Results and Discussion
We have previously reported [
2] the formation of 4-aminobicyclo[2.2.2]octan-2-ones from ammonium thiocyanates and benzylidene acetone in a one-pot reaction, proposing the following mechanism for this transformation: benzylidene acetone (
1) reacts with dialkylammonium thiocyanates
2a-d in an initial step to give the enammonium salts
3a-d, followed by a Diels-Alder reaction of the latter with unreacted
1. The thus formed 4-acetylcyclohex-1-enylammonium salts
4a-d then cyclize to give the bicyclic compounds
5a-d (
Scheme 1).
Recently, Ramachary
et al. [
3] have reported amine-catalyzed self-Diels-Alder reactions of α,β‑unsaturated ketones giving cyclohexanones. Therefore, the following alternative mechanism also ought to be considered to account for the formation of compounds
5a-d: the first step in this case is an amine catalyzed Diels-Alder reaction to give cyclohexanone
6, followed by the formation of enamine salts
4a-d which subsequently cyclize as mentioned above to afford the final bicyclo[2.2.2]octan-2-one products
5a-d (
Scheme 2).
We started our investigations with the synthesis of diketone
6 via an amine-catalyzed Diels-Alder reaction [
3] giving selectively the symmetric diketone
7. Its diastereoisomer
8 was obtained by the reaction of
1 with 2-trimethylsilyloxy-4-phenyl-1,3-butadiene (
9) [
4]. The regioselective formation of enamines
10b-d,
11b and
12b was observed for the reaction of both diketones
7 and
8 with secondary amines by standard methods. In the case of diketone
8, an unseparable mixture of compounds
11b and
12b was produced (
Scheme 3).
The enamine
10b and the mixture of
11b and
12b were exposed to the reaction conditions which are described by Morita and Kobayashi for the formation of 1-methyl-4-morpholinobicyclo-[2.2.2]octan-2-one from 4-acetyl-4-methyl-1-morpholino-1-cyclohexene [
5]. However, the enamine
10b decomposed to the diketone 7 whereas the mixture of enamines
11b and
12b decomposed to a mixture of unseparable products. No 4-aminobicyclooctanone derivatives were detectable in these reaction mixtures by NMR experiments.
The diketone
7 was next refluxed with dimethylammonium thiocyanate in toluene at 160°C or in dimethylformamide at 200°C at a water separator but no reaction was observed. For the reaction of
7 with morpholinium thiocyanate in refluxing toluene we observed no formation of a bicyclic compound either. Diketone
8 reacts with morpholinium thiocyanate under the same conditions to give small but detectable amounts of
5b. The reaction of diketone
8 with morpholine in refluxing benzene under the catalysis of 4-toluenesulfonic acid yielded a mixture of
11b and
12b accompanied by small amounts of
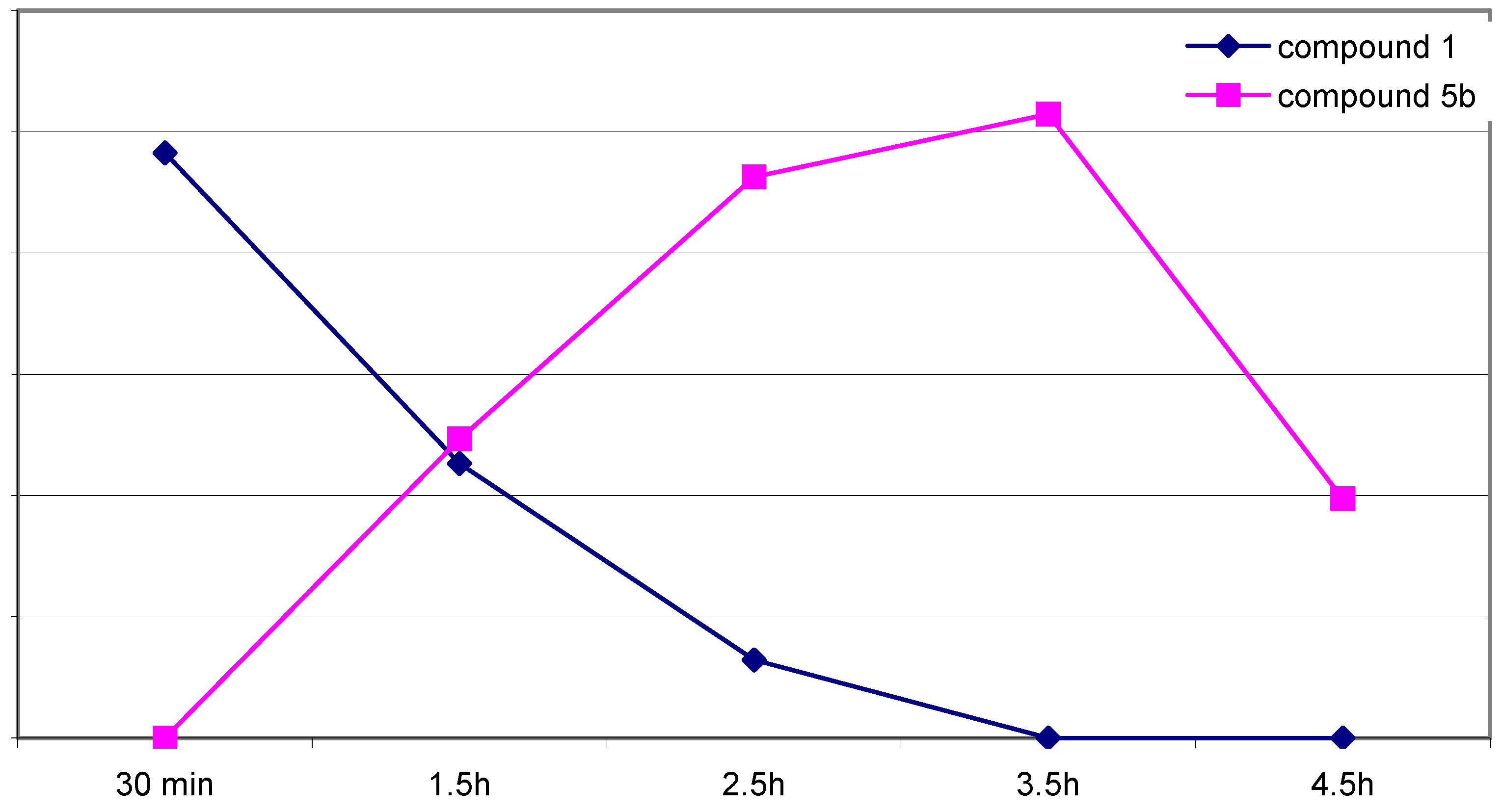
5b. We monitored the reaction of benzylidene acetone with morpholinium thiocyanate in toluene using GC-MS methods. Each hour, we took a sample which was extracted with 2N NaOH and water to remove salts. The concentrations of benzylidene acetone and
5b formed during the progress of the reaction were calculated as the areas under the respective curves and are shown in
Figure 1.
Figure 1.
The course of reaction expressed as areas under the curve (ordinate).
Figure 1.
The course of reaction expressed as areas under the curve (ordinate).
Obviously the concentration of 5b increases parallel to the decrease of the concentration of 1. After 3.5 hours the maximum amount of 5b is reached and after this point, decomposition of 5b takes place. In addition to that, we found only very small amounts of 7, 8, 10b and 11b/12b and no significant change of concentrations was observed for these compounds during the course of the reaction. From these results, we assume that a formation of 5b via diketone 8 is possible.
Besides, the diketones
8 the ammonium salts
3a-d might be key intermediates during the formation of bicyclo-octanones. 4-Phenyl-3-buten-2-one-
N-phenylimine was prepared by Brady
et al. [
6] by refluxing benzylidene acetone with aniline in benzene catalyzed by zinc chloride. The formation of cyclic products was not reported. However, when we replaced aniline by morpholine, we detected moderate amounts of
5b and small amounts of the diketones
7 and
8 instead of the expected imine.
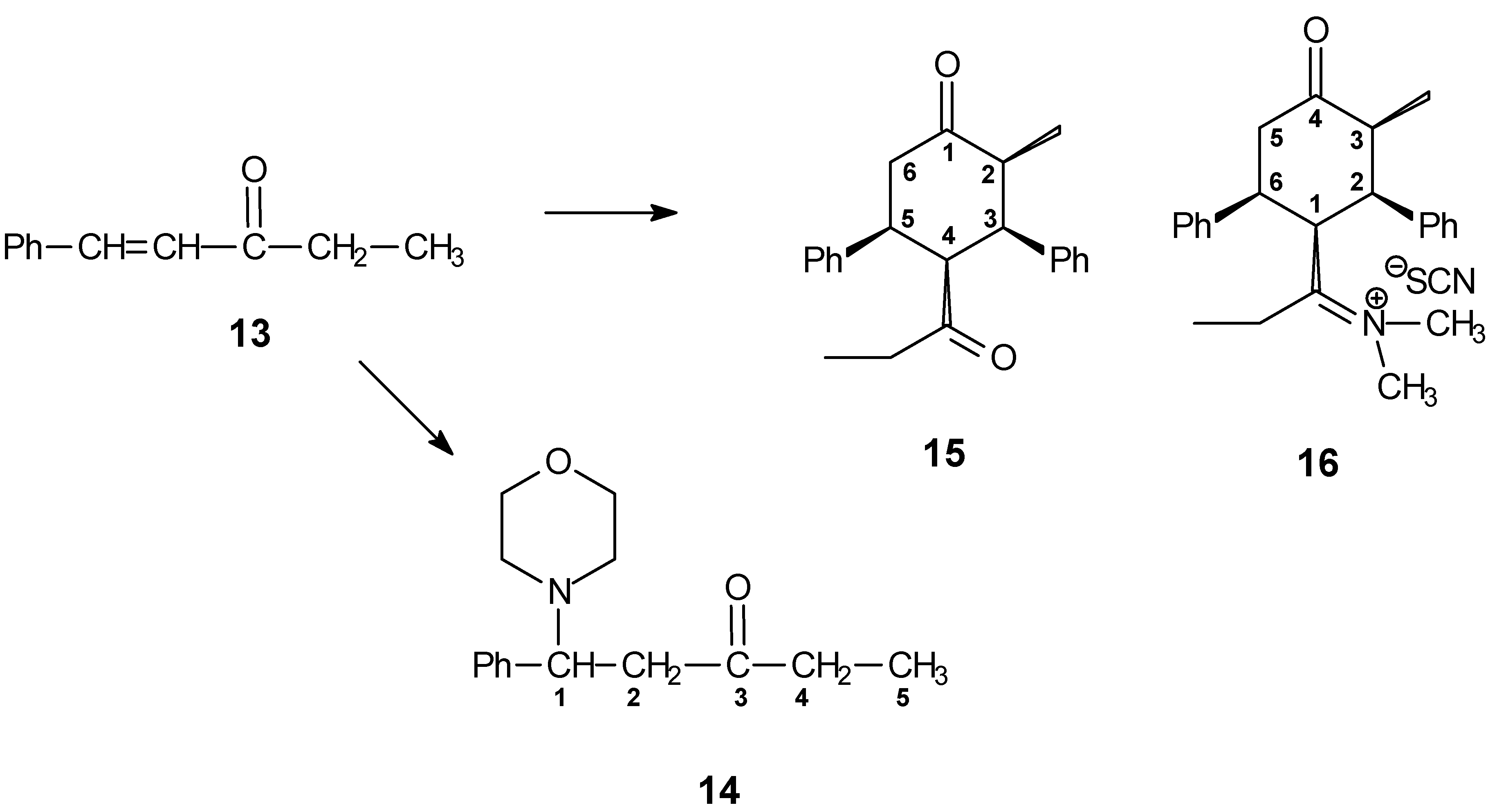
When ethyl styryl ketone
(13) is used instead of benzylidene acetone
(1) the reaction with morpholine under the same conditions gives compound
14, which is not stable, especially in an alkaline medium. The reaction of
13 with dimethylammonium thiocyanate in refluxing toluene yielded compounds
15 and
16, which were isolated by sequential crystallization from ethanol (
Scheme 5). The structure of
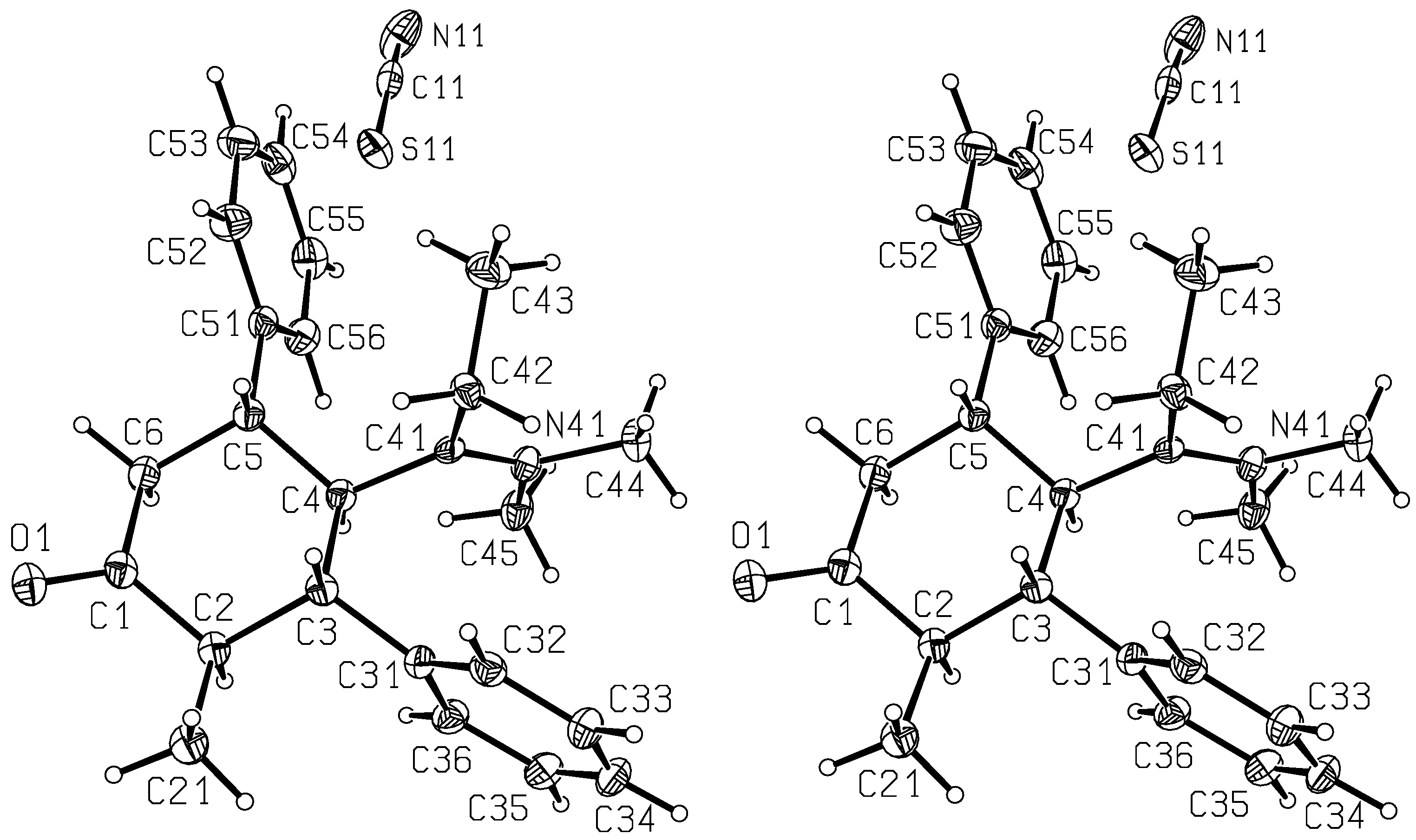
16 was elucidated with the aid of a single crystal structure analysis (
Figure 2 and
Figure 3).
Figure 2.
Stereoscopic ORTEP [
7] plot of
16 showing the atomic numbering scheme. The probability ellipsoids are drawn at the 50% probability level.
Figure 2.
Stereoscopic ORTEP [
7] plot of
16 showing the atomic numbering scheme. The probability ellipsoids are drawn at the 50% probability level.
Figure 3.
Stereoscopic ORTEP [
7] plot of the packing of
16. The atoms are drawn with arbitrary radii.
Figure 3.
Stereoscopic ORTEP [
7] plot of the packing of
16. The atoms are drawn with arbitrary radii.
Besides,
10b-d were reduced regioselectively with Pd on charcoal to a mixture of 4-amino-cyclohexanones
17b-d and
18b-d. Only compounds
17b-d were isolated in pure form by crystallization from ethanol (
Scheme 3). The structure of
17b-d was determined with the aid of NMR spectroscopy. Small coupling constants (3Hz) of H-5
ax and H-3
ax to H-4 in the
1H spectra of
17b indicate the equatorial position of H-4. Furthermore a NOE of 8.6% was observed from H-1 to H-3
ax and H-5
ax indicating the equatorial position of the acetyl group (
Figure 4).
Figure 4.
NOEs observed in compound 17b
Figure 4.
NOEs observed in compound 17b
Experimental
General
Melting points were obtained on an Electrothermal IA 9200 digital melting point apparatus and are uncorrected. IR spectra: infrared spectrometer system 2000 FT (Perkin Elmer). UV/VIS: Lambda 17 UV/VIS-spectrometer (Perkin Elmer). NMR spectra: Varian Inova 400 (300 K), 5 mm tubes, TMS resonance as internal standard. 1H- and 13C-resonances were assigned using 1H,1H- and 1H,13C-correlation spectra. HMBC spectra were optimized for 8 Hz. For NOE measurements oxygen was carefully removed by bubbling Ar through the solutions. 1H- and 13C-resonances are numbered as given in the formulas. MS: 70 eV electron impact: Varian MAT 711 spectrometer, Kratos profile spectrometer. GC-MS: HP-6890 (Hewlett-Packard) 70 eV electron impact. Microanalyses: EA 1108 CHNS-O apparatus (Carlo Erba), Microanalytical Laboratory at the Institute of Physical Chemistry, Vienna. Materials: Column-chromatography (CC): silica gel 60 (Merck, 70 - 230 mesh), pore-diameter 60 Å, thin-layer chromatography (TLC): TLC plates (Merck, silica gel 60 F254, 0.2 mm, 200 x 200 mm); the substances were detected in UV light at 254 nm.
(6RS,7RS)-(±)-4-Morpholino-6,7-diphenylbicyclo[2.2.2]octan-2-one (5b)
Benzylidene acetone (
1, 46 g, 0.31 mol) and morpholine (27.4 g, 0.31 mol) were dissolved in benzene (125 mL) and zinc chloride (200 mg) was added. The mixture was refluxed at a water separator at 140°C over night, cooled to room temperature and filtered. The solvent was evaporated
in vacuo giving a residue which was further purified by use of CC (eluent: 8:8:1 benzene/ chloroform/ethanol) affording
5b (15.4 g, 13.5%) as a yellowish resin. Spectral data corresponded well with those reported [
2].
Synthesis of (3RS, 5RS)-(±)-4-acetyl-3,5-diphenylcyclohexanone (8) and (3RS, 5SR)-(±)-4-acetyl-3,5-diphenylcyclohexanone (7).
Compound
8 was synthesized from 2-trimethylsilyloxy-4-phenyl-1,3-butadiene (
9) [
4] and benzylidene acetone (
1) following a reported procedure [
8]. Compound
7 was prepared via an amine catalyzed Diels-Alder reaction using pyrrolidine as catalyst [
3].
Synthesis of (2RS, 6SR)-(±)-1-(4-amino-2,6-diphenylcyclohex-3-en-1-yl) ethanones 10b-d
Compounds 7 (1 g) were dissolved in dry benzene (14 mL). A threefold molar amount of the secondary amine, activated 4Å molecular sieves (2 g) and 4-toluenesulfonic acid (40 mg) were added. The reaction mixture was refluxed over night at 100°C. After cooling to room temperature, benzene (30 mL) was added and the solution was extracted four times with water. After drying over Na2SO4 and filtration, the solvent was evaporated in vacuo and the residue recrystallized from ether.
(2RS, 6SR)-(±)-1-(4-Morpholino-2,6-diphenylcyclohex-3-en-1-yl) ethanone (10b)
Compound 7 (1 g, 3.4 mmol) and morpholine (894 mg, 10.3 mmol) gave 10b (804 mg, 65%) as white needles. Mp: 114°C (ether); IR (KBr) cm-1: 2957, 2918, 2891, 2853, 2823, 1704, 1651, 1492, 1452, 1378, 1356, 1262, 1205, 1190, 1162, 1119, 1039, 891, 767, 753, 703; UV (CH2Cl2): l (log e) = 236 (3.965) nm; 1H-NMR (CDCl3, 400 MHz)δ: 1.31 (s, 3H, CH3), 2.41 - 2.54 (m, 2H, 5-H), 2.79 - 2.93 (m, 4H, (NCH2)2), 3.04 (t, J = 11.0 Hz, 1H, 1-H), 3.24 (ddd, J = 11.4, 11.2, 5.9 Hz, 1H, 6-H), 3.70 - 3.74 (m, 4H, O(CH2)2), 3.82 (b, d, J = 10.0 Hz, 1H, 2-H), 4.69 (s, 1H, 3-H), 7.17 – 7.31 (m, 10H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 33.13 (CH3), 35.17 (C-5), 44.48 (C-6), 46.92 (C-2), 48.39 (N(CH2)2), 61.64 (C-1), 66.80 (O(CH2)2), 103.92 (C-3), 126.76, 126.92, 127.53, 127.76, 128.61, 128.69 (aromatic C), 142.92, 144.71, 144.84 (C-4, aromatic Cq), 212.32 (C=O) ppm; MS (EI+): m/z (%) = 361 (18.6) [M+], 318 (100.0), 215 (22.5), 185 (10.0), 157 (7.8), 129 (9.3), 91 (9.7), 43 (7.0); Anal. Calcd for C24H27NO2 (361.48): C 79.74, H 7.53, N 3.87; found: C 79.50, H 7.58, N 3.69; HRMS (EI+) for C24H27NO2 (M+): Calcd 361.20418; Found 361.20589.
(2RS, 6SR)-(±)-1-(2,6-Diphenyl-4-pyrrolidinocyclohex-3-en-1-yl) ethanone (10c)
Compound 7 (1 g, 3.4 mmol) and pyrrolidine (730 mg, 10.3 mmol) gave 10c (803 mg, 68%) as white needles. Mp: 145°C (ether); IR (KBr) cm-1: 2905, 2821, 1705, 1631, 1494, 1455, 1392, 1368, 1351, 1315, 1266, 1164, 758, 699; UV (CH2Cl2): l (log e) = 235 (3.863) nm; 1H-NMR (CDCl3, 400 MHz)δ: 1.30 (s, 3H, CH3), 1.82 - 1.89 (m, 4H, 2CH2), 2.51 - 2.66 (m, 2H, 5-H), 3.02 (t, J = 11.2 Hz, 1H, 1-H), 3.03 - 3.08 (m, 4H, (NCH2)2), 3.28 (ddd, J = 11.5, 11.5, 5.7 Hz, 1H, 6-H), 3.84 (b, d, J = 10.0 Hz, 1H, 2-H), 4.25 (s, 1H, 3-H), 7.17 – 7.30 (m, 10H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 24.67 ((CH2)2), 33.01 (CH3), 35.94 (C-5), 44.42 (C-6), 47.26 (C-2), 47.48 (N(CH2)2), 61.84 (C-1), 96.94 (C-3), 126.50, 126.79, 127.63, 127.82, 128.43, 128.64 (aromatic C), 142.29, 143.31, 145.83 (C-4, aromatic Cq), 212.61 (C=O) ppm; MS (EI+): m/z (%) = 345 (14.3) [M+], 302 (100.0), 199 (34.9), 184 (8.5), 129 (7.8), 91 (7.0), 43 (6.2); Anal. Calcd for C24H27NO (345.48): C 83.44, H 7.88, N 4.05; found: C 83.27, H 8.09, N 3.99; HRMS (EI+) for C24H27NO (M+): Calcd 345.20926; Found 345.21098.
(2RS, 6SR)-(±)-1-(4-Piperidino-2,6-diphenylcyclohex-3-en-1-yl) ethanone (10d)
Compound 7 (1 g, 3.4 mmol) and piperidine (874 mg, 10.3 mmol) gave 10d (775 mg, 63%) as white needles. Mp: 156°C (ether); IR (KBr) cm-1: 2932, 2853, 2794, 1703, 1631, 1493, 1454, 1390, 1353, 1230, 1215, 1201, 1165, 1125, 758, 703; UV (CH2Cl2): l (log e) = 237 (3.954) nm; 1H-NMR (CDCl3, 400 MHz)δ: 1.32 (s, 3H, CH3), 1.48 - 1.53 (m, 2H, CH2), 1.56 - 1.59 (m, 4H, (CH2)2), 2.41 - 2.57 (m, 2H, 5-H), 2.77 - 2.91 (m, 4H, (NCH2)2), 3.02 (t, J = 11.0 Hz, 1H, 1-H), 3.24 (ddd, J = 11.6, 11.4, 5.3 Hz, 1H, 6-H), 3.83 (b, d, J = 10.2 Hz, 1H, 2-H), 4.67 (s, 1H, 3-H), 7.18 – 7.30 (m, 10H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 24.48 (CH2), 25.74 ((CH2)2), 33.01 (CH3), 35.95 (C-5), 44.69 (C-6), 47.15 (C-2), 49.02 (N(CH2)2), 61.86 (C-1), 103.57 (C-3), 126.62, 126.79, 127.54, 127.80, 128.53, 128.63 (aromatic C), 143.22, 145.16, 145.49 (C-4, aromatic Cq), 212.48 (C=O) ppm; MS (EI+): m/z (%) = 359 (17.8) [M+], 316 (100.0), 268 (9.7), 213 (25.6), 198 (13.2), 136 (12.8), 115 (7.8), 91 (7.8), 43 (6.2); Anal. Calcd for C25H29NO (359.51): C 83.52, H 8.13, N 3.90; found: C 83.25, H 8.25, N 3.79; HRMS (EI+) for C25H29NO (M+): Calcd 359.22491; Found 359.22582.
Reaction of ethyl styryl ketone (13) with dimethylammonium thiocyanate
Ethyl styryl ketone (13, 30 g, 0.187 mol) and dimethylammonium thiocyanate (7.2g, 0.095 mol) were suspended in dimethylformamide (120 mL) and refluxed at 220°C for 6h at a water separator. After cooling to ambient temperature the solvent was evaporated under reduced pressure and the residue was dissolved in a small amount of hot ethanol. Compound 15 crystallized first and was filtered off by suction. The iminium salt 16 crystallized from the filtrate.
(2RS, 3RS, 4RS, 5SR)-(±)-2-Methyl-3,5-diphenyl-4-propionylcyclohexanone (15)
Yield: 980 mg (3.2%) Mp: 197°C (ethanol); the spectral data exactly matched those reported [
9].
(1RS, 2SR, 3RS, 6SR)-(±)-N,N-Dimethyl-1-(3-methyl-4-oxo-2,6-diphenylcyclohexyl)-propan-1-iminium thiocyanate (16)
Yield: 2.178 g (5.6%) Mp: 212°C (ethanol); yellow prisms. IR (KBr) cm-1: 3060, 2987, 2972, 2956, 2933, 2051, 1709, 1649, 1494, 1455, 1428, 1352, 1336, 1226, 1074, 759, 708; UV (CH3OH): l (log e) = 214 (4.117) nm; 1H NMR (DMSO-d6, 400 MHz) δ: 0.69 (d, J = 6.5 Hz, 3H, CH3), 0.75 (t, J = 7.4 Hz, 3H, CH2CH3), 2.47 (dd, J = 13.3, 3.9 Hz, 1H, 5-H), 2.77 (dq, J = 14.6, 7.3 Hz, 1H, CH2CH3), 2.94 (dq, J = 14.6, 7.3 Hz, 1H, CH2CH3), 3.00 (s, 3H, NCH3), 3.10 - 3.17 (m, 2H, 3-H, 5-H), 3.26 (dd, J = 11.5, 11.1 Hz, 1H, 2-H), 3.31 (s, 3H, NCH3), 3.68 (ddd, J = 13.1, 10.8, 4.0 Hz, 1H, 6-H), 4.88 (dd, J = 10.9, 10.7 Hz, 1H, 1-H), 7.27 – 7.46 (m, 10H, aromatic H) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 9.59 (CH2CH3), 11.88 (CH3), 24.10 (CH2CH3), 45.15 (NCH3), 45.26 (C-6), 45.39 (NCH3), 48.01 (C-3), 48.25 (C-5), 51.91 (C-2), 53.77 (C-1), 127.25, 128.03, 128.13, 129.08, 129.18 (aromatic C), 139.56, 140.87 (aromatic Cq), 195.26 (C=N), 207.57 (C-4) ppm; Anal. Calcd for C25H30N2OS (406.58): C 73.85, H 7.44, N 6.89, S 7.89; found: C 73.63, H 7.70, N 6.95, S 7.60.
X-ray diffraction data of 16
All the measurements were performed using graphite-monochromatized Mo K
α radiation at 95(2)K: C
24H
30NO
+ SCN
-,
Mr 406.57, orthorhombic, space group P b c a, a = 9.777(2)Å, b = 15.746(3)Å, c = 28.318(5)Å, V = 4359.5(14)Å
3, Z = 8, d
calc = 1.239g cm
-3, μ = 0.167mm
-1. A total of 4771 reflections were collected (Θ
max = 26.0°), from which 4272 were unique (R
int = 0.0360), with 2847 having I > 2σ(I). The structure was solved by direct methods (SHELXS-97) [
10] and refined by full-matrix least-squares techniques against
F2 (SHELXL-97) [
11]. The non-hydrogen atoms were refined with anisotropic displacement parameters without any constraints. The H atoms were refined with common isotropic displacement parameters for the H atoms bonded to the same acyclic C atom or to the same ring. The H atoms of the tertiary C-H groups were refined with all X-C-H angles equal at a C-H distance of 1.00Å. The H atoms of the CH
2 groups were refined with idealized geometry with approximately tetrahedral angles and C-H distances of 0.99Å. The H atoms of the methyl groups were refined with idealized geometry with tetrahedral angles, enabling rotation around the X-C bond, and C-H distances of 0.98 Å. The H atoms of the phenyl rings were put at the external bisector of the C-C-C angle at a C-H distance of 0.95Å. For 274 parameters final
R indices of R = 0.0652 and wR
2 = 0.1325 (GOF = 1.050) were obtained. The largest peak in a difference Fourier map was 0.234eÅ
-3. The final atomic parameters, as well as bond lengths and angles are deposited at the Cambridge Crystallographic Data Centre (CCDC 231557). These data can be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336033; e-mail:
deposit@ccdc.cam.ac.uk or www:
http://www.ccdc.cam.ac.uk).
RS-(±)-1-Morpholino-1-phenylpentan-3-one (14)
Ethyl styryl ketone (13, 53.19 g, 0.332 mol) and morpholine (14.46 g, 0.166 mol) were dissolved in benzene (25 mL) and zinc chloride (200 mg) was added. The mixture was refluxed at a water separator at 140°C over night, cooled to room temperature and the zinc chloride was filtered off. The solvent was evaporated in vacuo giving a resinous residue which was purified by CC with ether as eluent. The fractions containing larger amounts of product were dissolved in dichloromethane and 2M ethereal HCl was added and the solvent evaporated. After that, analytical amounts of a pink solid were crystallized from ethyl acetate. A further recrystallization from ethanol gave the hydrochloride of 14 (980 mg, 2.4%) as white powder which was used for biological testing and as an analytical sample. The easily decomposed base was freed with neutral washed Amberlite IRA-420 ion exchanger (Fluka) in ethanol. Mp: 156°C (HCl, ethanol); IR (HCl, KBr) cm-1: 2943, 2901, 2866, 2672, 2610, 2574, 2557, 2473, 1720, 1712, 1456, 1379, 1132, 1081, 767, 706; UV (HCl, CH3OH): l (log e) = 208 (3.891) nm; 1H-NMR (CDCl3, 400 MHz) δ: 0.95 (t, J = 7.2 Hz, 3H, 5-H), 2.26 (dq, J = 14.6, 7.2 Hz, 1H, 4-H), 2.33 - 2.45 (m, 5H, 4-H, N(CH2)2), 2.76 (dd, J = 15.2, 7.4 Hz, 1H, 2-H), 3.02 (dd, J = 15.2, 6.9 Hz, 1H, 2-H), 3.62 - 3.65 (m, 4H, O(CH2)2), 3.94 (dd, J = 7.1, 7.4 Hz, 1H, 1-H), 7.23 – 7.33 (m, 5H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 7.56 (C-5), 36.72 (C-4), 45.88 (C-2), 50.64 (N(CH2)2), 65.73 (C-1), 67.18 (O(CH2)2), 127.51, 128.27, 128.32 (aromatic C), 139.18 (aromatic Cq), 209.58 (C-3) ppm; GC-MS (70 eV): m/z (%) = 247 (2.0) [M+], 218 (1.0), 176 (100.0), 131 (9.8), 103 (17.6), 77 (8.8), 57 (18.6); Anal. Calcd for C15H22NO2Cl (283.80): C 63.48, H 7.81, N 4.94, Cl 12.49; found: C 63.18, H 7.98, N 4.86, Cl 12.77; HRMS (EI+) for C15H21NO2 (M+): Calcd 247.15723; Found 247.15609.
Synthesis of (2RS, 6SR)-(±)-1-(4-amino-2,6-diphenylcyclohexan-1-yl) ethanones 17b-d
The enamines 10b-d were dissolved in ethanol and Pd/C (10%) was added. The reaction mixtures were shaken over night in a Paar hydrogenator low pressure vessel under a H2 atmosphere (50 psi) at room temperature. The catalyst was filtered off and the solvent was evaporated in vacuo. The residue was dissolved in hot ethanol and compounds 11b-d crystallized upon cooling.
(2RS, 6SR)-(±)-1-(4-Morpholino-2,6-diphenylcyclohexan-1-yl) ethanone (17b)
Compound 10b (150 mg, 0.41 mmol) and Pd/C (10%, 100 mg) in ethanol (50 mL) gave 17b as white needles (72 mg, 48%). Mp: 211°C (ethanol); IR (KBr) cm-1: 2965, 2951, 2854, 2806, 1703, 1493, 1453, 1355, 1271, 1168, 1122, 754, 702, 693; UV (CH2Cl2): l (log e) = 230 (2.973), 259 (2.675) nm; 1H-NMR (CDCl3, 400 MHz) δ: 1.24 (s, 3H, CH3), 1.81 (ddd, J = 12.9, 11.5, 2.4 Hz, 2H, 3Hax, 5Hax), 2.20 (b, d, J = 13.3 Hz, 2H, 3-Heq, 5-Heq), 2.42 - 2.52 (m, 5H, 4-H, (NCH2)2), 3.02 (t, J = 11.0 Hz, 1H, 1-H), 3.35 (ddd, J = 13.0, 11.0, 2.2 Hz, 2H, 2-H, 6-H), 3.80 - 3.84 (m, 4H, O(CH2)2), 7.16 – 7.30 (m, 10H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 32.71 (CH3), 35.25 (C-3, C-5), 40.96 (C-2, C-6), 50.75 (N(CH2)2), 59.12 (C-4), 64.00 (C-1), 67.17 (O(CH2)2), 126.68, 127.39, 128.60 (aromatic C), 143.59 (aromatic Cq), 211.83 (C=O) ppm; MS (EI+): m/z (%) = 363 (24.0) [M+], 320 (69.8), 202 (100.0), 129 (10.1), 113 (25.6), 103 (6.6), 91 (24.8), 55 (7.0), 43 (14.0); Anal. Calcd for C24H29NO2 (363.49): C 79.30, H 8.04, N 3.85; found: C 79.02, H 7.86, N 3.83; HRMS (EI+) for C24H29NO2 (M+): Calcd 363.21983; Found 363.22092.
(2RS, 6SR)-(±)-1-(2,6-Diphenyl-4-pyrrolidinocyclohexan-1-yl) ethanone (17c)
Compound 10c (776 mg, 2.2 mmol) and Pd/C (10%, 100 mg) in ethanol (50 mL) gave 17c (593 mg, 76%) as white needles. Mp: 192°C (ethanol); IR (KBr) cm-1: 2939, 2902, 2786, 1702, 1493, 1455, 1352, 1343, 1169, 1078, 755, 698; UV (CH2Cl2): l (log e) = 231 (3.320) nm; 1H-NMR (CDCl3, 400 MHz) δ: 1.28 (s, 3H, CH3), 1.79 - 1.86 (m, 6H, 3-Hax, 5-Hax, 2CH2), 2.12 (d, b, J = 13.0 Hz, 2H, 3-Heq, 5-Heq), 2.47 (t, J = 2.7 Hz, 1H, 4-H), 2.52 - 2.58 (m, 4H, (NCH2)2), 3.02 (t, J = 11.1 Hz, 1H, 1-H), 3.44 (ddd, J = 13.0, 11.1, 3.1 Hz, 2H, 2-H, 6-H), 7.15 – 7.29 (m, 10H, aromatic H) ppm; 13C‑ NMR (CDCl3, 100 MHz) δ: 23.83 ((CH2)2), 32.46 (CH3), 38.17 (C-3, C-5), 41.25 (C-2, C-6), 51.90 (N(CH2)2), 60.12 (C-4), 63.85 (C-1), 126.54, 127.51, 128.56 (aromatic C), 144.07 (C-4, aromatic Cq), 212.17 (C=O) ppm; MS (EI+): m/z (%) = 347 (18.6) [M+], 304 (79.8), 186 (100.0), 115 (10.0), 97 (36.4), 91 (22.4), 69 (12.4), 43 (11.6); Anal. Calcd for C24H29NO (347.50): C 82.95, H 8.41, N 4.03; found: C 82.70, H 8.56, N 3.93; HRMS (EI+) for C24H29NO (M+): Calcd 347.22491; Found 347.22395.
(2RS, 6SR)-(±)-1-(2,6-Diphenyl-4-piperidinocyclohexan-1-yl) ethanone (17d)
Compound 10d (502 mg, 1.4 mmol) and Pd/C (10%, 340 mg) in ethanol (160 mL) gave 17d (237 mg, 47%) as white needles. Mp: 208°C (ethanol); IR (KBr) cm-1: 2970, 2933, 2750, 1703, 1493, 1452, 1353, 1164, 1077, 752, 702; UV (CH2Cl2): l (log e) = 233 (3.193), 302 (1.838) nm; 1H-NMR (CDCl3, 400 MHz) δ: 1.27 (s, 3H, CH3), 1.42 - 1.46 (m, 2H, CH2), 1.63 - 1.69 (m, 4H, CH2)2), 1.76 (ddd, J = 13.9, 12.9, 2.6 Hz, 2H, 3-Hax, 5-Hax), 2.23 (b, d, J = 12.7 Hz, 2H, 3-Heq, 5-Heq), 2.40 - 2.48 (m, 5H, 4-H, (NCH2)2), 3.01 (t, J = 11.2 Hz, 1H, 1-H), 3.38 (ddd, J = 13.1, 10.8, 2.3 Hz, 2H, 2-H, 6-H), 7.15 – 7.29 (m, 10H, aromatic H) ppm; 13C-NMR (CDCl3, 100 MHz) δ: 24.64 (CH2), 26.28 ((CH2)2), 32.36 (CH3), 36.24 (C-3, C-5), 41.09 (C-2, C-6), 51.30 (N(CH2)2), 59.00 (C-4), 64.27 (C-1), 126.57, 127.48, 128.57 (aromatic C), 144.03 (aromatic Cq), 211.93 (C=O) ppm; MS (EI+): m/z (%) = 361 (17.1) [M+], 318 (55.0), 200 (100.0), 129 (6.0), 111 (25.2), 91 (14.7), 43 (7.8); Anal. Calcd for C25H31NO (361.52): C 83.06, H 8.64, N 3.87; found: C 82.82, H 8.71, N 3.81; HRMS (EI+) for C25H31NO (M+): Calcd 361.24056; Found 361.24066.