Results and Discussion
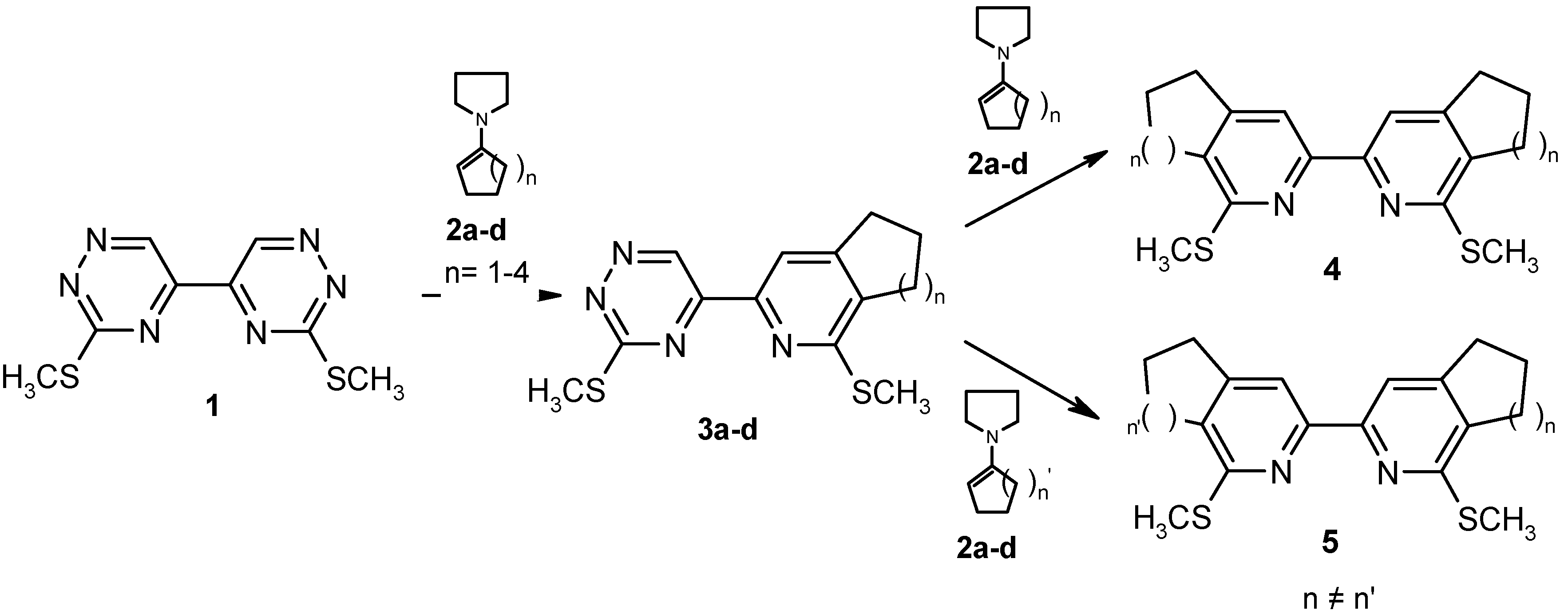
This paper describes the extension of the method to the synthesis of annulated 2,2’-bipyridine analogues
8, consisting of the two different heterocyclic units – 2,6-naphthyridine and cycloalkeno[c]pyridine (
Scheme 2). The preparation of such compounds could involve the regiospecific conversion of the parent 3,3’-bis(methylsulfanyl)-5,5’-bi-1,2,4-triazine (
1) to the single cycloaddition product
7 and the subsequent treatment of the latter with the cyclic enamine
2a,b (route
a), or alternatively, the IDA reaction of the easily accessible
3a,b with the appropriate dienophile
6 (route
b).
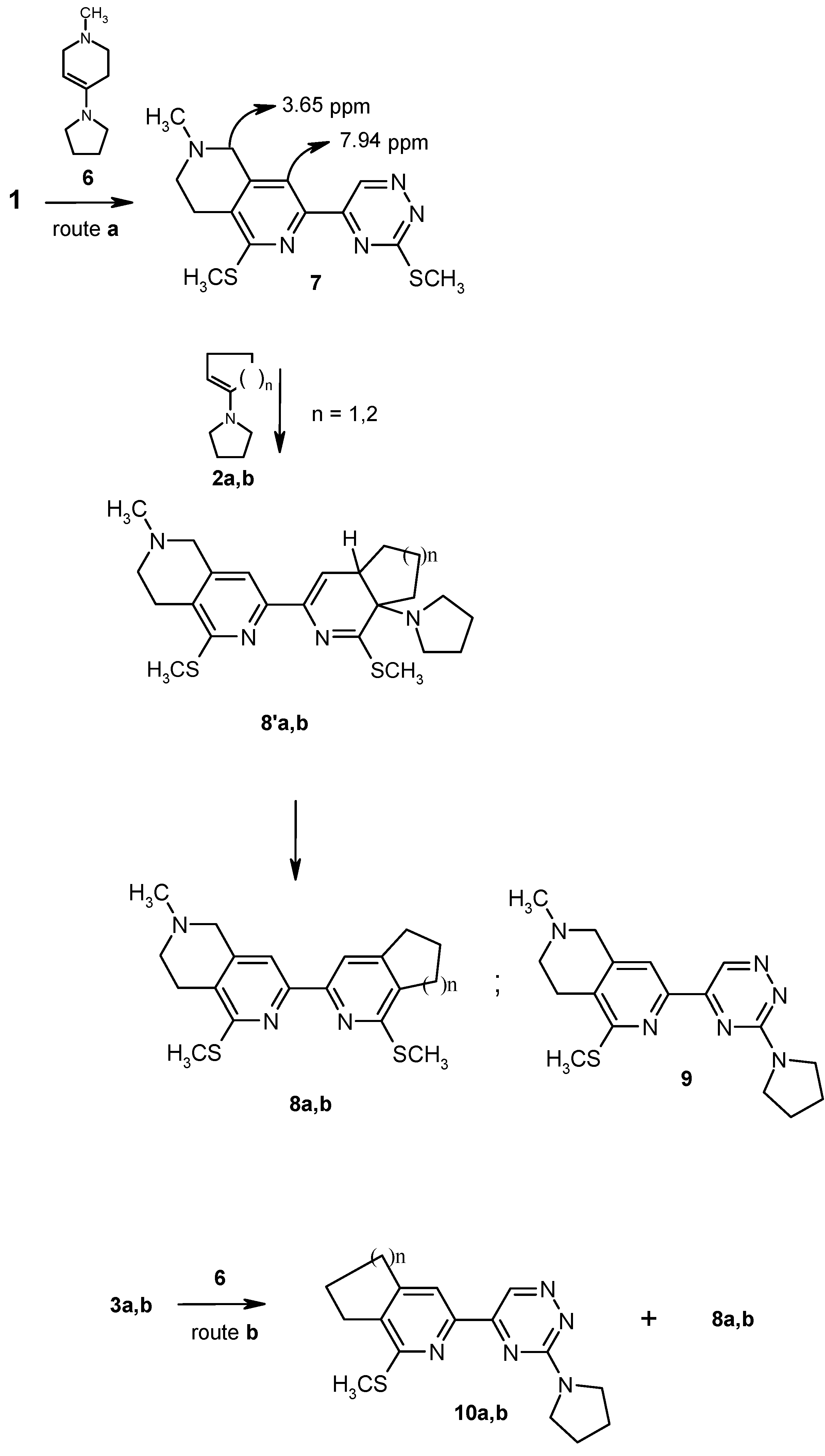
Compound
7 was obtained via the single step [4+2]cycloaddition/retro cycloaddition reaction of easily available compound
1 [
16] with 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydropyridine (
6). The reaction was carried out with 1.5 molar excess of
6 in the boiling dioxane for 1h. The product
7 precipitated from the crude reaction mixture in 92 % yield. In the reaction of
1 with
6 the formation of two isomeric 2,6- or 2,7-tetrahydronaphthyridine derivatives can be anticipated considering it involves the use of an unsymmetric enamine. However, the reaction gives only one reaction product in the excellent yield. Its
1H-NMR spectrum exhibited two signals of isolated aromatic protons at δ=9.97 and δ=7.94 ppm respectively. The former signal belongs to 1,2,4-triazine and the latter one is attributed to the pyridine hydrogen. The separate signal of isolated methylene group appears at δ=3.65, and the multiplet at δ=2.77 can only corresponds to four vicinal hydrogens in saturated pyridine ring. Nuclear Overhauser Enhancement difference spectroscopy provided an unambigous assignment for this compound. It was found that irradiation of a proton in aromatic pyridine ring (δ=7.94 ppm) led to significant NOE for the signal of the isolated methylene group in the saturated pyridine ring (δ=3.65). These results and the lack of such interactions with the multiplet at δ=2.77 provide evidence for spatial closeness of the pyridine hydrogen and CH
2 and confirm the 2,6-tetrahydronaphthyridine derivative structure of
7.
Heating
7 with an excess of 1-pyrrolidino-1-cyclopentene (
2a, n=1) at 100 ºC for 15 hours gives annulated 2,2’-bipyridine dihydroanalogue
8’a as a reaction intermediate. The latter is simply converted into
8a by heating with acetic acid in boiling toluene for 1 hour. Using less reactive 1‑pyrrolidino-1-cyclohexene (
2b, n=2) [
11] and the similar reaction conditions, the annulated 2,2’-bipiridine analogue
8b with a cyclohexene ring attached is prepared in low yield, while the corresponding pyrrolidino derivative
9 is obtained as a main product. This compound is also obtained by treatment of 3,3’-bis(methylsulfanyl)-5,5’-bi-1,2,4-triazine (
1) with an excess of
6 without solvent at 100 °C. Compound
9 is obviously formed by conventional nucleophilic replacement of methylsulfanyl group in 1,2,4-triazine part of
7. The single annulation products
3a (n=1) and
3b (n=2) undergo to small extent Diels-Alder reaction with 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydro-pyridine (
6) to yield compounds
8a and
8b in low yield. Reaction of such derivatives has been found to result in a rather nucleophilic substitution of methylsulfonyl group in
3a and
3b giving compounds
10a and
10b respectively (
Table 1). These results suggest that compound
6 is less reactive and less stable as a dienophile in comparison to cycloalkeno derived enamines
2a and
2b.
Table 1.
Yields of prepared compounds.
Table 1.
Yields of prepared compounds.
| Entry | Reaction | 8a | 8’a | 8b | 9 | 10a | 10b |
|---|
| 1 | 7 + 2a | <1% | 60% | - | - | - | - |
| 2 | 3a + 6 | 23% | 8% | - | - | 30% | - |
| 3 | 7 + 2b | - | - | 10% | 50% | - | - |
| 4 | 3b + 6 | - | - | 5% | - | - | 64% |
| 5 | 1 + 6 | - | - | - | 72% | - | - |
| 6 | 8’a+HOAc | 98% | - | - | - | - | - |
In order to help us better characterize the cycloaddition reactions of
3a,b with
6 as well as cyclic enamines
2a,b with
7, we calculated the energy differences (∆E) between the LUMO of diene and HOMO of dienophile, using the AM1 semiempirical method [
17]. The results are presented in
Table 2. A better orbital overlap should be obtained between the HOMO orbital of cyclic enamines
2a,b and the LUMO orbital of the
7 than in combination of 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydropyridine
6 with
3a,b because the energy gaps are smaller and range from 0.02 to 0.05 eV.
Table 2.
Energy Values of the HOMO of Dienophiles Calculated by the AM1 Semiempirical method, and LUMOdiene – HOMOdienophile Energy Differences (∆E)
Table 2.
Energy Values of the HOMO of Dienophiles Calculated by the AM1 Semiempirical method, and LUMOdiene – HOMOdienophile Energy Differences (∆E)
| Dienophile EHOMO (eV) | Comp. | ∆E |
|---|
| 6: -8.2212 | | (ELUMO diene 7a – EHOMO dienophile 2a,b) | (ELUMO diene 3a,b – EHOMO dienophile 6) |
| 2a: -8.0790 | 8a | 7.1193 | 7.1491 |
| 2b: -8.0823 | 8b | 7.1226 | 7.1740 |
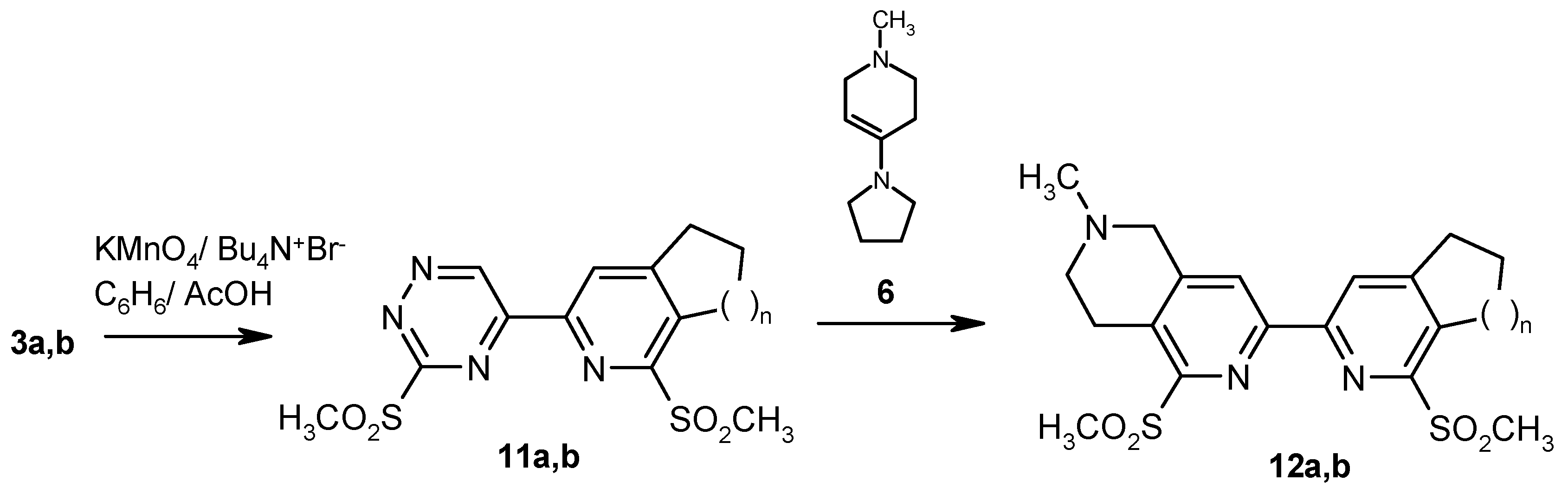
From the above data it is clear that compounds
3a,b are not suitable intermediates for the synthesis of bipyridine derivatives
8a,b. It is well known however that introduction of electron-withdrawing substiuents increases deficiency in the 1,2,4-triazine ring and enhances its reactivity in Diels-Alder reaction [
12]. We have therefore explored the reaction between
6 and methylsulfonyl derivatives
11a,b, easily available by oxidation of
3a,b with KMnO
4 under phase transfer catalysis conditions [
18] (
Scheme 3). The reaction of
11a,b with heterocyclic enamine
6 is complete within 1 hour at room temperature leading to unsymmetrical annulated 2,2’-bipyridine analogues
12a and
12b in good yield (
Scheme 3).
Experimental
General
Melting points are uncorrected. IR spectra were measured with a Magna IR-760 spectrophotometer. The
1H-NMR spectra were recorded in deuteriochloroform on a Varian-Gemini 200 MHz spectrometer. Mass spectra were measured with an AMD 604 (AMD Intectra GmbH, Germany). Column chromatography was performed on silica gel (230-400 mesh, 60 Merck). All solvents used were dried and distilled according to standard procedures [
21]. Merck 60F
254 plates were used for analytical (TLC) chromatography. 3-Methylsulfanyl-1,2,4-triazine (
1) [
22], 1-methyl-sulfonyl-3-(3-methylsulfonyl-1,2,4-triazin-5-yl)-6,7-dihydro-5H-cyclopenta[c]pyridine (
11a) and 1‑methylsulfonyl-3-(3-methyl-sulfonyl-1,2,4-triazin-5-yl)-5,6,7,8-tetrahydroisioquinoline (
11b)[
20], 1‑methyl-4-pyrrolidin-1-yl-1,2,3,4-tetra-hydropyridine (
6) and 1-pyrrolidino-1-cyclopentene (
2a) and 1‑pyrrolidino-1-cyclohexene (
2b) [
23] were prepared according to literature methods. Naphthyridine derivatives
8a,b and
12a,b are air sensitive and decompose upon standing.
2-Methyl-5-methylsulfanyl-7-(3-methylsulfanyl-1,2,4-triazin-5-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (7): A solution of 1 (1.25 g, 4.96 mmol) and 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydropyridine (6, 1.2 g, 7.23 mmol) in dry dioxane (20 mL) was refluxed for 1 h. The solvent was evaporated under reduced pressure and the residue was recrystallized from ethanol-water to give 1.4 g (92 %) of 7 as an orange solid. M. p. 205-206 ºC; IR (KBr) γ cm-1 1165, 1440, 1540, 2800, 2960; 1H-NMR δ: 2.42 (s, 3H), 2.60-2.90 (m, 4H), 2.65 (s, 3H), 2.80 (s, 3H), 3.65 (s, 2H), 7.94 (s, 1H), 9.97 (s, 1H); HRMS (EI): m/z calc. for C14H17N5S2 (M+): 319.09253, found 319.09604.
2-Methyl-5-methylsulfanyl-7-(3-pyrrolidin-1-yl-1,2,4-triazin-5-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (9): A mixture of 3,3’-bis(methylsulfanyl)-5,5’-bi-1,2,4-triazine (1, 0.50 g, 1.98 mmol) and 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydropyridine (6, 3 mL) was heated at 100 ºC for 16 h. After cooling the mixture was purified by column chromatography using first chloroform and next chloroform-acetone (50:1) to give 0.69 g (72 %) of 9 as a yellow solid. M. p. 205-206 ºC; IR (KBr) γ cm-1 1251, 1485, 1538, 2873, 2942; 1H-NMR δ: 2.03-2.07 (m, 4H), 2.47 (s, 3H), 2.66 (s, 3H), 2.76 (s, 4H), 3.60 (s, 2H), 3.74 (broad s, 4H), 7.84 (s, 1H), 9.54 (s, 1H); HRMS (EI): m/z calc for C17H22N6S (M+): 342.16267, found 342.16317.
2-Methyl-5-methylsulfanyl-7-(1-methylsulfanyl-6,7-dihydro-5H-cyclopenta[c]pyridin-3-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (8a) and 2-methyl-5-methylsulfanyl-7-(1-methylsulfanyl-7a-pyrrolidin-1-yl-5,6,7,7a-tetrahydro-4aH-cyclopenta[c]pyridin-3-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (8’a):
The synthesis of compound 8a was accomplished by two different routes (a and b, respectively):
Route a: A mixture of 7 (0.36 g, 1.18 mmol) and 2a (2.5 mL) was heated at 100 ºC for 16 h. After cooling the mixture was purified by column chromatography using first chloroform and next chloroform-acetone (50:1) to give 8a and 8’a (0.30 g, 60 %). The mixture of the crude dihydro derivative 8’a (0.20g, 0.47 mmol), toluene (10 mL) and HOAc (1mL) was refluxed for 1 h. After cooling the precipitated solid was filtered off and recrystallized from ethanol to give 0.16 g (98 %) of 8a as a yellow solid.
8a: M. p. 209-211 ºC; IR (KBr) γ cm-1 1200, 1404, 1576, 2766, 2945; 1H-NMR δ: 2.17 (qui, 2H, J=7.4 Hz), 2.48 (s, 3H), 2.70 (s, 6H), 2.78 (s, 4H), 2.83 (t, 2H, J=7.5 Hz), 3.02 (t, 2H, J=7.5 Hz), 3.62 (s, 2H), 7.84 (s, 1H), 8.06 (s, 1H); HRMS (EI): m/z calc for C19H23N3S2 (M+): 357.13334, found 357.13433.
8’a: M. p. 170-171 ºC; IR (KBr) γ cm-1 1240, 1303, 1575, 2803, 2947; 1H-NMR δ: 1.54-1.60 (m, 2H), 1.66-1.71 (m, 5H), 2.09-2.35 (m, 4H), 2.45 (s, 3H), 2.49 (s, 4H), 2.62 (s, 3H), 2.51-2.75 (m, 4H), 2.72 (m, 3H), 3.55 (s, 2H), 6.91 (d, 1H, J=6.1 Hz), 7.51 (s, 1H); HRMS (EI): m/z calc for C23H32N4S2 (M+): 428.20684, found 428.20728.
Route b: A mixture of 3a (0.26 g, 0.89 mmol) and 6 (2 mL) was heated at 100 ºC for 16 h. After cooling the mixture was purified by column chromatography using chloroform and next chloroform: acetone (50:1) to give 8a (0.08 g, 23 %) and 1-methylsulfanyl-3-(3-pyrrolidin-1-yl-1,2,4-triazin-5-yl)-6,7-dihydro-5H-cyclopenta[c]pyridine (10a, 0.085 g, 30 %). M. p. 220-221ºC; IR (KBr) γ cm-1 1289, 1396, 1534, 2870, 2942; 1H-NMR δ: 2.0-2.1 (m, 4H), 2.09 (qui, 2H, J=7.4 Hz), 2.68 (s, 3H), 2.88 (t, 2H, J=6.1 Hz), 2.98 (t, 2H, J=6.0 Hz), 3.74 (broad s, 4H), 8.05 (s, 1H), 9.54 (s, 1H); HRMS (EI): m/z calc for C16H20N5S (M+ H) calculated 314.1434 found 314.1443.
2-Methyl-5-methylsulfanyl-7-(1-methylsulfanyl-5,6,7,8-tetrahydroisoquinolin-3-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (8b):
The synthesis of compound 8b has been accomplished by two different routes (a or b).
Route a: A mixture of 7 (0.36 g, 1.18 mmol) and 2b (2.5 mL) was heated at 100 ºC for 16 h. After cooling the mixture was purified by column chromatography using first chloroform and next chloroform-acetone (50:1) to give 8b (0.042 g, 10 %) and 9 (0.19 g, 50%).
8b: M. p. 210-212 ºC; IR (KBr) γ cm-1 1292, 1377, 1573, 2766, 2947; 1H-NMR δ: 1.78-1.90 (m, 4H), 2.48 (s, 3H), 2.59-2.65 (t, 2H, J=6.1Hz), 2.67 (s, 3H), 2.69 (s, 3H), 2.77 (s, 4H), 2.80 (t, 2H, J=6.0 Hz), 3.62 (s, 2H), 7.83 (s, 1H), 7.86 (s, 1H); HRMS (EI): m/z calc for C20H25N3S2 (M+): 371.14899, found 371.14830.
Route b: A mixture of 3b (0.29 g, 0.95 mmol) and 6 (2 mL) was heated at 100 ºC for 32h. After cooling the mixture was purified by column chromatography using first chloroform and next chloroform-acetone (50:1) to give 8b (0.001 g, 5 %) and 1-methylsulfanyl-3-(3-pyrrolidin-1-yl-1,2,4-triazin-5-yl)-5,6,7,8-tetrahydroisoquinoline (10b, 0.02 g, 64 %). M. p. 131-132 ºC; IR (KBr) γ cm-1 1289, 1396, 1534, 2870, 2942; 1H-NMR δ: 1.79-1.92 (m, 4H), 2.03-2.17 (m, 4H), 2.62 (t, 2H, J=6.1 Hz), 2.66 (s, 3H), 2.80 (t, 2H, J=6.0 Hz), 3.75 (broad s, 4H), 7.86 (s, 1H), 9.55 (s, 1H); HRMS (EI): m/z calc for C17H21N5S (M+): 327.15177, found 327.15139.
2-Methyl-5-methylsulfonyl-7-(1-methylsulfonyl-6,7-dihydro-5H-cyclopenta[c]pyridin-3-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (12a): A mixture of 11a (1.24 g, 3.5 mmol) and 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydro-pyridine (6, 1.16 g, 6.98 mmol) in dioxane (16 mL) was stirred at room temperature for 1 h. The crude product was purified by column chromatography using chloroform-acetone (10:1) as eluent, to give 0.83 g (56 %) of 12a as a yellow solid. M. p. 199-200 ºC; IR (KBr) γ cm-1 1200, 1404, 1576, 2766, 2945; 1H-NMR δ: 1.95-2.1 (m, 2H), 2.24 (qui, 2H, J=7.5 Hz), 2.49 (s, 3H), 2.77 (t, 2H, J=7.5 Hz), 3.07 (t, 2H, J=7.6 Hz), 3.37 (s, 3H), 3.39-3.48 (m, 2H), 3.48 (s, 3H), 3.73 (s, 2H), 8.20 (s, 1H), 8.32 (s, 1H); HRMS (LSIMS): m/z calc for C19H24O4N3S2 (M+H): 422.12082, found 422.12017.
2-Methyl-5-methylsulfonyl-7-(1-methylsulfonyl-5,6,7,8-tetrahydroisoquinolin-3-yl)-1,2,3,4-tetrahydro-2,6-naphthyridine (12b): A mixture of 11b (0.56 mg, 1.54 mmol) and 1-methyl-4-pyrrolidin-1-yl-1,2,3,6-tetrahydro-pyridine (6, 0.52 g, 3.08 mmol) in dioxane (16 mL) was stirred at room temperature for 0.5 h under nitrogen. The solvent was evaporated under reduced pressure. The crude product was purified by column chromatography using chloroform-acetone (10:1) as eluent, to give 0.58 g (62 %) of 12b as a yellow solid. M. p. 285-286 ºC; IR (KBr) γ cm-1 1128, 1302, 1586, 2931, 3011; 1H‑NMR δ: 1.78-1.90 (m, 6H), 2.50 (s, 3H), 2.78 (t, 2H, J=5.9 Hz), 2.90-3.00 (m, 2H), 3.25-3.40 (m, 2H), 3.48 (s, 6H), 3.76 (s, 2H), 8.06 (s, 1H), 8.10 (s, 1H); HRMS (LSIMS): m/z calc for C20H26O4N3S2 (M+ H): 436.13647, found 436.13755.