Addition to Electron Deficient Olefins of α-Oxy Carbon- Centered Radicals, Generated from Cyclic Ethers and Acetals by the Reaction with Alkylperoxy- λ3-iodane
Abstract
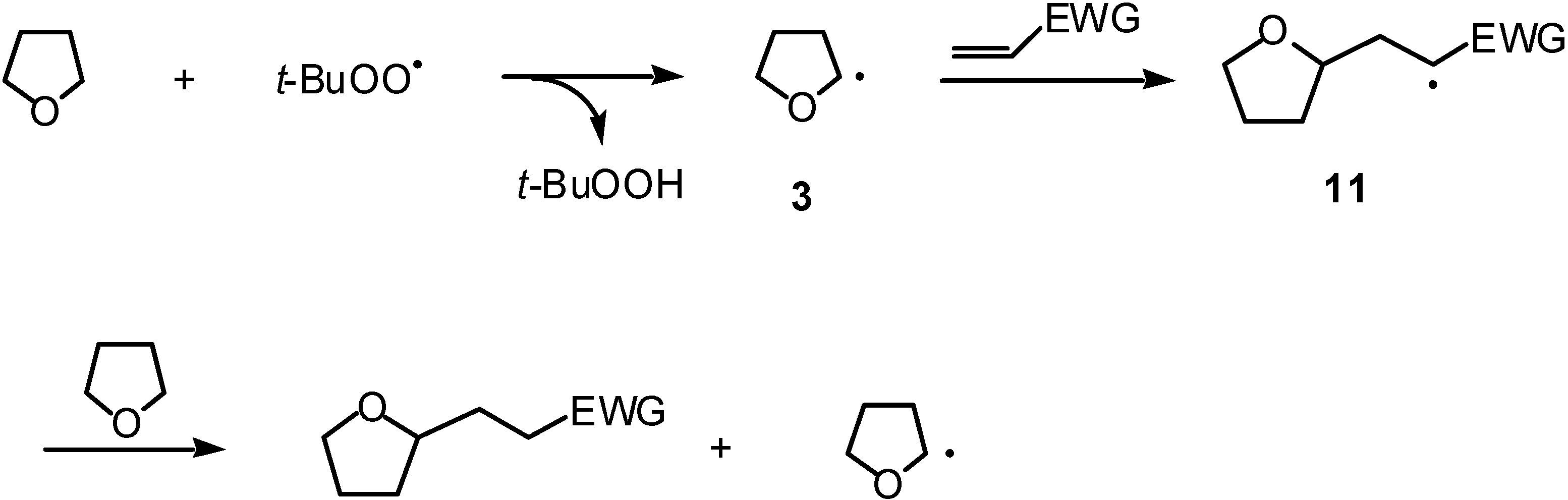
:Introduction
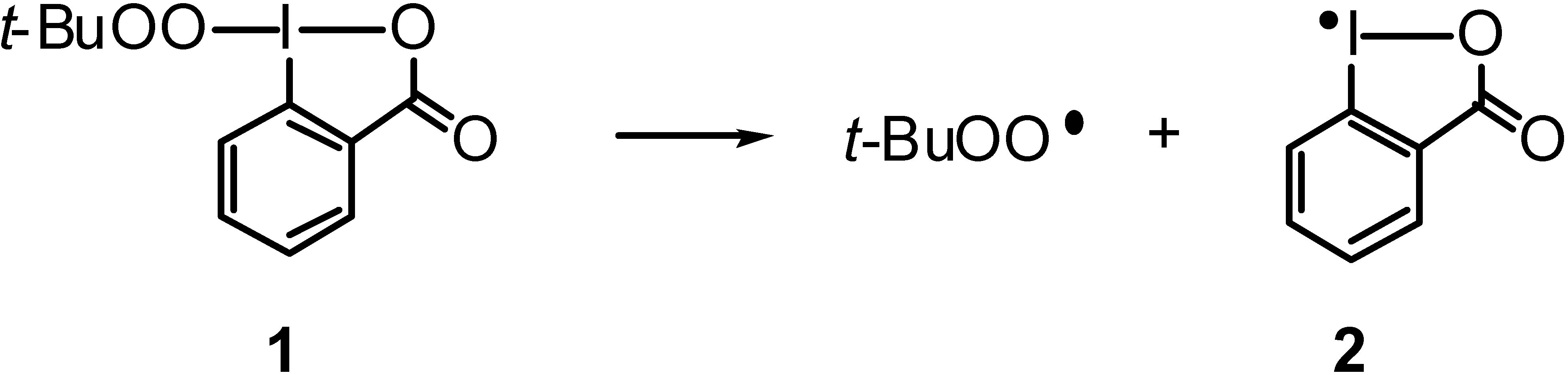
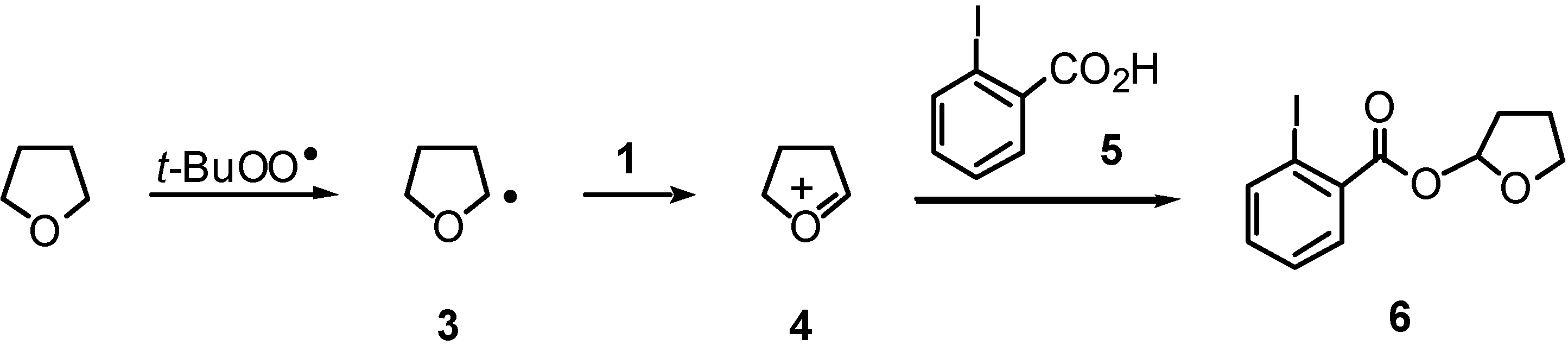
Results and Discussion
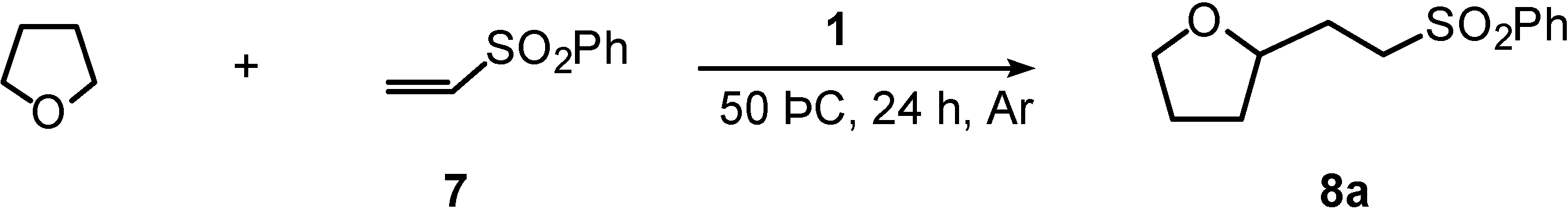
| Entry | λ3-Iodane 1 (equiv) | Ether | Product | ||
|---|---|---|---|---|---|
| Structure | 8 | Yield (%) b | |||
| 1 | 0.3 | THF |  | 8a | 66 |
| 2 | 1 | THF |  | 8a | 83 |
| 3 | 1 | THP |  | 8b | 50 |
| 4 | 0.3 | 1,3-dioxolane |  | 8c | 76 |
| 5 | 1 | 1,4-dioxane |  | 8d | 52 |
| 6 | 1 c | 1,3-dioxolane |  | 8e | 0 |
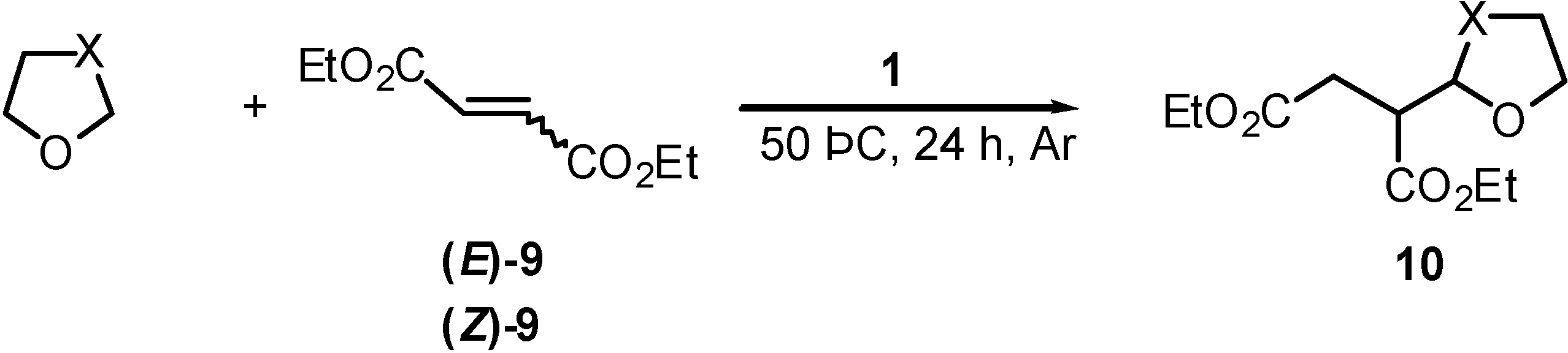
| Entry | Olefin | Ether | Product | |
|---|---|---|---|---|
| 10 | Yield (%) b | |||
| 1 | (E)-9 | THF | 10a | 47 (53) c |
| 2 | (Z)-9 | THF | 10a | 61 (70) c |
| 3 | (E)-9 | 1,3-dioxolane | 10b | 81 |
| 4 | (Z)-9 | 1,3-dioxolane | 10b | 88 |
Experimental
General
General Procedure for Addition of Cyclic Ethersto Phenyl Vinyl Sulfone (2). A Typical Example: 2-[2-(Phenylsulfonyl)ethyl]tetrahydrofuran (8a) (Table 1, Entry 2).
General Procedure for Addition of Cyclic Ethers to Unsaturated Ester (9). A Typical Example: Diethyl (Tetrahydrofuran-2-yl)succinate (10a) (Table 2, Entry 1).
References
- For reviews, see: Ochiai, M. Topics in Current Chemistry; Wirth, T., Ed.; Springer: Berlin, 2003; Vol. 224, pp. 5–68. [Google Scholar] Ochiai, M. TCI Mail 1999, No. 104. 2–11. Zhdankin, V.V. Rev. Heteroatom Chem. 1997, 17, 133–151. Zhdankin, V.V.; Stang, P.J. Chem. Rev. 2002, 102, 2523–2584. Muraki, T.; Togo, H.; Yokoyama, M. Rev. Heteroatom Chem. 1997, 17, 213–243.
- Ochiai, M.; Nakanishi, A.; Ito, T. J. Org. Chem. 1997, 62, 4253–4259. Ochiai, M.; Kajishima, D.; Sueda, T. Heterocycles 1997, 46, 71–76. Ochiai, M.; Kajishima, D.; Sueda, T. Tetrahedron Lett. 1999, 40, 5541–5544.
- Ochiai, M.; Nakanishi, A.; Yamada, A. Tetrahedron Lett. 1997, 38, 3927–3930.
- Ochiai, M.; Ito, T.; Takahashi, H.; Nakanishi, A.; Toyonari, M.; Sueda, T.; Goto, S.; Shiro, M. J. Am. Chem. Soc. 1996, 118, 7716–7730. Ochiai, M.; Ito, T.; Masaki, Y.; Shiro, M. J. Am. Chem. Soc. 1992, 114, 6269–6270.
- Ochiai, M.; Sueda, T. Tetrahedron Lett. 2004, 45, 3557–3559.
- Rosenthal, I.; Elad, D. Tetrahedron 1967, 23, 3193–3204.
- Matthews, D.P.; McCarthy, J.R. J. Org. Chem. 1990, 55, 2973–2975. Boivin, J.; Crepon, E.; Zard, S.Z. Bull. Soc. Chim. Fr. 1992, 129, 145–150. Togo, H.; Aoki, M.; Yokoyama, M. Chem. Lett. 1992, 2169–2172. Togo, H.; Aoki, M.; Yokoyama, M. Tetrahedron 1993, 49, 8241–8256.
- Shtarev, A.B.; Tian, F.; Dolbier, W.R.; Smart, B.E. J. Am. Chem. Soc. 1999, 121, 7335–7341. Malatesta, V.; Ingold, K.U. J. Am. Chem. Soc. 1981, 103, 609–614. Malatesta, V.; Scaiano, J.C. J. Org. Chem. 1982, 47, 1455–1459.
- Sueda, T.; Fukuda, S.; Ochiai, M. Org. Lett. 2001, 3, 2387–2390.
- Watanabe, Y.; Tsuji, Y.; Takeuchi, R. Bull. Chem. Soc. Jpn. 1983, 56, 1428–1430. Tsujimoto, S.; Sakaguchi, S.; Ishii, Y. Tetrahedron Lett. 2003, 44, 5601–5604. Hirano, K.; Sakaguchi, S.; Ishii, Y. Tetrahedron Lett. 2002, 43, 3617–3620. Rosenthal, I.; Elad, D. J. Org. Chem. 1968, 33, 805–811. Kim, Y.H.; Yang, S.G. Rev. Heteroatom Chem. 1999, 20, 69–96.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Sueda, T.; Takeuchi, Y.; Suefuji, T.; Ochiai, M. Addition to Electron Deficient Olefins of α-Oxy Carbon- Centered Radicals, Generated from Cyclic Ethers and Acetals by the Reaction with Alkylperoxy- λ3-iodane. Molecules 2005, 10, 195-200. https://doi.org/10.3390/10010195
Sueda T, Takeuchi Y, Suefuji T, Ochiai M. Addition to Electron Deficient Olefins of α-Oxy Carbon- Centered Radicals, Generated from Cyclic Ethers and Acetals by the Reaction with Alkylperoxy- λ3-iodane. Molecules. 2005; 10(1):195-200. https://doi.org/10.3390/10010195
Chicago/Turabian StyleSueda, T., Y. Takeuchi, T. Suefuji, and M. Ochiai. 2005. "Addition to Electron Deficient Olefins of α-Oxy Carbon- Centered Radicals, Generated from Cyclic Ethers and Acetals by the Reaction with Alkylperoxy- λ3-iodane" Molecules 10, no. 1: 195-200. https://doi.org/10.3390/10010195




