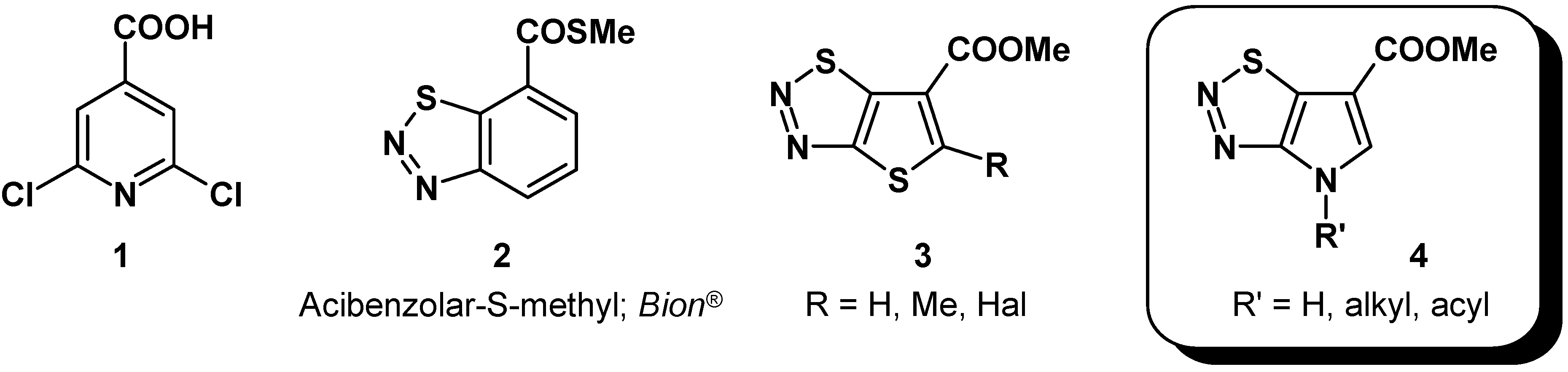
Results and Discussion
Synthesis of alkyl substituted pyrrolo-thiadiazoles
4a (R = Bn) and
4b (R = Me) started from itaconic acid or its dimethylester (
Scheme 2). Cyclization towards the pyrrolidine system
5a/b was optimized based on a protocol by Feldkamp and coworker [
11].
From our previous experiences in the thieno-series we had already expected that efficient conversion to the hydrazone precursor for the Hurd-Mori reaction required formation of a thiocarbonyl species. Consequently, lactams
5a/b were transformed into the corresponding thiolactams
6a/b using Lawesson’s reagent. [
12] As work-up conditions for reactions involving this reagent are troublesome, sometimes, we found
Kugelrohr distillation as a most convenient form to purify the products.
Thiolactams 6a/b gave clean conversion to the corresponding hydrazono cyclization precursors 7a/b by treatment with ethyl carbazate upon refluxing in THF in the presence of Hg(OAc)2. In the absence of this reagent, the reaction progress was significantly decreased. In addition, equilibrium concentrations are shifted by precipitation of HgS.
Scheme 2.
i) R’ = H: BnNH2, 130°C; then MeOH, H2SO4, 88% (5a); ii) R’ = Me: MeNH2/H2O, MeOH, 95% (5b); iii) Lawesson’s reagent, THF, 98% (6a), 87% (6b); iv) EtOOCNHNH2, Hg(OAc)2, THF, 75% (7a), 81% (7b); v) SOCl2, CHCl3, 25% (4a), 15% (4b).
The actual Hurd-Mori cyclization towards the alkyl-thiadiazoles gave fully aromatized products 4a/b with both precursors. However, it turned out to be disappointing with yields of 25% (4a) and 15% (4b), respectively, even under optimized reaction conditions. The pyrrolidino-precursors required significantly harsher conditions (chloroform at reflux) to display reasonable conversion rates. However, at such temperatures the stability of the starting material and/or of intermediate products becomes a limiting factor. In all optimization experiments significant decomposition was observed by darkening of the reaction mixtures and/or precipitation of insoluble material.
Although we could demonstrate access to N-alkyl-pyrrolo-thiadiazole-carboxylates 4a and 4b in principal, the chosen strategy did not seem suitable for scale-up in order to provide larger quantities for derivatization studies. We attribute the limited stability to the presence of a relatively electron rich nitrogen species in all cyclization precursors and intermediates. Consequently, another option to overcome the poor cyclization yields could be by changing the protecting group at the pyrrolidine system to an electron withdrawing substituent.
In order to confirm this hypothesis, we started a second series from 5-oxopyrrolidin-3-carboxylate methylester (
Scheme 3). [
13,
14] Conversion to the thiolactam
8 was again accomplished in excellent yields using Lawesson’s reagent. Introduction of the carbamate protecting group gave compound
6c in only moderate yields, as complete conversion could not be achieved. Based on consumed starting material almost quantitative transformation can be accomplished. Reaction with ethyl carbazate did not require presence of a mercury additive and produced compound
7c in excellent yield.
Scheme 3.
i) Lawesson’s reagent, THF, 99%; ii) MeOOCCl, TEA, DMAP, CH2Cl2, 30%; iii) EtOOCNHNH2, CH2Cl2, 98%; iv) SOCl2, CH2Cl2, 94%; v) SiO2, MeOH, 99%.
In contrast to the alkyl precursors, Hurd-Mori reaction with 7c required cooling and progressed smoothly to fully aromatized thiadiazole 4c. No significant side products were observed and compound 4c was isolated after simple recrystallization in 94% yield. Final deprotection of the pyrrolo nitrogen was accomplished by treatment of a methanolic solution of 4c with silica gel to afford 4d quantitatively.
Experimental
General
Unless otherwise noted, chemicals were purchased from commercial suppliers and used without further purification. All solvents were distilled prior to use. Flash column chromatography was performed on silica gel 60 from Merck (40-63μm). Melting points were determined using a Kofler-type Leica Galen III micro hot stage microscope and are uncorrected. NMR-spectra were recorded from CDCl3 or DMSO-d6 solutions on a Bruker AC 200 (200 MHz) spectrometer and chemical shifts are reported in ppm using TMS as internal standard. Combustion analysis was carried out in the Microanalytic Laboratory, University of Vienna.
1-Benzyl-5-oxopyrrolidine-3-carboxylic acid methyl ester (5a).
Itaconic acid (36.11 g, 0.28 mol) and benzylamine (29.74 g, 0.28 mol) were heated in substance to 130°C and the water formed was distilled off during 3 hours. Crystals of crude 1-benzyl-5-oxopyrrolidine-3-carboxylic acid (55.13 g, 0.25 mol, 90 %) were collected by filtration after treatment of the reaction mixture with ice/water and dried
in vacuo. This material was dissolved in methanol (200 mL) and treated with conc. sulfuric acid (28.66 g, 0.27 mol) at reflux for 3 hours. The reaction solution was concentrated to approx. half volume, subsequently neutralized with 10N NaOH, 2N NaOH, and satd. NaHCO
3-solution, and repeatedly extracted with dichloromethane. The combined organic layers were washed with water, dried over sodium sulfate, and concentrated to give 56.92g (88% overall) of
5a [
11] as colorless crystals; m.p. 71-73°C;
1H-NMR (CDCl
3): 2.90-3.10 (m, 2H, H-4), 3.18-3.37 (m, 1H, H-3), 3.58 (d, J=8Hz, 2H, H-2), 3.69 (s, 3H, OCH
3), 4.45-4.50 (m, 2H, PhCH
2), 7.14-7.38 (m, 5H, arom. H);
13C-NMR (DMSO-
d6): 33.4 (t, C-4), 35.1 (d, C-3), 45.3 (t, PhCH
2), 48.1 (t, C-2), 52.0 (q, OCH
3), 127.3 (d, C-4`), 127.6 (d, C-3`+ C-5`), 128.5 (d, C-2`+ C-6`), 136.6 (s, C-1`), 171.7 (s,
COOCH
3), 173.4 (s, C-5).
1-Methyl-5-oxopyrrolidine-3-carboxylic acid methyl ester (5b).
Dimethyl itaconate (30g, 0.19mol) was added to aqueous methylamine solution (17.2 mL, 15.53 g, 40% solution, 0.2 mol) at 5-10°C and maintained at this temperature for another hour. Afterwards, the mixture was warmed to room temperature and stirred for 16 hours, concentrated and distilled
in vacuo to give 29.0 g (95%) of
5b [
11] as a colorless liquid; b.p. 160-161°C/24mbar;
1H-NMR (CDCl
3): 2.60-2.75 (m, 2H, H-4), 2.86 (s, 3H, NCH
3), 3.16-3.38 (m, 1H, H-3), 3.40 (d, J=8Hz, 2H, H-2), 3.66 (s, 3H, OCH
3);
13C-NMR (CDCl
3): 28.0 (q, NCH
3), 32.5 (t, C-4), 34.4 (d, C-3), 49.8 (t, C-2), 51.0 (q, OCH
3), 171.0 (s,
COOCH
3), 172.2 (s, C-5).
1-Benzyl-5-thioxopyrrolidine-3-carboxylic acid methyl ester (6a).
Lactam 5a (56.9 g, 0.244 mol) and Lawesson’s reagent (49.35 g, 0.15 mol) were stirred at room temperature for 30 min in dry THF (400 mL). The solvent was removed in vacuo and the crude material was Kugelrohr distilled to give 59.6 g (98%) of 6a as beige oil; b.p. 155-165°C/0.001-0.005mbar; nD20=1.5880; 1H-NMR (CDCl3): 3.11-3.40 (m, 3H, H-3, H-4), 3.69 (s, 3H, OCH3), 3.61-3.91 (m, 2H, H-2), 4.90-5.00 (m, 2H, PhCH2), 7.20-7.40 (m, 5H, arom. H); 13C-NMR (CDCl3): 37.0 (d, C-3), 47.1 (t, C-4), 51.0 (t, PhCH2), 52.1 (q, OCH3), 55.3 (t, C-2), 127.8 (d, C-4`), 127.9 (d, C-3`+ C-5`), 128.5 (d, C-2`+C-6`), 134.2 (s, C-1`), 172.1 (s, COOCH3), 198.7 (s, C-5); Anal. for C13H15NO2S (249.33): calc.: C 62.63%, H 6.06%, N 5.62%; found.: C 62.75%, H 6.30%, N 5.57%.
1-Methyl-5-thioxopyrrolidine-3-carboxylic acid methyl ester (6b).
Lactam 5b (29.0 g, 0.18 mol) and Lawesson’s reagent (60.0 g, 0.18 mol) were stirred at room temperature for 30 min in dry THF (400 mL). The solvent was removed in vacuo and the crude material was Kugelrohr distilled to give 26.0 g (87%) of 6b as beige oil; b.p. 100°C/0.01-0.02mbar; 1H-NMR (CDCl3): 3.28 (s, 3H, NCH3), 3.20-3.50 (m, 3H, H-3, H-4), 3.76 (s, 3H, OCH3), 3.86-4.12 (m, 2H, H-2); 13C-NMR (CDCl3): 33.9 (d, C-3), 35.9 (q, NCH3), 46.0 (t, C-4), 51.2 (q, OCH3), 57.3 (t, C-2), 171.4 (s, COOCH3), 196.8 (s, C-5); Anal. for C7H11NO2S (173.23): calc.: C 48.53%, H 6.40%, N 8.09%; found.: C 48.56%, H 6.32%, N 8.06%.
5-Thioxopyrrolidine-3-carboxylic acid methyl ester (8).
Methyl 5-oxopyrrolidine-3-carboxylate (5.0 g, 34.9 mmol) and Lawesson’s reagent (14.1 g, 34.9 mmol) were stirred at room temperature for 2 hours in dry THF (50 mL). The solvent was removed in vacuo and the residue was purified by flash column chromatography (triethylamine impregnated silica gel, LP/EtOAc = 3:2), followed by recrystallization from toluene to give 5.51g (99%) of 8 as colorless crystals; m.p. 106-109°C; 1H-NMR (CDCl3): 3.10-3.30 (m, 2H, H-4), 3.40-3.55 (m, 1H, H-3), 3.75 (s, 3H, OMe), 3.83 (dd, J=11.3Hz, 5.5Hz, 1H, H-2), 3.96 (dd, J=11.3Hz, 6.5Hz, 1H, H-2), 8.85 (bs, 1H, NH); 13C-NMR (CDCl3): 40.7 (t, C-4), 45.8 (d, C-3), 51.5 (q, OCH3), 52.6 (t, C-2), 172.4 (s, COOCH3), 203.0 (s, C-5); Anal. for C6H9NO2S (159.21): calc.: C 45.27%, H 5.70%, N 8.80%; found.: C 45.33%, H 5.71%, N 8.67%.
5-Thioxopyrrolidine-1,3-dicarboxylic acid dimethyl ester (6c).
Thiolactam 8 (4.50 g, 28.3 mmol), triethylamine (2.86 g, 28.3 mmol), and DMAP (3.45 g, 28.3 mmol) were dissolved in dry dichloromethane (100 mL) and treated with methyl chloroformate (5.34 g, 56.5 mmol) in dry dichloromethane (20 mL) at -60 to -70°C. After complete addition the reaction mixture was warmed to room temperature, stirred for 12 hours, and subsequently concentrated in vacuo. Flash column chromatography (silica gel, LP/EtOAc = 1:1) gave 3.13 g of recovered starting material 8 and 1.80 g (30 %, 96% based on consumed 8) of 6c as beige crystals; m.p. 89-91°C; 1H-NMR (CDCl3): 3.20-3.41 (m, 3H, H-3, H-4), 3.76 (s, 3H, C-3-COOCH3), 3.89 (s, 3H, N1-COOCH3), 4.24-4.44 (m, 2H, H-2); 13C-NMR (CDCl3): 37.4 (d, C-3), 50.4 (t, C-4), 52.5 (q, C3-COOCH3), 53.6 (q, N1-COOCH3), 54.8 (t, C-2), 151.6 (s, N1-COOCH3), 171.7 (s, C3-COOCH3), 204.0 (s, C-5); Anal. for C8H11NO4S (217.24): calc.: C 44.23%, H 5.10%, N 6.45%; found.: C 44.45%, H 4.92%, N 6.35%.
1-Benzyl-5-(2-ethoxycarbonyl-2-hydrazinyl-1-yliden)-pyrrolidine-3-carboxylic acid methyl ester (7a).
Thiolactam 6a (59.6 g, 0.24 mol) and ethyl carbazate (24.9 g, 0.24 mol) were dissolved in dry THF (100 mL) and treated with Hg(OAc)2 (76.2 g, 0.24 mol). The mixture was stirred at room temperature for 16 hours, then charcoal was added and the suspension was filtered through a pad of Celite®. The resulting solution was concentrated and the residue was recrystallized from diisopropyl ether to give 57.5 g (75%) of 7a as colorless crystals; m.p. 103-107°C; 1H-NMR (CDCl3): 1.29 (t, J=7Hz, 3H, OCH2CH3), 2.90-3.25 (m, 3H, H-3, H-4), 3.43 (d, J=8Hz, 2H, H-2), 3.69 (s, 3H, OCH3), 4.19 (q, J=7Hz, 2H, OCH2CH3), 4.45-4.55 (m, 2H, PhCH2), 6.78 (bs, 1H, NH), 7.20-7.40 (m, 5H, arom. H); 13C-NMR (CDCl3): 14.7 (q, OCH2CH3), 30.0 (t, C-4), 37.0 (d, C-3), 47.2 (t, PhCH2), 50.7 (t, C-2), 52.0 (q, OCH3), 59.5 (t, OCH2CH3), 127.0 (d, C-4`), 127.8 (d, C-3`+ C-5`), 128.3 (d, C-2`+ C-6`), 137.2 (s, C-1`), 154.7 (s, COOEt), 162.6 (s, C-5), 173.1 (s, COOMe); Anal. for C16H21N3O4 (319.36): calc.: C 60.18%, H 6.63%, N 13.16%; found.: C 59.92%, H 6.48%, N 13.03%.
1-Methyl-5-(2-ethoxycarbonyl-2-hydrazinyl-1-yliden)-pyrrolidine-3-carboxylic acid methyl ester (7b).
Thiolactam 6b (11.0 g, 63 mmol) and ethyl carbazate (6.6 g, 63 mmol) were dissolved in dry THF (100 mL) and treated with Hg(OAc)2 (20.24 g, 63 mmol). The mixture was stirred at room temperature for 16 hours, then charcoal was added and the suspension was filtered through a pad of Celite®. The resulting solution was concentrated to give 12.35 g (81%) of 7b as orange, highly viscous oil, which is sufficiently pure for the subsequent cyclization. Additional chromatographic purification (silica gel, LP/EtOAc = 1:1) gives beige crystals of m.p. 95-96°C; 1H-NMR (CDCl3): 1.25 (t, J=7Hz, 3H, OCH2CH3), 2.88 (s, 3H, NCH3), 2.90-3.20 (m, 3H, H-3, H-4), 3.55 (d, J=8Hz, 2H, H-2), 3.72 (s, 3H, OCH3), 4.16 (q, J=7Hz, 2H, OCH2CH3), 6.58 (bs, 1H, NH); 13C-NMR (CDCl3): 14.2 (q, OCH2CH3), 29.9 (t, C-4), 31.0 (q, NCH3), 37.2 (d, C-3), 51.8 (q, OCH3), 52.5 (t, C-2), 60.4 (t, OCH2CH3), 155.4 (s, COOEt), 165.2 (s, C-5), 172.6 (s, COOMe); Anal. for C10H17N3O4 (243.26): calc.: C 49.38%, H 7.04%, N 17.27%; found.: C 49.63%, H 7.05%, N 17.22%.
5-(Ethoxycarbonyl-2-hydrazinyl-1-yliden)-pyrrolidine-1,3-dicarboxylic acid dimethyl ester (7c).
Thiolactam 6c (1.42 g, 6.54 mmol) and ethyl carbazate (0.68 g, 6.54 mmol) were refluxed in dry dichloromethane (30 mL) for 21 hours. The mixture was concentrated in vacuo and the oily residue was dissolved in hot EtOAc. Pure 7c crystallizes upon storage at -35°C for 48 hours, yielding 1.85 g (99%) as colorless crystals; m.p. 90-92°C; 1H-NMR (CDCl3): 1.30 (t, J=7Hz, 3H, OCH2CH3), 2.61 (bs, 1H, NH), 2.95-3.25 (m, 3H, H-3, H-4), 3.74 (s, 3H, C3-COOCH3), 3.83 (s, 3H, N1-COOCH3), 3.95 (dd, J=18.5Hz, J=6.6 Hz, 1H, H-2), 4.03 (dd, J=18.5Hz, J=8.1Hz, 1H, H-2), 4.21 (q, J=7Hz, 2H, OCH2CH3); 13C-NMR (CDCl3): 14.4 (q, OCH2CH3), 29.8 (t, C-4), 36.9 (d, C-3), 49.8 (t, C-2), 52.5 (q, C3-COOCH3), 53.3 (q, N1-COOCH3), 61.7 (t, OCH2CH3), 151.8 (s, N1-COOCH3), 154.6 (s, COOCH2CH3), 171.9 (s, C3-COOCH3).
4-Benzyl-pyrrolo[2,3-d][1,2,3]thiadiazole-6-carboxylic acid methyl ester (4a).
Compound 7a (1.0 g, 3.13 mmol) was dissolved in dry chloroform (10 mL) and added to a refluxing mixture of thionyl chloride (2.6 g, 21.9 mmol) and dry chloroform (10 mL). After complete addition, refluxing was continued for another 16 hours. The mixture was concentrated in vacuo and the residue was taken up with dichloromethane. The organic layer was washed with satd. NaHCO3-solution and water, dried over sodium sulfate, and concentrated. Pure 4a (0.21 g, 25%) was obtained after flash column chromatography (silica gel, LP/EtOAc = 10:1) and subsequent recrystallization from diisopropyl ether as colorless crystals; m.p. 150-151°C; 1H-NMR (CDCl3): 3.89 (s, 3H, OCH3), 5.60 (s, 2H, PhCH2), 7.30-7.40 (m, 5H, arom. H), 7.85 (s, 1H, H-5); 13C-NMR (CDCl3): 51.8 (q, OCH3), 52.3 (t, PhCH2), 107.0 (s, C-6), 128.2, 128.8, 129.1 (3 d, C-2’, C-6’, C-3’, C-5’, C-4’), 130.8 (s, C-6a), 134.7 (s, C-1’), 135.8 (d, C-5), 161.0, 163.0 (2 s, CO, C-3a); Anal. for C13H11N3O2S (273.31): calc.: C 57.13%, H 4.06%, N 15.37%; found.: C 57.27%, H 4.03%, N 15.42%.
4-Methyl-pyrrolo[2,3-d][1,2,3]thiadiazole-6-carboxylic acid methyl ester (4b).
Compound 7b (0.5 g, 2.06 mmol) was dissolved in dry chloroform (10 mL) and added to a refluxing mixture of thionyl chloride (2.6 g, 21.9 mmol) and dry chloroform (10 mL). After complete addition, refluxing was continued for another 16 hours. The mixture was concentrated in vacuo and the residue was taken up with dichloromethane. The organic layer was washed with satd. NaHCO3-solution and water, dried over sodium sulfate, and concentrated. Pure 4b (0.06 g, 15%) was obtained after flash column chromatography (silica gel, LP/EtOAc = 10:1) and subsequent recrystallization from diisopropyl ether as colorless crystals; m.p. 161-165°C; 1H-NMR (CDCl3): 3.90 (s, 3H, OCH3), 4.17 (s, 3H, NCH3), 7.82 (s, 1H, H-5); 13C-NMR (CDCl3): 34.9 (q, NCH3), 51.9 (q, OCH3), 106.6 (s, C-6), 130.7 (s, C-6a), 137.1 (s, C-5), 161.4, 163.1 (2 s, CO, C-3a); Anal. for C7H7N3O2S (197.22): calc.: C 42.63%, H 3.58%, N 21.31%; found.: C 41.88%, H 3.44%, N 20.78%.
Pyrrolo[2,3-d][1,2,3]thiadiazole-4,6-dicarboxylic acid dimethyl ester (4c).
Compound 7c (1.7 g, 5.9 mmol) was dissolved in dry dichloromethane (20 mL) and added to a cooled mixture (-10 to -5°C) of thionyl chloride (4.9 g, 41.4 mmol) and dry dichloromethane (70 mL) over a period of 60 min. After complete addition, the mixture was warmed to room temperature and stirred overnight. The mixture was concentrated in vacuo and the residue was taken up with dichloromethane, treated with an excess of triethylamine and concentrated again. The residue was taken up with dichloromethane and washed with water. The organic layer was dried over sodium sulfate and concentrated. Recrystallization from EtOAc gave 1.34 g (94%) of pure 4c as colorless crystals; m.p. 152-154°C; 1H-NMR (CDCl3): 3.95 (s, 3H, C6-COOCH3), 4.24 (s, 3H, N1-COOCH3), 8.38 (s, 1H, H-5); 13C-NMR (CDCl3): 52.4 (q, C6-COOCH3), 55.6 (q, N1-COOCH3), 110.8 (s, C-6), 133.8 (d, C-5), 134.2 (s, C-6a), 148.6 (s, N1-CO), 157.9 (s, C-3a), 161.8 (s, COO); Anal. for C8H7N3O4S (241.23): calc.: C 39.83%, H 2.92%, N 17.42%; found.: C 39.91%, H 3.01%, N 17.27%.
Pyrrolo[2,3-d][1,2,3]thiadiazole-6-carboxylic acid methyl ester (4d).
A suspension of product 4c (0.40 g, 1.66 mmol) and silica gel (5 g) in dry methanol (20 mL) was stirred at room temperature for 3 days. The silica gel was removed by filtration and the obtained solution was concentrated in vacuo. Pure 4d (0.30 g, 99%) was obtained without further purification as colorless crystals; m.p. 218-220°C; 1H-NMR (CDCl3 + 10% DMSO-d6): 3.90 (s, 3H, OCH3), 7.99 (s, 1H, H-5), 8.78 (bs, 1H, H-4); 13C-NMR (CDCl3 + 10% DMSO-d6): 51.2 (q, OCH3), 106.5 (s, C-6), 130.2 (s, C-6a), 134.4 (d, C-5), 160.9 (s, C-3a), 163.0 (s, COO); Anal. for C6H5N3O2S (183.19): calc.: C 39.34%, H 2.75%, N 22.94%; found.: C 39.63%, H 2.65%, N 22.67%.