (d, C-1), 128.42 (d, Ar-CH), 129.30 (d, Ar-CH), 129.94 (d, Ar-CH), 133.19 (s, Ar-C), 165.68 (s, - OCOAr).
General procedure for the hydrolysis of methyl-4-O-benzoyl-6-bromo-2,3-dideoxy-D-erythro- hexopyranoside (6) and Methyl-4-O-benzoyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (13).
Compounds 6 or 13 (1 mmol) were dissolved in absolute methanol (50 mL) and stirred for 3h at room temperature with anhydrous sodium carbonate (3 mol). The solids were removed by filtration and the filtrate was evaporated under reduced pressure. The residue was further purified by flash column chromatography on silica gel to obtain methyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (7) and methyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (14).
Methyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (7). Viscous liquid; TLC: Rf: 0.3 (hexane- EtOAc = 7:3); IR (CHCl3) υ (cm-1): 3408,2928, 2864, 1372, 1340, 1315, 1123, 1094, 1014, 982, 956, 902, 864 ; 1H-NMR δ (ppm): 1.69-2.01 (m, 4H, H-2a, H-2e, H-3a, H-3e), 2.20 (bs, 1H, 4-OH), 3.40 (s, 3H, OCH3), 3.50-3.70 (m, 3H, H-5, H-6a, H-6b), 3.77 (d, J4,5 = 9.09 Hz, H-4), 4.75 (bs, 1H, H-1); 13C-NMR δ (ppm): 27.45 (t, C-3), 29.13 (t, C-2), 34.21 (t, C-6), 54.64 (q, -OCH3), 68.38 (d, C-4), 72.41 (d, C-5), 97.50 (d, C-1).
Methyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (14). Viscous liquid; TLC: Rf: 0.3 (hexane- EtOAc = 7:3); IR (CHCl3) υ (cm-1): 3410, 2928, 2866, 1376, 1343, 1316, 1128, 1098, 1017, 986, 962; 1H-NMR δ (ppm): 1.67-2.10 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.42 (s, 3H, OCH3), 3.37-3.55 (m, 3H, 4-OH, H-6a, H-6b), 3.90 (bs, 1H, H-4), 3.99 (distorted t, J = 6.20Hz, 1H, H-5), 4.76 (bs, 1H, H-1); 13C-NMR δ (ppm): 23.37 (t, C-3), 25.68 (t, C-2), 31.87 (t, C-6), 54.94 (q, -OCH3), 65.04 (d, C-4), 70.58 (d, C-5), 98.45 (d, C-1).
General procedure for the mesitylation of methyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (7) and methyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (14).
Compound 7 (or 14) (1 mmol) was dissolved in dry dichloromethane (5 mL) in a two-necked round-bottomed flask. To this solution, methansesulfonyl chloride (1.2 mmol) and triethyl amine (2 mmol) were added at 0 °C. The reaction mixture was stirred for 3 h at ambient temperature. The reaction mixture was diluted with CH2Cl2 (2 x 25 mL), washed with water (3 x 25 mL), dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain methyl-4-O-methanesulfonyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (8) and methyl-4-O-methanesulfonyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (15).
Methyl-4-O-methanesulfonyl-6-bromo-2,3-dideoxy-D-erythro-hexopyranoside (8). Viscous liquid; TLC: Rf: 0. 7 (hexane-EtOAc = 1:1); IR (CHCl3) υ (cm-1): 2944, 2864, 1356, 1174, 1129, 1094, 905, 960, 908, 889, 867, 832 ; 1H-NMR δ (ppm): 1.72-2.15 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.06 (s, 3H, -SO2CH3), 3.36 (s, 3H, OCH3), 3.45-3.70 (m, 2H, H-6a, H-6b), 3.87 (d, J = 2.5Hz, 1H, H-5), 4.51 (d, J = 5.5Hz, 1H, H-4), 4.70 (bs, 1H, H-1); 13C-NMR δ (ppm): 25.32 (t, C-3), 28.89 (t, C-2), 32.68 (t, C-6), 38.98 (q, -OSO2CH3), 54.94 (q, -OCH3), 68.97 (d, C-5), 76.61 (d, C-4), 97.43 (d, C-1).
Methyl-4-O-methanesulfonyl-6-bromo-2,3-dideoxy-D-threo-hexopyranoside (15). Vscous liquid; TLC: Rf: 0. 5 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 2948, 2864, 1358, 1176, 1123, 1098, 908, 958, 908; 1H-NMR δ (ppm): 1.72-2.24 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.10 (s, 3H, -SO2CH3 of α), 3.15 (s, 3H, -SO2CH3 of β), 3.41 (s, 3H, OCH3 of α), 3.44 (s, 3H, OCH3 of β), 3.28-3.70 (m, 2H, H-6a, H-6b), 4.06 (t, J5,6a = J5,6b = 6.07Hz, 1H, H-5), 4.78 (bs, 1H, H-1), 4.92 (bs, 1H, H-4); 13C-NMR δ (ppm): 23.35 (t, C-3), 23.85 (t, C-2), 30.34 (t, C-6), 38.65 (q, -OSO2CH3), 54.94 (q, -OCH3), 69.12 (d, C-5), 74.53 (d, C-4), 98.05 (d, C-1).
Methyl-6-O-triphenylmethyl-2,3-dideoxy-D-erythro-hexopyranoside (18). Methyl-2,3-dideoxy-D-erythro-hexopyranoside (4) (1 mmol) was dissolved in dry DMF (5 mL) in a two necked round bottomed flask. To this solution, triphenylmethyl chloride (1.1 mmol), triethyl amine (2 mmol) and catalytic amount DMAP (25 mg) were added. The reaction mixture was stirred for 36 h at room temperature. The solution was poured in to ice water (25 mL) and extracted with dichloromethane (3x 25 mL). The organic layer was washed successively with saturated ammonium chloride solution (3 x 25 mL) and water (3 x 25 mL), dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain (18). Semi solid; TLC: Rf: 0.5 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 3504(b), 2928, 2862, 1600, 1484, 1443, 1369, 1321, 1126, 1081, 988, 944, 899; 1H-NMR δ (ppm): 1.69-1.83 (m, 4H, H-2a, H-2e, H-3a, H-3e), 2.74 (bs, 1H, 4-OH), 3.41 (s, 3H, OCH3), 3.43-3.60 (m, 4H, H-4, H-5, H-6a, H-6b), 4.61 (bs, 1H, H-1), 7.20-7.32 (m, 9H, Ar-H), 7.43-7.46 (m, 6H, Ar-H); 13C-NMR δ (ppm): 28.79 (t, C-3), 29.96 (t, C-2), 54.39 (q, -OCH3), 66.05 (t, C-6), 69.05 (d, C-5), 70.88 (d, C-4), 87.48 (s, -OC(Ph)3), 97.18 (d, C-1), 127.15 (d, Ar-CH), 127.92 (d. Ar-CH), 128.56 (d, Ar-CH), 143.38 (s, Ar-C), 143.57 (s, Ar-C).
Methyl-4-O-methanesulfonyl-6-triphenylmethyl-2,3-dideoxy-D-erythro-hexopyranoside (19). Methyl-6-O-triphenylmethyl-2,3-dideoxy-D-erythro-hexopyranoside (18) (1 mmol) was dissolved in dry dichloromethane (5 mL) in a two-necked round-bottomed flask. To this solution, methansesulfonyl chloride (1.2 mmol) and triethyl amine (2 mmol) were added at 0 °C. The reaction mixture was brought to room temperature over a period of 1.5 h. The reaction mixture was diluted with CH2Cl2 (2 x 25 mL), washed with water (3 x 25 mL), dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain (19). Semi solid; TLC: Rf: 0.6 (hexane-EtOAc = 8:2); IR (CHCl3) υ (cm-1): 2928, 1600, 1488, 1446, 1356, 1174, 1129, 972, 956; 1H-NMR δ (ppm): 1.69-2.27 (m, 4H, H-2a, H-2e, H-3a, H-3e), 2.55 (s, 3H, 4-OSO2CH3), 3.40 (s, 3H, OCH3), 3.26-3.57 (m, 2H, H-6a, H-6b), 3.86 (dd, J4,5 = 9.7Hz, J5,6a = 2.96Hz, 1H, H-5), 4.64 (td, J4,5 = J3a,4 = 9.99Hz, J3e,4 = 5.51Hz, 1H, H-4), 4.77 (s, 1H, H-1), 7.05-7.28 (m, 9H, Ar-H), 7.45-7.48 (m, 6H, Ar-H); 13C-NMR δ (ppm): 25.89 (t, C-3), 28.81 (t, C-2), 37.93 (q, -OSO2CH3), 54.50 (q, -OCH3), 62.57 (t, C-6), 69.28 (d, C-5), 75.61 (d, C-4), 86.58 (s, -OC(Ph)3), 97.14 (d, C-1), 125.73 (d, Ar-CH), 127.04 (d, Ar-CH), 127.74 (d, Ar-CH), 128.70 (d, Ar-CH), 143.57 (s, Ar-C).
Methyl-4-azido-6-O-triphenylmethyl-2,3-dideoxy-D-threo-hexopyranoside (20). Methyl-4-O- methanesulfonyl-6-triphenylmethyl-2,3-dideoxy-D-erythro-hexopyranoside (19) (1 mmol) was dissolved in dry DMF (3 mL) in a two necked round bottomed flask. To this solution were added sodium azide (1.2 mmol) and one or two drops of water, just to solubilise the azide. The reaction mixture was heated at 110 °C for 6 h, then poured in to ice water (25 mL) and extracted with dichloromethane (5 x 25 mL). The organic layer was dried over anhydrous sodium sulphate and concentrated. The residue was purified by flash column chromatography on silica gel to obtain (20). Solid (m.p. = 69 °C); TLC: Rf: 0.6 (hexane-EtOAc = 8:2); IR (CHCl3) υ (cm-1): 3056, 2928, 2112, 1484, 1443, 1366, 1331, 1264, 1206, 1180, 1126, 1078, 1033, 995, 953, 921, 899, 707, 630; 1H-NMR δ (ppm): 1.69-2.13 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.15 (dd, J6a,6b = 13.18Hz, J5,6a = 5.86Hz, 1H, H-6a), 3.20-3.39 (m, 1H, H-6b), 3.33 (s, 3H, OCH3), 3.73 (s, 1H, H-5), 3.87 (td, J4,5 = J3a,4 = 6.1Hz, J3e,4 = 1.47Hz, 1H, H-4), 4.67 (d, J1,2e = 2.93Hz, 1H, H-1), 7.20-7.31 (m, 9H, Ar-H), 7.42-7.82 (m, 6H, Ar-H); 13C-NMR δ (ppm): 22.60 (t, C-3), 24.40 (t, C-2), 54.67 (q, -OCH3), 56.77 (d, C-4), 63.67 (t, C-6), 68.13 (d, C-5), 86.91 (s, -OC(Ph)3), 97.07 (d, C-1), 127.06 (d, Ar-CH), 127.82 (d, Ar-CH), 128.64 (d, Ar-CH), 128.74 (d, Ar-CH), 143.91 (s, Ar-C).
Methyl-4-(N-t-butoxycarbonyl)-6-O-triphenylmethyl-2,3-dideoxy-D-threo-exopyranoside (21). Methyl-4-azido-6-O-triphenylmethyl-2,3-dideoxy-D-threo-hexopyranoside (20) (1 mmol) was dissolved in EtOAc (5 mL) in a two necked round bottomed flask. To this solution, a catalytic amount of 10% Pd / C catalyst (25mg) and di-t-butylcarbonate (1.2 mmol) were added. Hydrogen gas was passed through the solution using a balloon. When there was no more uptake of hydrogen (~ 12h), the reaction was stopped and the catalyst filtered. The solvent was evaporated in a rotary evaporator under reduced pressure and the residue was purified by flash column chromatography on silica gel to obtain pure product (21). Semi solid; TLC: Rf: 0.5 (hexane-EtOAc = 8:2); IR (CHCl3) υ (cm-1): 3440(s), 2992, 2960, 1708, 1484, 1449, 1385, 1356, 1328, 1161, 1116, 1068, 1030, 1001, 905, 630; 1H-NMR δ (ppm): 1.36 (s, 9H, C(CH3)3), 1.60-2.03 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.09-3.17 (m, 2H, H-6a, H-6b), 3.42 (s, 3H, OCH3), 3.71 (bs, 1H, H-5), 4.05 (bs, H-4), 4.72 (bs, 1H, H-1), 5.13 (d, J 8.3Hz, 1H, -NH), 7.20-7.30 (m, 9H, Ar-H), 7.44-7.84 (m, 6H, Ar-H); 13C-NMR δ (ppm): 23.87 (t, C-3), 24.49 (t, C-2), 28.35 (q, -OC(CH3)3), 45.88 (d, C-4), 54.62 (q, -OCH3), 64.54 (t, C-6), 69.00 (d, C-5), 79.11 (s, OC(CH3)3 of α), 86.65 (s, OC(CH3)3 of β), 98.07 (d, C-1), 126.95 (d, Ar-CH), 127.77 (d, Ar-CH), 128.68 (d, Ar-CH), 143.98 (s, Ar-C), 155.35 (s, -OCO(CH3)3).
Methyl-4-(N-t-butoxycarbonyl)-2,3-dideoxy-D-threo-hexopyranoside (22). To methyl-4-(N-t- butoxycarbonyl)-6-O-triphenylmethyl-2,3-dideoxy-D-threo-exopyranoside (21) (1 mmol) taken in a round bottomed flask, was added a solution of aq. 80% acetic acid (2 mL) and the contents stirred for 16 h at room temperature. The reaction mixture was poured into cold water (10 mL) and extracted with chloroform (5x 25 mL). The organic layer was washed repeatedly with water (3 x 25 mL), saturated hydrogen carbonate (3 x 25 mL) and again with water (3 x 25 mL). The organic layer was evaporated in a rotary evaporator under reduced pressure and the residue was purified by flash column chromatography on silica gel to obtain pure product (22). Viscous liquid; TLC: Rf: 0.2 (hexane-EtOAc = 7:3); IR (CHCl3) υ (cm-1): 3540(bs), 2992, 2960, 1706, 1488, 1449, 1386, 1361, 1328, 1164, 1110, 1066, 1028, 908; 1H-NMR δ (ppm): 1.36 (s, 9H, C(CH3)3), 1.67-2.17 (m, 4H, H-2a, H-2e, H-3a, H-3e), 3.19-3.70 (m, 2H, H-6a, H-6b), 3.35 (s, 3H, OCH3), 3.83-3.98 (m, 2H, H-4, H-5), 4.76 (bs, 1H, H-1), 5.15 (d, J = 8.68Hz, 1H, -NH); 13C-NMR δ (ppm): 23.06 (t, C-3), 24.70 (t, C-2), 28.23 (q, -OC(CH3)3), 44.33 (d, C-4), 54.67 (q, -OCH3), 61.75 (t, C-6), 69.33 (d, C-5), 80.17 (s, -OC(CH3)3), 97.74 (d, C-1), 157.03 (s, -OCO(CH3)3).