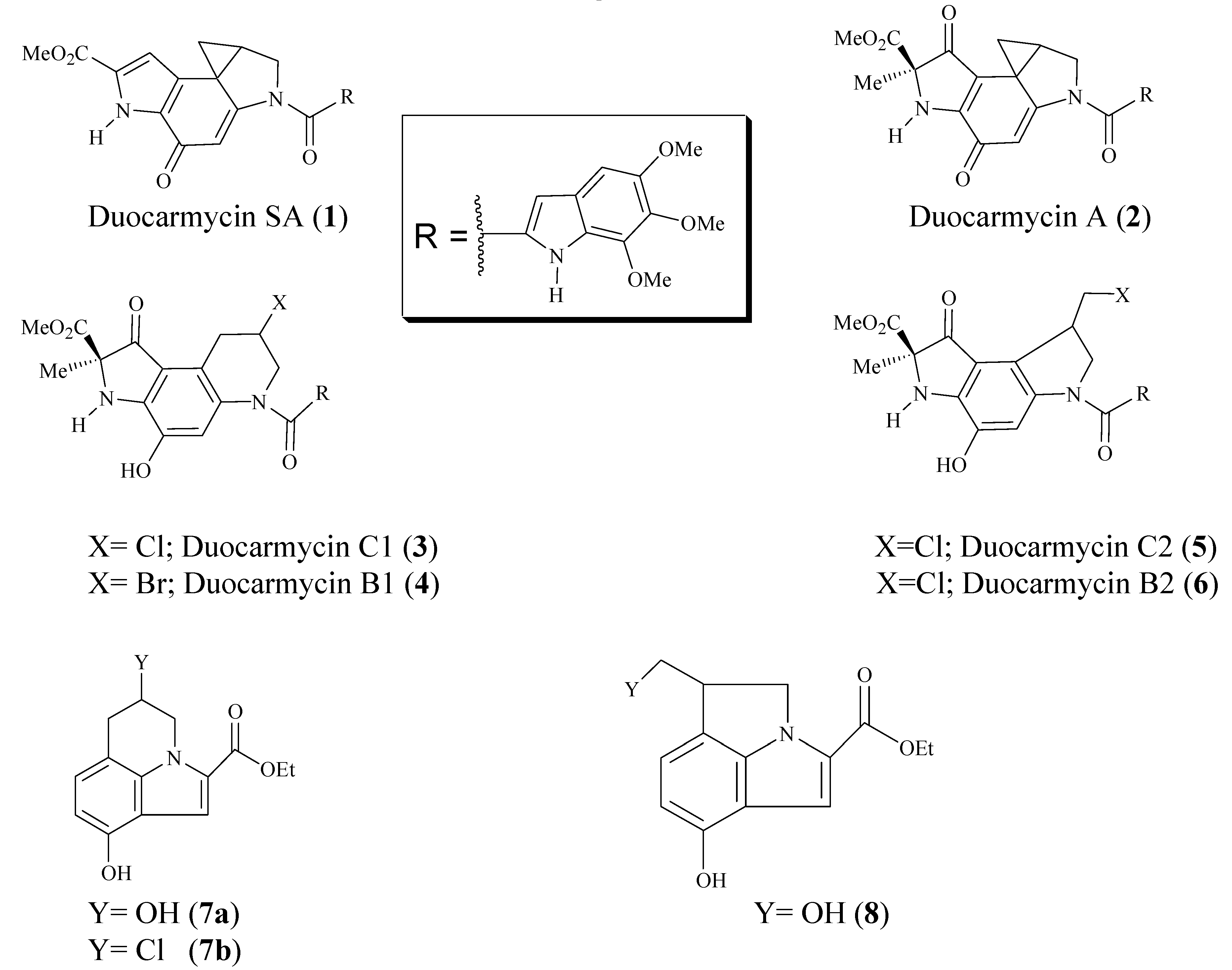
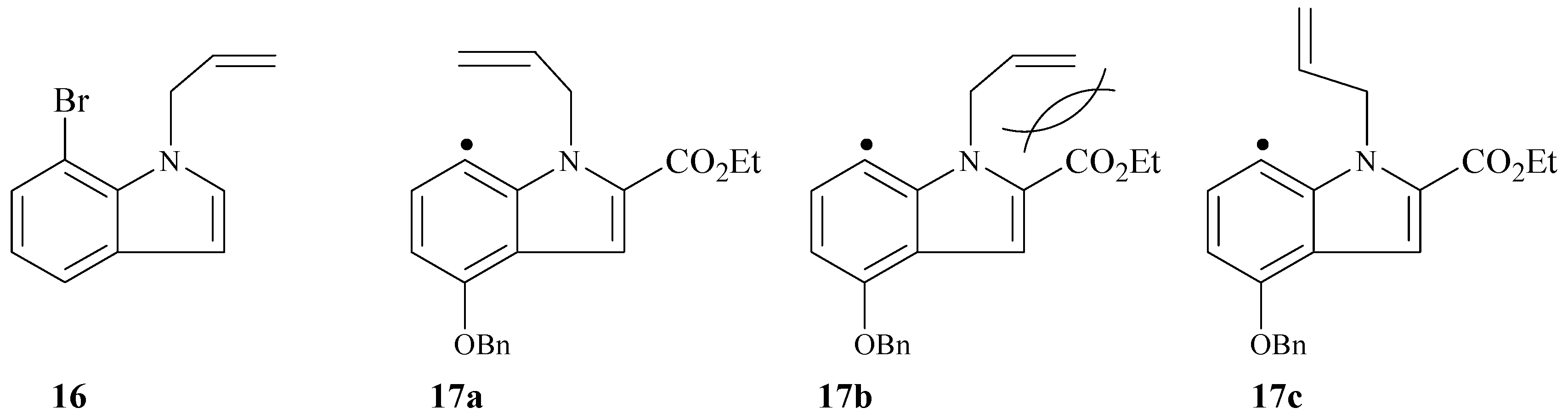
General procedure for the preparation of ethyl N-substituted indole carboxylates 12a-f.
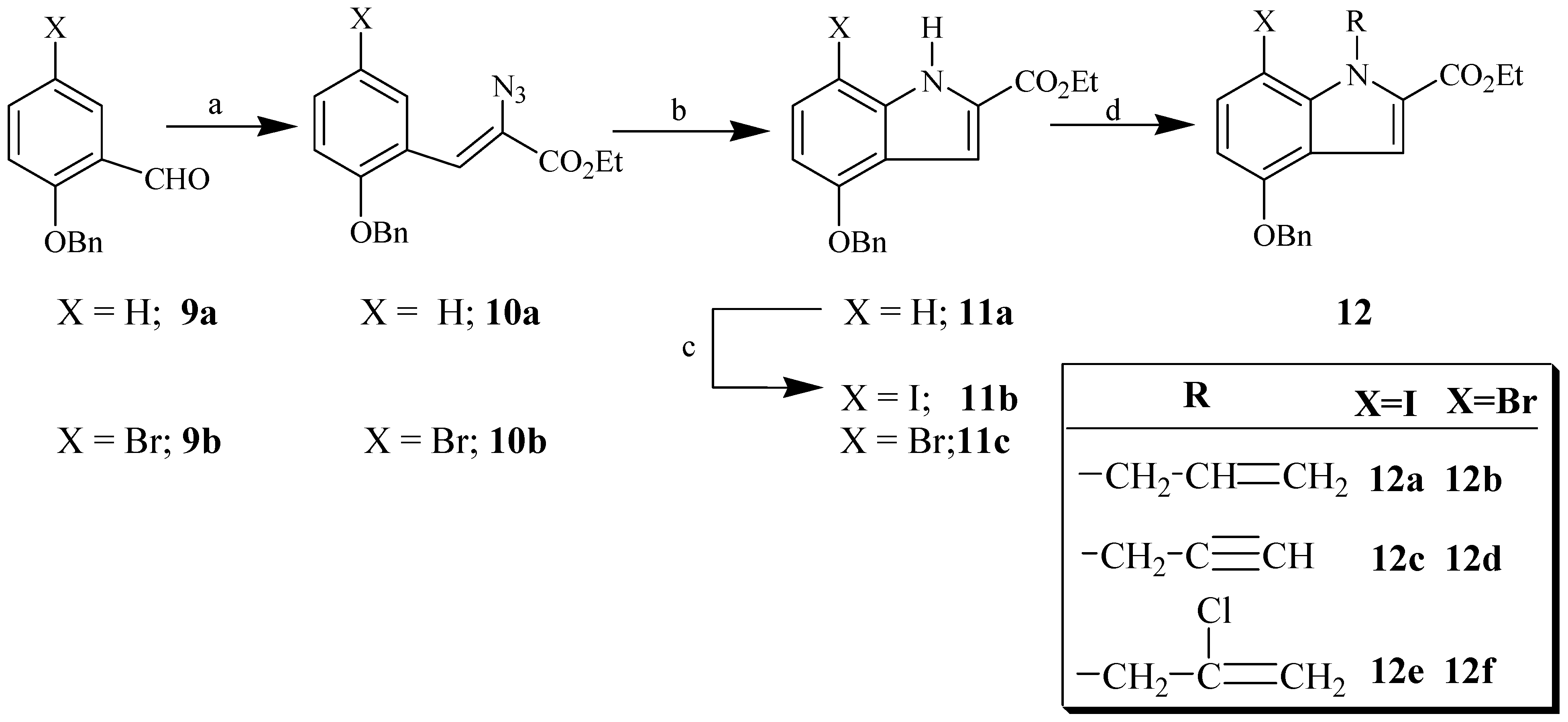
A mixture of indole 11b or 11c (4.0 mmol), potassium carbonate (8.0 mmol), sodium iodide (2.00 mmol) and the appropriate alkyl halide (16.0 mmol) in DMF (100 mL) was stirred at 50 °C for 24 h. The solvent was evaporated to dryness. The residue was washed with ethyl acetate (4x50mL). The combined organic layers were washed with brine solution, dried (MgSO4), and concentrated in vacuo. The resulting products were purified by column chromatography on silica gel (1-2.5% ethyl acetate in hexane) to give the desired ethyl N-substituted indole carboxylate.
Ethyl 1-allyl-4-(benzyloxy)-7-iodo-1H-indole-2-carboxylate (12a). Prepared from 11b and allyl chloride as a white solid (95%): mp. 85-86°C; IR (KBr, cm-1) 1716 (C=O), 1638 and 1599 (C=C); 1H-NMR (250 MHz, CDCl3) δ 7.69 (d, J = 7.5 Hz, 1H, Ar-H), 7.50-7.30 (br m, 6H, Ar-H), 6.34 (d, J = 7.5 Hz, 1H, Ar-H), 6.03 (m, 1H, vinylic CH), 5.78 (m, 2H, -NCH2), 5.17 (s, 2H, -OCH2Ph), 5.08 (br d, J = 10 Hz, 1H, vinylic CH2), 4.63 (br d, J = 17.5 Hz, 1H, vinylic CH2), 4.33 (q, J = 7.5 Hz, 2H, -OCH2CH3), and 1.38 (t, J = 7.5 Hz, 3H, -CH3); 13C-NMR (62.9 MHz, CDCl3) δ 161.63, 154.26, 139.12, 138.20, 136.87, 135.89, 128.81, 128.29, 127.99, 127.66, 120.82, 115.33, 109.50, 103.74, 70.25, 62.72, 60.88, 46.04, and 14.53. Anal. Calcd for C21H20INO3: C, 54.68; H, 4.37; N, 3.04; found C, 54.60; H, 4.29; N, 2.99; EIMS (m/z rel intensity) calc for C21H20INO3: 461; found 461 (M+, 40).
Ethyl 1-allyl-4-(benzyloxy)-7-bromo-1H-indole-2-carboxylate (12b). Prepared from 11c and allyl chloride as a white solid (90%): mp. 74-76°C; IR (KBr, cm-1) 1715 (C=O, ester), 1646 and 1606 (C=C); 1H-NMR (300 MHz, CDCl3) δ 7.50-7.25 (br m, 7H, Ar-H), 6.36 (d, J = 8.4 Hz, 1H, Ar-H), 5.97 (m, 1H, vinylic CH), 5.68 (m, 2H, -NCH2), 5.10 (s, 2H, -OCH2Ph), 4.99 (br d, J = 10.5 Hz, 1H, vinylic CH2), 4.64 (br d, J = 17.2 Hz, 1H, vinylic CH2), 4.26 (q, J = 7.1 Hz, 2H, -OCH2CH3), and 1.39 (t, J = 7.2 Hz, 3H, -CH3); 13C-NMR (62.9 MHz, CDCl3) 161.42, 153.06, 136.70, 136.00, 135.71, 131.23, 128.62, 128.09, 127.86, 127.47, 120.69, 115.15, 109.50, 102.37, 95.37, 70.40, 60.69, 46.70, 14.33;EIMS (m/z rel intensity) calc for C21H20NO379Br: 413; found 413 (M+, 24).
Ethyl 4-(benzyloxy)-7-iodo-1-prop-2-ynyl-1H-indole-2-carboxylate (12c). Prepared from 11b and propargylic chloride as a white solid (94%): mp. 132-133°C; IR (KBr, cm-1) 3274 (Csp-H), 2114 (Csp-Csp), 1712 (C=O), and 1597 (C=C); 1H-NMR (200 MHz, CDCl3) δ 7.7 (d, 1H, Ar-H) 7.5-7.3 ( br m, 6H, Ar-H), 6.4 (d, 1H, Ar-H), 5.9 (br d, 2H, -NCH2), 5.2 (s, 2H, -OCH2Ph), 4.4 (q, 2H, OCH2CH3), 2.3 (t, 1H, acetylinic CH), and 1.4 (t, 3H, -CH3); 13C-NMR (75.5 MHz, CDCl3) 161.47, 154.08, 139.09, 138.37, 136.60, 128,64, 128.12, 127.57, 12745, 120.98, 110.23, 104.13, 80.14, 73.10, 70.11, 60.922, 34.83, 14.32; Anal. Calcd for C21H18INO3: C, 54.92; H 3.95; N, 3.05; found C, 55.6; H, 4.05; N, 3.25; EIMS (m/z rel intensity) calc for C21H18INO3: 459; found 459 (M+, 35).
Ethyl 4-(benzyloxy)-7-bromo-1-prop-2-ynyl-1H-indole-2-carboxylate (12d). Prepared from 11c and propargyl chloride as a white solid (100%): mp. 100-101°C; IR (KBr, cm-1) 3275 (Csp-H), 2114 (Csp-Csp), 1713 (C=O) and 1606 (C=C); 1H-NMR (300 MHz, CDCl3) δ 7.52-7.30 (br m, 7H, Ar-H), 6.44 (d, J = 8.4 Hz, 1H, Ar-H), 5.92 (br d, J = 2.4 Hz, 2H, -NCH2), 5.16 (s, 2H, -OCH2Ph), 4.39 (q, J = 7.2 Hz, 2H, -OCH2CH3), 2.30 (t, J = 2.4 Hz, 1H, acetylinic CH), and 1.39 (t, J = 7.1 Hz, 3H, -CH3); 13C-NMR (75.5 MHz, CDCl3) 161.43, 153.08, 136.61, 136.07, 131.50, 128.64, 128.12, 127.49, 127.45, 120.96, 110.35, 102.96, 95.35, 80.12, 72.57, 70.17, 60.93, 35.28, 14.32; Anal. Calcd for C21H18BrNO3: C, 61.18; H, 4.40; N, 3.40; found C, 61.40; H, 4.60; N, 3.42; EIMS (m/z rel intensity) calc for C21H18NO379Br: 411; found 411 (M+, 18).
Ethyl 4-(benzyloxy)-1-(2-chloro-allyl)-7-iodo-1H-indole-2-carboxylate (12e). Prepared from 11b and 2,3-dichloropropene as a white solid (92%): mp. 114-116°C; IR (KBr, cm-1) 1712 (C=O), 1646 and 1598 (C=C); 1H-NMR (200 MHz, CDCl3) δ 7.7 (d, 1H, Ar-H), 7.5-7.3 (br m, 6H, Ar-H), 6.4 (d, 1H, Ar-H), 5.9 (s, 2H, -NCH2), 5.2 (d, 1H, vinylic CH2) 5.2 (s, 2H, -OCH2Ph), 4.4 (d, 1H, vinylic CH2), 4.3 (q, 2H, -OCH2CH3), and 1.4 (t, 3H, -CH3); 13C-NMR (75.5 MHz, CDCl3) 161.18, 154.08, 139.29, 138.71, 137.99, 13654, 128.66, 128.17, 127.70, 127.50, 120.62, 111.62, 109.92, 104.06, 70.16, 60.91, 49.36, 14.31 ; EIMS (m/z rel intensity) calc for C21H18INO335Cl: 495; found 495 (M+, 2).
Ethyl 4-(benzyloxy)-7-bromo-1-(2-chloro-allyl)-1H-indole-2-carboxylate (12f). Prepared from 11c and 2,3-dichloropropene as a white solid (100%): mp. 102-103°C; IR (KBr, cm-1) 1704 (C=O), 1643 and 1604 (C=C); 1H-NMR (300 MHz, CDCl3) δ 7.56-7.36 (br m, 7H, Ar-H), 6.47 (d, J = 8.7 Hz, 1H, Ar-H), 5.86 (s, 2H, -NCH2), 5.23 (d, J = 1.2 Hz, 1H, vinylic CH2) 5.19 (s, 2H, -OCH2Ph), 4.51 (d, J = 2.1 Hz, 1H, vinylic CH2), 4.35 (q, J = 7.2 Hz, 2H, -OCH2CH3), and 1.39 (t, J = 7.2 Hz, 3H, -CH3); 13C-NMR (75.5 MHz, CDCl3) δ 161.39, 153.27, 138.90, 136.75, 131.88, 128.89, 128.40, 127.88, 127.75, 120.83, 111.46, 110.29, 103.09, 95.61, 70.40, 61.16, 50.17, and 14.54. Anal. Calcd for C21H19BrClNO3: C, 56.21; H, 4.27; N, 3.12; found C, 56.27; H, 4.23; N, 3.09.
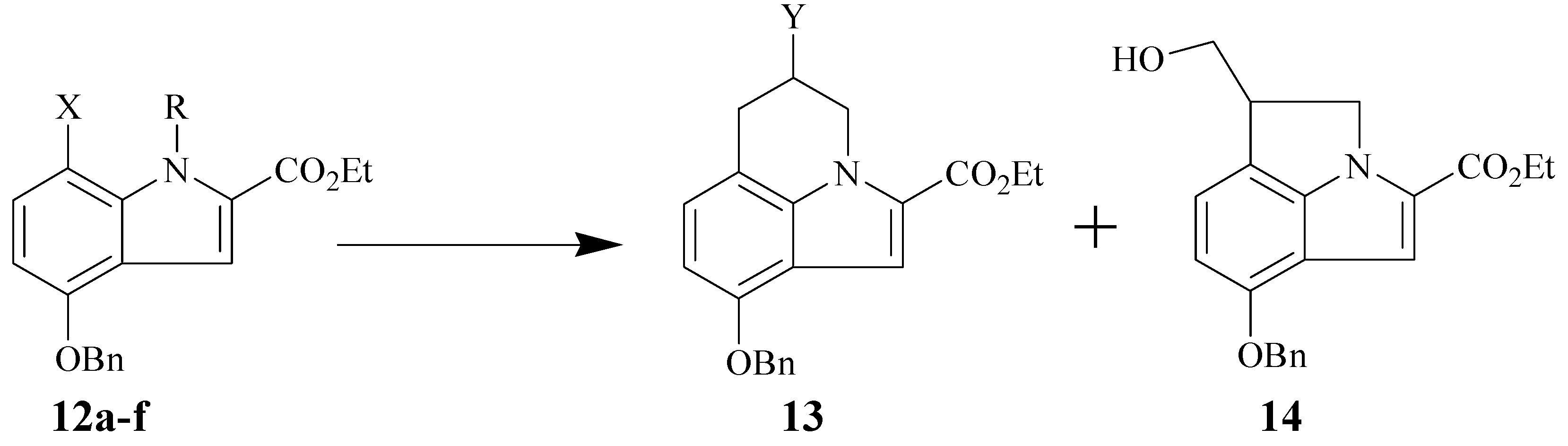
Ethyl 9-(benzyloxy)-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-2-carboxylate (13a). A solution of allyl compound 12a or 12b (0.72 mmol), AIBN (0.02 g) and Bu3SnH (1.45 mmol) in benzene (50 mL) was degassed with N2 gas and the solution was heated at reflux for 1.3 h. After cooling to room temperature, the solvent was removed under reduced pressure, and the residue obtained was purified by chromatography, to give the six-membered ring compound 13a as a colorless oil (82-84%): IR (CCl4, cm-1) 1708 (C=O) and 1604 (C=C); 1H-NMR (300 MHz, CDCl3) δ 7.50-7.30 (br m, 6H, Ar-H), 6.88 (d, J = 7.7 Hz, 1H, Ar-H), 6.43 (d, J = 7.7 Hz, 1H, Ar-H), 5.17 (s, 2H, -OCH2Ph), 4.50 (t, J = 5.8 Hz, 2H, -NCH2), 4.33 (q, J = 7.1 Hz, 2H, -OCH2CH3), 2.87 (t, J = 5.9 Hz, 2H, benzylic CH2), 2.17 (m, J = 6.0 Hz, 2H, -CH2), and 1.38 (t, J = 7.1 Hz, 3H, -CH3); 13C-NMR (75.5 MHz, CDCl3) δ 162.21, 151.77, 137.88, 137.37, 128.47, 127.76, 127.33, 126.05, 121.90, 115.98, 115.92, 107.09, 100.89, 69.89, 60.31, 44.11, 24.19, 23.22, and 14.39; Anal. Calcd for C21H21NO3: C, 72.20; H, 6.31; N, 4.18; found C, 72.8; H, 6.54; N, 4.22; EIMS (m/z rel intensity) calc for C21H21NO3: 335; found 335 (M+, 21).
Ethyl 9-(benzyloxy)-5-hydroxy-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-2-carboxylate (13b).
Method A: A solution of 12a (0.47 g, 1.02 mmol), TEMPO (98%, 0.65 g, 4.08 mmol) and Bu3SnH (97%, 0.32 g, 1.07 mmol) in 50 mL of freshly distilled benzene was degassed with N2 for 5 min, and then heated at reflux. After 15 min of reflux, an additional 3 equiv of TEMPO (3×0.16 g) in 9 mL of benzene and 3 equiv of Bu3SnH (3×0.30 g) in 9 mL of benzene were added successively over the next 40 min. After 15 min, another 1.5 equiv of TEMPO (0.24 g) in 5 mL of benzene was added, followed by the addition of another 1.0 equiv of Bu3SnH (0.32 g) in 5 mL of benzene. The mixture was kept at reflux temperature for additional 80 min, cooled to room temperature, and concentrated in vacuo. The crude product was passed over short column of silica gel to remove excess Bu3SnH and polar impurities. The crude product was then dissolved in 60 mL of a 3:1:1 mixture of HOAc: THF: H2O and treated with Zn powder (0.88 g, 13.46 mmol). The resulting suspension was kept at 70°C with continuous stirring. After 90 min, another amount of Zn powder (0.26 g, 4.00 mmol) was added as one portion followed by stirring for another 90 min. The solution was allowed to cool to room temperature, filtered off, and evaporated under reduced pressure. Water (80 mL) was added to the residue and the resulting aqueous solution was extracted with ethyl acetate (3×20 mL). The combined organic layers was washed with brine, dried, and concentrated in vacuo. The product was purified by column chromatography on silica gel (10-25% ethyl acetate in hexane) to give the alcohol derivative 13b as a white solid (0.23 g, 60%): mp. 143-144°C); IR (KBr, cm-1) 3447 (OH), 1685 (C=O), and 1604 (C=C); 1H-NMR (250 MHz, CDCl3) δ 7.50-7.20 (br m, 6H, Ar-H), 6.87 (d, J = 7.7 Hz, 1H, Ar-H), 6.40 (d, J = 7.7 Hz, 1H, Ar-H), 5.10 (s, 2H, -OCH2Ph), 4.62-4.35 (br m, 3H, -NCH2 and –CHOH), 4.29 (q, J = 7.12 Hz, 2H, -OCH2CH3), 3.10 (dd, J = 15.8 Hz and J’ = 2.5 Hz, 1H, benzylic CH2), 2.90 (dd, J = 15.9 Hz and J’ = 5.1 Hz, 1H, benzylic CH2), 1.93 (s, 1H, OH, D2O exchangeable), and 1.31 (t, J = 7.1 Hz, 3H, -CH3); 13C-NMR (62.9 MHz, CDCl3) δ 162.04, 152.25, 137.21, 136.98, 128.49, 127.83, 127.33, 126.41, 123.80, 115.80, 111.76, 107.78, 101.60, 69.98, 64.79, 60.84, 50.43, 32.71, and 14.35; Anal. Calcd for C21H21NO4: C, 71.78; H, 6.02; N, 3.99; found C, 71.49; H, 5.91; N, 3.91; EIMS (m/z rel intensity) calc for C21H21NO4: 351; found 351 (M+, 7).
Method B: A solution of 12c (0.37 g, 0.90 mmol), Bu3SnH (97%, 1.00 g, 3.33 mmol) and AIBN (40 mg) in freshly distilled benzene (75 mL) was bubbled with N2 gas for 5 min and then heated at reflux for 2.5 h. The reaction mixture was cooled, and the solvent was removed in vacuo to afford the crude product as yellow oil. The oily product was dissolved in THF (7.5 mL), cooled to 0°C. Then 1M BH3-THF solution (2.7 mL) was added in one portion. The reaction mixture was allowed to warm to room temperature, and stirred for 3.0 h. The reaction mixture was cooled 0°C and treated sequentially with water (2.7 mL), 2N aqueous sodium hydroxide (1.35 mL, 2.7 mmol), and 30% aqueous hydrogen peroxide (0.81 mL, 8.1 mmol). The reaction mixture was allowed to warm to room temperature, and stirred for 3.0 h. The reaction mixture was poured to ethyl acetate (50 mL). The organic layer was washed with brine (2×15 mL), dried, and concentrated invacuo. The residue was purified by column chromatography on silica gel (10-25% ethyl acetate in hexane) to give the alcohol derivative 13b as a white solid (0.16 g, overall 50%). This compound was also obtained in 35% yield from 12d.