Results and Discussion
The starting materials, 3,7- and 1,7-dibromodibenzosuberone
7 and
8 were prepared by the method previously developed in our laboratory, via regioselective bromination of commercially available dibenzosuberone [
22]. As the regiochemistry of the substitution process in the dibenzosuberone ring generally results in
ortho and
para substituted isomers with respect to an alkyl group, our method yields
para,
para as well as
ortho,
para substituted isomers. In this manner the 3,7- and 1,7-dibromoderivatives have been prepared as the main products and then used for the preparation of their respective
N,
N-dialkylaminopropyl and
O-alkyloxime derivatives.
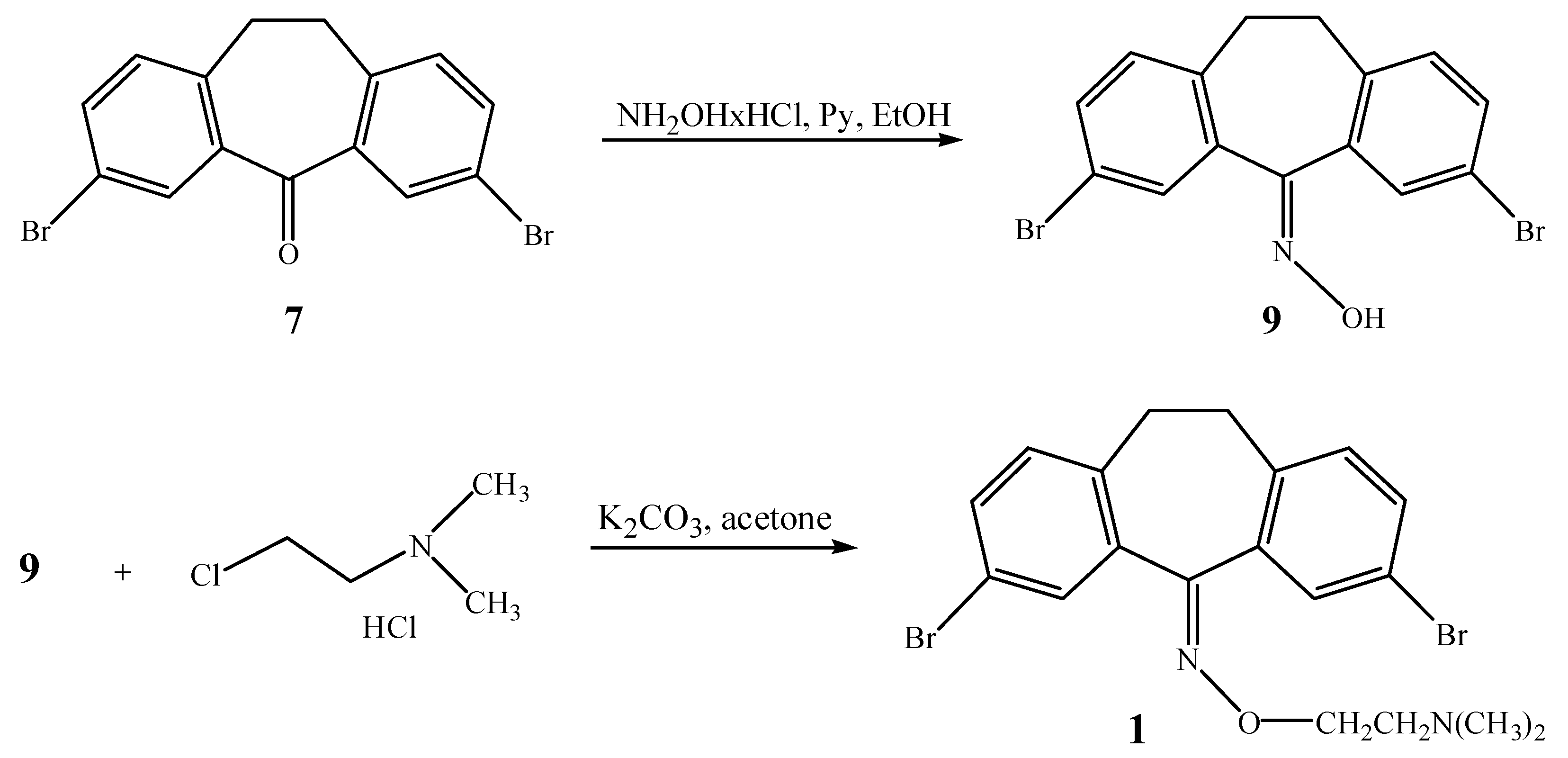
Among the other possible synthetic paths to the 3,7-dibromo derivative of noxiptiline (
1), first we applied an indirect one to avoid the direct alkylation of the oxime
9 and possible formation of an
N‑alkyl oxime instead of expected
O-alkyl one. Unfortunately this method, based on the
O-alkyl hydroxylamine prepared by
O-alkylation of hydroxylamine protected with a phtalimide group, failed at the
N-hydroxyphtalimide alkylation stage. Abandoning the indirect approach we then used the selective
O-alkylation of the oxime
9 under mild conditions (solvent, basic salt with an appropriate cation), as shown in
Scheme 1. The oxime of 3,7-dibromodibenzosuberone (
9) was thus prepared in five days by a method reported for hindered ketones [
23], using two portions of four equivalents of hydroxylamine hydrochloride and pyridine. The next step, regioselective
O-alkylation of oxime
9 [
24,
25,
26], was performed in acetone with dimethylaminoethylchloride hydrochloride and potassium carbonate as the base. By this procedure
O-alkylated compound
1 was obtained exclusively, with an overall yield of 87%. Since the alkylation of oximes as bidentate nucleophiles depends on the structural characteristics of the substrate, in the case of compound
9 alkylation at the less hindered oxygen atom was favored.
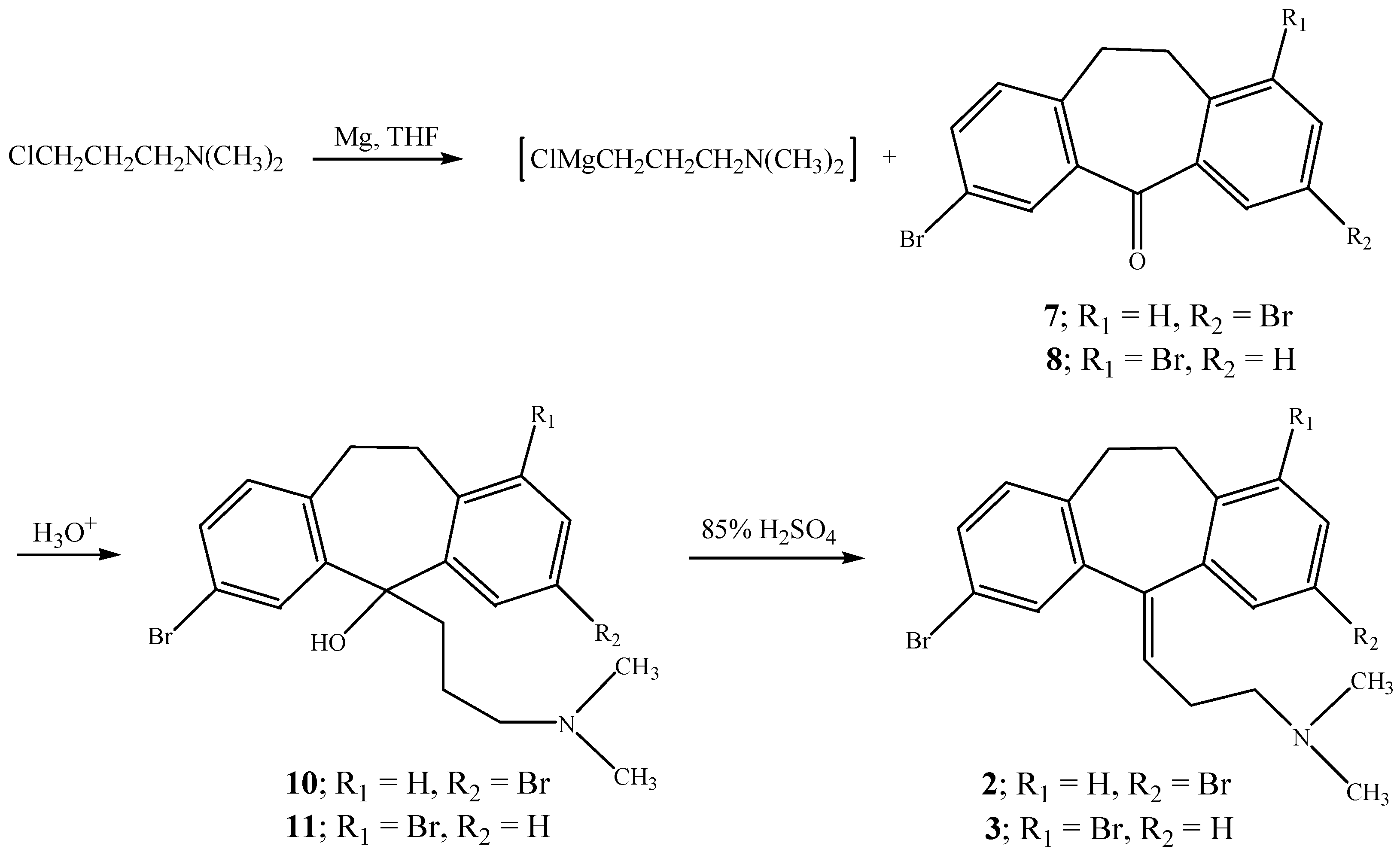
3,7- (
2) and 1,7-dibromo derivatives,
3, of amitriptyline were prepared by a two-step synthesis according to
Scheme 2.
Attachment of a dimethylaminopropylic functionality to the skeleton of brominated dibenzo-suberone was achieved by a Grignard reaction [
27,
28,
29,
30]. Since the required Grignard reagent 3‑dimethylamino-1-propylmagnesium chloride is not easy to obtain, very strictly controlled reaction conditions have to be employed. The free base is freshly prepared separately and the reaction is initiated with a more reactive alkyl halogenide and iodine. The reaction was carried out for 26 hours at room temperature and isolated compounds
10 and
11 were purified by chromatography on SiO
2. After chromatographic separation 65% of the 3,7- and 32% of 1,7-dibromo derivatives
10 and
11 were isolated, respectively.
Dehydration of compounds
10 and
11, obtained by the previous Grignard reaction, was carried out by stirring for a few hours at 4 ºC with 85% sulfuric acid. Starting with compound
10, 3,7-dibromoamitriptyline (
2) was obtained in 94% yield, but with the 1,7-dibromodibenzosuberone
11 a mixture of
syn (Z) and
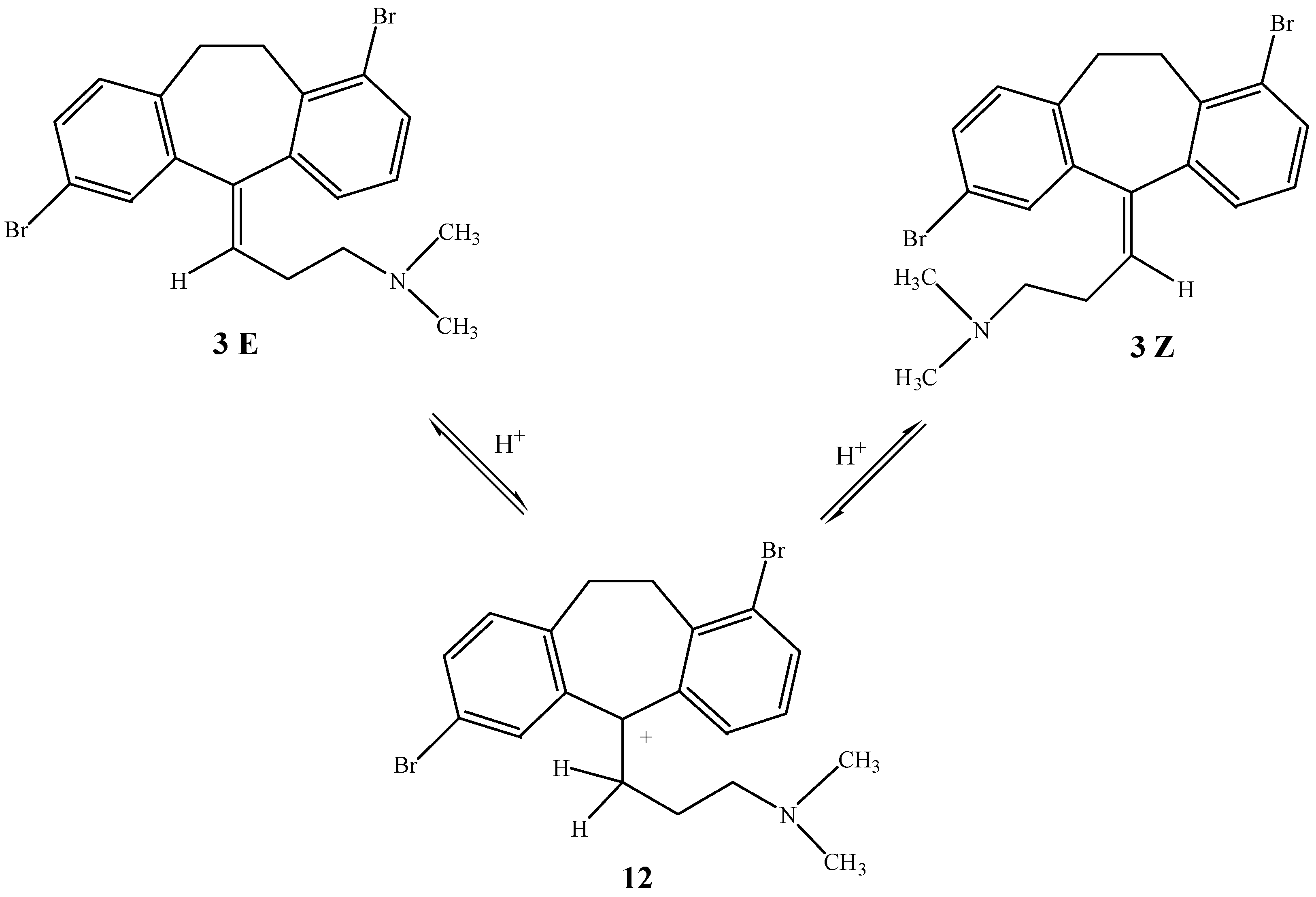
anti (E) isomers with respect to the 1-Br atom, was obtained. We tried to separate the isomers by chromatography using various mixtures of solvents, but unfortunately without success. Although rotation around the double bond is impossible, the dimethylaminopropyl chain connected to the tricyclic ring in the carbocation
12 rotates freely (
Scheme 3). Therefore, we tried to convert compound
3 to the thermodynamically more stable isomer by prolonged stirring in sulfuric acid. We anticipated that implied conclusion that in the thermodynamically controlled reaction the less sterically hindered
anti-isomer
3E would be obtained. Indeed, under these conditions the pure
E isomer
3E is formed in good yield.
Experimental
General
IR spectra were recorded on a Perkin-Elmer Spectrum One spectrophotometer, 1H-NMR and 13C-NMR spectra were recorded on a Bruker 600 instrument. Shifts are given in ppm downfield from TMS used as an internal standard. All the mass spectra were obtained on a FTMS Finnigan 2001 DD spectrometer. TLC analyses were performed on Merck (Darmstadt, Germany) DC-Alufolien with Kieselgel 60254. Elemental analyses were done at the Central Analytical Service (CAS) of the Ruđer Bošković Institute.
3,7-Dibromo-5-hydroxyimino-10,11-dihydro-5H-dibenzo[a,d]cyclohepta-1,4-diene (9).
To a solution of compound 7 (10.98 g, 30 mmol) in abs. ethanol (100 mL) hydroxylamine hydrochloride (8.33 g, 120 mmol) and pyridine (9.67 g, 120 mmol) were added. The reaction mixture was stirred under reflux for 24 hours, then an additional four equivalents of hydroxylamine hydrochloride (8.33 g, 120 mmol) and pyridine (9.67 g, 120 mmol) were added. After stirring under reflux for 100 hours the reaction mixture was cooled, poured into water (300 mL), acidified with conc. hydrochloric acid and extracted with ethyl acetate (3x100 mL). The extracts were dried over anhydrous sodium sulfate and the solvent was evaporated. Hot n-hexane (100 mL) was added to the residue and the precipitated colorless crystals (10.18 g, 89%) were filtered off; mp 228-230 ºC; Rf=0.50 (dichloromethane as eluent); IR (KBr) ν 3240, 2930, 1760, 1590 (C=N), 1565, 1475, 1420, 1405, 1385, 1355, 1320, 1290, 1260, 1175, 1160, 1100, 1075, 1010, 965, 940, 910, 890, 830, 815, 780, 775, 755, 715, 690, 660, 630 cm-1; 1H-NMR (DMSO-d6), δ: 2.89-2.96 (m, 4H, Hbenzylic), 7.08 (d, 1H, Harom, J=8.3 Hz), 7.24 (d, 1H, Harom, J=8.3 Hz), 7.43-7.61 (m, 4H, Harom), 11.70 (s, 1H, =NOH) ppm; 13C‑NMR (DMSO-d6), δ: 30.56, 32.43, 118.43, 118.69, 130.50, 130.92, 131.02, 131.55, 131.69, 132.79, 135.87, 136.32, 137.57, 137.67, 152.94 (C=NOH) ppm.
3,7-Dibromo-5-(dimethylaminoethyloxyimino)-10,11-dihydro-5H-dibenzo[a,d]cyclohepta-1,4-diene (1).
This compound was prepared by heating the ketoxime 9 (2.1 g, 5.51 mmol), 2-dimethylamino-ethylchloride hydrochloride (0.95 g, 6.61 mmol) and potassium carbonate (1.68 g, 12 mmol) in acetone for 15 hours under an inert atmosphere. After cooling to room temperature, the precipitate was filtered off and the solvent was removed from the filtrate under reduced pressure. A brown oil was obtained, which crystallized on cooling. The yield was 2.44 g (98%); Rf=0.54 (9:1 dichloromethane-methanol); IR (KBr) ν 2920, 2810, 2760, 2040, 1580, 1550 (C=N), 1440, 1380, 1350, 1320, 1290, 1250, 1160, 1070, 1020 (C-O), 990 (N-O), 910, 880, 810 cm-1; 1H-NMR (CDCl3) δ: 2.32 (s, 6H, N(CH3)2), 2.72 (t, 2H, CH2, J=5.8 Hz), 3.02-3.06 (m, 4H, Hbenzylic C-10,11), 4.36 (t, 2H, CH2, J=5.8 Hz), 6.99 (d, 1H, Harom C-9,1, J=8.5 Hz), 7.12 (d, 1H, Harom C-9,1, J=8.2 Hz), 7.39 (dd, 2H, Harom C-8,2, J=1.9 Hz), 7.57 (d, 1H, Harom C-4,6, J=1.9 Hz), 7.72 (d, 1H, Harom C-4,6, J=1.9 Hz) ppm; 13C-NMR (CDCl3) δ: 31.21 (C-10,11), 32.77 (C-10, 11), 45.70 (N(CH3)2), 57.75 (CH2), 72.91 (CH2), 119.13 (C-3,7), 119.64 (C-3,7), 129.70 (C-1), 131.33 (C-4,6), 131.96 (C-2,8,9), 154.81 (C=NOR) ppm; Anal. Calcd. (C19H20Br2N2O): C 50.46, H 4.46, Br 35.34, N 6.20; Found: C 50.60, H 4.61, Br 35.54 and N 6.04%.
Grignard reaction with 3,7- and 1,7- dibromo derivatives 7 and 8: preparation of dry tetrahydrofuran for use in the Grignard reaction:
To Mg-turnings (1.72 g, 70 mmol), a few milliliters of THF (just enough to cover the Mg) and a small crystal of iodine were added. A separately prepared solution of ethyl bromide (5.0 mL) in THF (20 mL) was added dropwise into the Mg-suspension at such a rate that the reaction mixture kept boiling. After the whole solution was added, refluxing of the reaction mixture was continued for 30 minutes. Additional THF (130 mL) was then added and refluxing was maintained for 2 hours. When the Mg-turnings had mostly reacted, the THF was distilled off in a closed system.
Preparation of the free base
Sodium hydroxide and 3-dimethylamino-1-propylchloride hydrochloride were dissolved separately in water (10 mL). These two solutions were mixed and the pH was adjusted to ~14. After extraction with dichloromethane (3x30 mL), the extracts were dried over anhydrous sodium sulfate and the solvent was removed to afford 3.34 g (53%) of the free base.
Grignard reaction
A solution of the free base was prepared by dissolving N,N‑dimethylamino-1-propyl-chloride (2.5 g) in dry THF (20 mL). After that, CaH2 (1.0 g) was added, the suspension was stirred for one hour, and filtered. This freshly prepared filtrate was added dropwise over 30 minutes to a small volume of dry THF (20 mL), a crystal of iodine and Mg-turnings (0.51 g). The reaction mixture was heated with stirring for 2 hours. The solution of the Grignard reagent was cooled to 0 °C and a solution of 3,7-(7) or 1,7-dibromodibenzosuberone (8) (3.66 g) in THF (50 mL) was added dropwise. The obtained mixture was stirred at room temperature overnight, then poured into a saturated solution of sodium chloride and extracted with dichloromethane (3x30 mL). The extract was dried over anhydrous sodium sulfate and the solvent was evaporated. The Grignard reaction products 10 and 11 were purified by column chromatography on SiO2 with dichloromethane/methanol 9:1 as eluent. 3,7-Dibromo-5-(hydroxy-5-N,N-dimethylaminopropyl)-10,11-dihydro-5H-dibenzo[a,d]cycloheptane (10): 2.93 g (64.7%), mp 145.5-146.5 °C; Rf= 0.47 (9:1 dichloromethane/methanol); IR (KBr) ν 3090 (OH), 2940, 2920, 2850, 2820, 2780, 2700, 2630, 2330, 1900, 1795, 1580, 1560, 1460, 1420, 1400, 1385, 1350, 1335, 1290, 1265 (C-N), 1245, 1230, 1205, 1185, 1170, 1150, 1110, 1100, 1065, 1050, 1035, 1005, 950, 920, 900, 880, 845, 820, 790, 775, 755, 735, 700, 680, 650, 635, 615 cm-1; 1H-NMR (CDCl3) δ: 1.32 (t, 2H, CH2, J=5.2 Hz), 2.17-2.25 (m, 8H, N(CH3)2+CH2), 2.43-2.47 (m, 2H, CH2), 2.85-2.96 (m, 2H, Hbenzylic), 3.32-3.43 (m, 2H, Hbenzylic), 6.93 (d, 2H, Harom, J=8.0 Hz), 7.23-7.27 (m, 2H, Harom), 8.19 (d, 2H, Harom, J=1.9 Hz) ppm; 13C-NMR (CDCl3) δ: 22.15, 32.68, 44.15, 44.95, 59.50, 75.39 (COH), 120.13, 129.75, 129.95, 131.89, 135.73, 147.37 ppm; Anal. Calcd. (C20H23Br2NO): C 53.00, H 5.12, Br 35.26, N 3.09; Found: C 53.07, H 5.04, Br 35.32 and N 2.97%. 1,7-dibromo-5-(hydroxy-5-N,N-dimethylaminopropyl)-10,11-dihydro-5H-dibenzo[a,d]cycloheptane (11): 1.50 g (33.1%) of product 11 was obtained, mp 164.5-167.0 °C; Rf=0.31 (9:1 dichloromethane/methanol); IR (KBr) ν 3030 (OH), 2970, 2940, 2900, 2840, 2760, 2680, 2640, 2540, 2460, 2320, 1900, 1580, 1500, 1460, 1440, 1400, 1390, 1380, 1290, 1260 (C-N), 1240, 1200, 1160, 1140, 1100, 1040, 100, 950, 920, 890, 850, 820, 780, 750, 740, 690, 650 cm-1; 1H‑NMR (CDCl3) δ: 1.42 (d, 2H, CH2, C-13), J=4.7 Hz), 2.25 (s, 6H, N(CH3)2), 2.37-2.47 (m, 2H, CH2), 2.62-2.69 (m, 1H, CH2), 2.88-2.97 (m, 1H, CH2), 3.20-3.48 (m, 4H, Hbenzylic C-10,11), 6.98 (d, 1H, Harom, J=8.0 Hz), 7.06 (t, 1H, Harom, J=7.8 Hz), 7.26-7.29 (m, 1H, Harom), 7.48 (d, 1H, Harom, J=8.0 Hz), 8.07 (d, 1H, Harom, J=8.0 Hz), 8.17 (d, 1H, Harom, J=1.9 Hz) ppm; 13C-NMR (CDCl3) δ: 21.93, 31.92, 32.96, 43.61, 44.82 (N(CH3)2), 59.21, 75.80 (COH), 120.20, 125.98, 126.27, 126.91, 129.82, 129.88, 131.50, 131.87, 135.36, 135.56, 147.35, 147.44 ppm; Anal. Calcd. (C20H23Br2NO): C 53.00, H 5.12, Br 35.26, N 3.09; Found: C 53.01, H 4.99, Br 35.23 and N 2.93%.
Dehydration of products 10 and 11
The hydroxy derivatives 10 or 11 (0.2 g, 0.44 mmol) were dissolved in 85% sulfuric acid (20 mL) and stirred for 3 hours at 4 °C. After that, the reaction mixture was slowly diluted with cold water, made alkaline with sodium hydroxide and extracted with dichloromethane (3x30 mL). The dichloromethane solution obtained was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure to give, in the case of compound 10, 3,7-dibromo-5-(3-N,N-dimethyl-aminopropylidene)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene (2): 0.18 g (94.0%), mp 98.5-100.5 °C; Rf=0.34 (9:1 dichloromethane/methanol); IR (KBr) ν 2940, 2850, 2780, 2710, 1880, 1580, 1560, 1450, 1370, 1260 (C-N), 1220, 1150, 1070, 1050, 1025, 960, 920, 890, 870, 800, 770, 760, 750, 710, 670 cm‑1; 1H-NMR (CDCl3) δ: 2.19 (s, 6H, N(CH3)2), 2.21-2.42 (m, 4H, CH2+CH2 C-13,14), 2.70-2.95 (m, 2H, Hbenzylic C-10,11), 3.22-3.39 (m, 2H, Hbenzylic C-10,11), 5.90-5.95 (m, 1H, C=CH), 6.90 (d, 1H, Harom, J=8.2 Hz), 7.09 (d, 1H, Harom, J=8.0 Hz), 7.24-7.35 (m, 3H, Harom), 7.24 (s, 1H, Harom C-4,6, J=1.9) ppm; 13C-NMR (CDCl3) δ: 27.76, 31.04, 32.84, 38.73, 45.24 (N(CH3)2), 59.02, 119.25, 119.28, 129.65, 129.88, 130.38, 130.75, 131.04, 131.18, 131.53, 135.59, 137.94, 140.69, 141.29, 142.22; Anal. Calcd. (C20H21Br2N): C 55.19, H 4.86, Br 36.72, N 3.22; Found: C 55.24, H 4.76, Br 36.68 and N 3.19%. After dehydratation of compound 11 a mixture (0.2 g, 0.44 mmol) of the respective syn- and anti-isomers of 1,7-dibromo-5-(3-N,N-dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo[a,d]-cycloheptene (3) was obtained. The pure anti-isomer was obtained by prolonged stirring in cold 85% sulfuric acid at 4 °C for 3 hours and at room temperature for 8 hours. After chromatography 0.14 g (73%), Rf=0.39 (dichloromethane/methanol 9:1) of pure 3E were obtained; IR (KBr) ν 2929, 2857, 2817, 2780, 1673, 1584, 1558, 1477, 1449, 1361, 1264 (C-N), 1231, 1160, 1128, 1041, 969, 897, 817, 790, 739, 705 cm-1; 1H-NMR (CDCl3) δ: 2.22 (s, 6H, (N(CH3)2)), 2.32-2.34 (m, 2H, CH2), 2.41-2.44 (m, 2H, CH2), 2.78-2.90 (m, 2H, Hbenzylic), 3.21-3.31 (m, 2H, Hbenzylic), 5.87-5.92 (t, 1H, C=CH, J=7.0 Hz), 6.91-7.49 (m, 6H, Harom) ppm; 13C-NMR (CDCl3) δ: 27.45, 27.59, 30.80, 31.51, 34.59, 45.04 (N(CH3)2), 58.85, 119.26, 127.22, 127.96, 129.73, 131.53, 131.89, 135.49, 135.65, 141.05, 141.25, 142.09, 142.48 ppm; Anal. Calcd. (C20H21Br2N): C 55.19, H 4.86, Br 36.72, N 3.22; Found C 55.15, H 5.01, Br 36.94 and N 3.11%.