Empirical Data Confirm Autism Symptoms Related to Aluminum and Acetaminophen Exposure †
Abstract
:1. Introduction
2. Aluminum and Mercury in Vaccines
Mechanisms of Aluminum and Mercury Toxicity
3. Related Work
3.1. MMR Vaccine
3.2. Other Related Work
4. Our Studies with U.S. CDC VAERS Database
| Symptom | C1 | C2 | p-value |
|---|---|---|---|
| Anxiety | 49 | 2 | 0.011 |
| Constipation | 41 | 0 | 0.012 |
| Infection | 54 | 6 | 0.013 |
| Ear infection | 32 | 3 | 0.029 |
| Eczema | 18 | 0 | 0.044 |
| Premature | 20 | 1 | 0.046 |
| Asthma | 24 | 3 | 0.048 |
| Pneumonia | 19 | 1 | 0.050 |
4.1. Distribution of Vaccine Types in Autism versus Controls
| Pathogen | Percent Autism | Percent Not Autism | Ratio |
|---|---|---|---|
| MMR | 40.94 | 15.35 | 2.67 |
| Hep-B | 16.02 | 8.71 | 1.84 |
| HiB Titer | 15.02 | 8.40 | 1.80 |
| DTaP | 42.53 | 43.93 | 0.97 |
| Polio | 15.60 | 16.34 | 0.96 |
| Varicella | 15.77 | 16.68 | 0.95 |
| Pneumonia | 8.81 | 10.29 | 0.86 |
| Rotavirus | 0.25 | 3.29 | 0.076 |
| Total | 154.94 | 122.99 | 1.26 |

4.2. Relationship between Aluminum in Vaccines and Symptoms
| Symptom | C1 Before 2000 | C2 After 2000 | p-value | C1w/ Al+3 | C2 w/o Al+3 | p-value |
|---|---|---|---|---|---|---|
| Seizure | 636 | 3468 | 0.0000 | 2350 | 1023.2 | 0.00028 |
| Injection Site Reaction | 1961 | 4605 | 1.0E-8 | 3851 | 2584 | 0.000061 |
| Infection | 195 | 1552 | 1.0E-8 | 1358 | 927 | 0.0026 |
| Swelling | 8621 | 13218 | 1.0E-8 | 11406 | 8470 | 0.0000026 |
| Pain | 8153 | 12122 | 6.0E-8 | 8576 | 7099 | 0.00044 |
| Cellulitis | 760 | 1977 | 0.000001 | 2087 | 1089 | 0.000024 |
| Depression | 57 | 322 | 0.00023 | 334 | 143 | 0.0031 |
| Death | 210 | 558 | 0.0040 | 483 | 303 | 0.011 |
| Fatigue | 1222 | 1839 | 0.00080 | 1744 | 968 | 0.00011 |
| Insomnia | 81 | 195 | 0.0089 | 230 | 71 | 0.0025 |

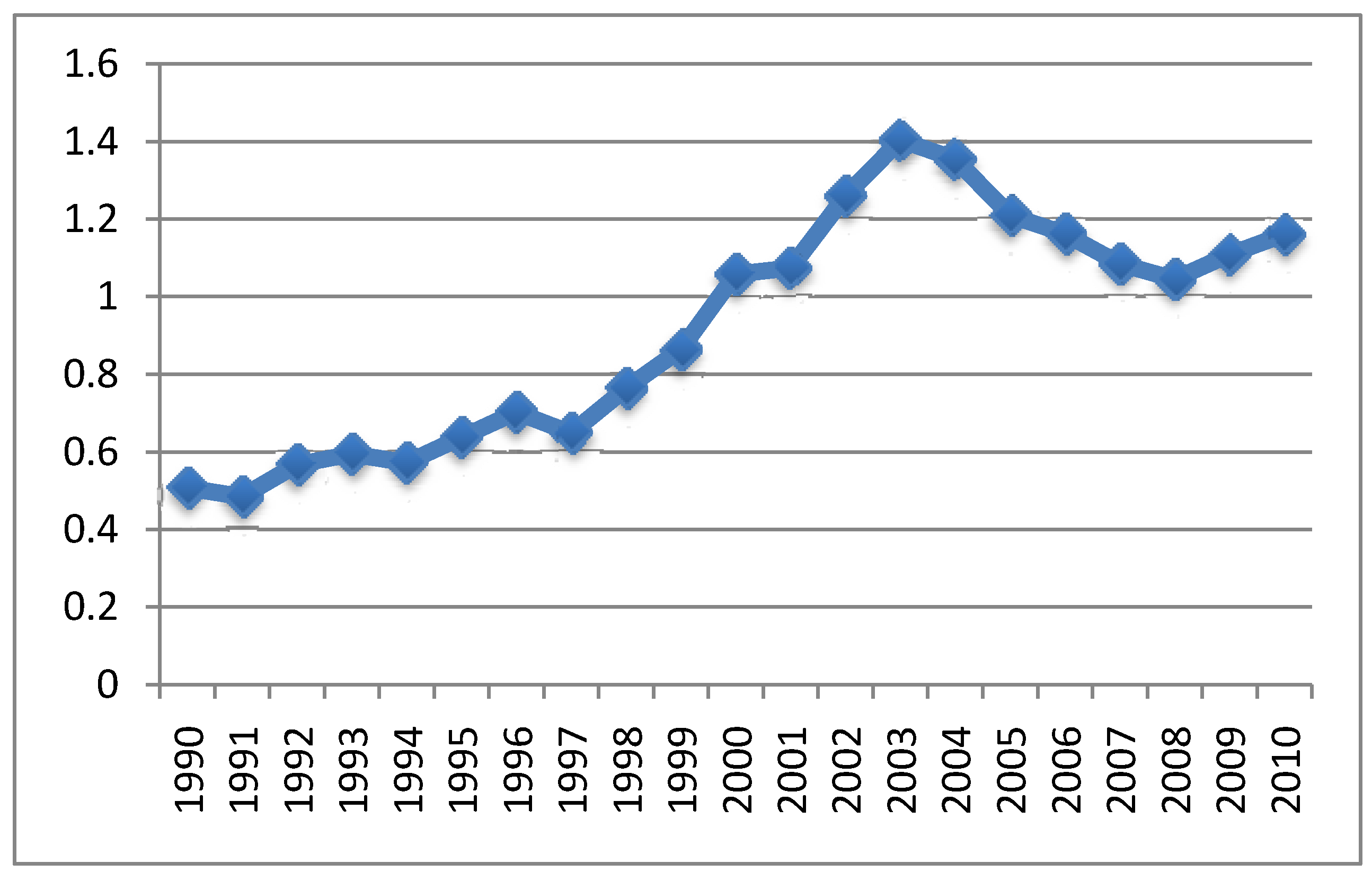
4.3. MMR Vaccine and Autism
| Symptom | C1 MMR | C2 not MMR | p-value |
|---|---|---|---|
| Rash | 2197 | 745 | 3.0E-8 |
| Chicken pox | 311 | 23 | 6.6E-5 |
| Mumps | 217 | 0 | 0.00012 |
| Face Oedema | 232 | 28 | 0.00036 |
| Measles | 59 | 4 | 0.0089 |
| Autism | 168 | 58 | 0.0067 |
| Cough | 191 | 90 | 0.014 |
| Fever | 1840 | 1584 | 0.024 |
| Hematoma | 52 | 12 | 0.026 |
| Conjunctivitis | 42 | 7 | 0.027 |
| Lymph Node Pain | 25 | 0 | 0.028 |
| Respiratory Infection | 50 | 16 | 0.042 |
| Blister | 327 | 232 | 0.043 |
4.4. Hepatitis B Vaccine and Autism
| Symptom | C1 Hep-B | C2 not Hep-B | p-value | C1w/ Al3+ | C2w/o Al3+ | p-value |
|---|---|---|---|---|---|---|
| Rash | 818 | 299 | 4.2E-5 | 11649 | 11109 | - |
| chicken pox | 80 | 3 | 0.0038 | 523 | 1152 | - |
| Autism | 108 | 1 | 0.0014 | 556 | 443 | 0.06 |
| Macule | 163 | 75 | 0.016 | 4702 | 3098 | 0.000016 |
| Cellulitis | 56 | 6 | 0.012 | 2087 | 1089 | 0.000024 |
| Blister | 188 | 53 | 0.0030 | 4275 | 3066 | 0.00015 |
| Seizure | 179 | 115 | 0.051 | 3331 | 2350 | 0.00028 |
| Infection | 78 | 12 | 0.0085 | 1358 | 927 | 0.0026 |
| Abscess | 74 | 26 | 0.029 | 1205 | 918 | 0.012 |
| Death | 38 | 6 | 0.030 | 483 | 303 | 0.011 |
| Low appetite | 32 | 2 | 0.025 | 368 | 252 | 0.031 |
4.5. Limitations of the VAERS Database and the Experiments
5. Discussion
6. Conclusion
Acknowledgements
References
- Dawson, G.; Toth, K.; Abbott, R.; Osterling, J.; Munson, J.; Estes, A.; Liaw, J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 2004, 40, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Wills, S.; van de Water, J. The immune response in autism: a new frontier for autism research. J. Leukoc. Biol. 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Castellani, M.L.; Conti, C.M.; Kempuraj, D.J.; Salini, V.; Vecchiet, J.; Tete, S.; Ciampoli, C.; Conti, F.; Cerulli, G.; Caraffa, A.; et al. Autism and immunity: Revisited study. Int. J. Immunopathol. Pharmacol. 2009, 22, 15–19. [Google Scholar] [PubMed]
- Ratajczak, H.V. Theoretical aspects of autism: Causes—a review. J. Immunotoxicol. 2011, 8, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Oller, J.W., Jr. The antithesis of entropy: Biosemiotic communication from genetics to human language with special emphasis on the immune systems. Entropy 2010, 12, 631–705. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Croen, L.A.; Daniels, J.; Giarelli, E.; Grether, J.K.; Levy, S.E.; Mandell, D.S.; Miller, L.A.; Pinto-Martin, J.; Reaven, J.; et al. The epidemiology of autism spectrum disorders. Annu. Rev. Publ. Health 2007, 28, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Baio, J. Prevalence of Autism Spectrum Disorders Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008; Morbidity and Mortality Weekly Report; Centers for Disease Control and Prevention: Atlanta, GA, 2012. [Google Scholar]
- Oller, J.W., Jr.; Oller, S.D. Autism: The Diagnosis, Treatment, and Etiology of the Undeniable Epidemic; Jones and Bartlett Publishers: Sudbury, MA, USA, 2010. [Google Scholar]
- Stankovic, M.; Lakic, A.; Ilic, N. Autism and autistic spectrum disorders in the context of new DSM-V classification, and clinical and epidemiological data. Srp. Arh. Celok. Lek. 2012, 140, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Russo, J.P.; Yang, S.; Roohi, J.; Blaxill, M.; Kahler, S.G.; Cremer, L.; Hatchwell, E. Autism and environmental genomics. Neurotoxicology 2006, 27, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Law, P.; Law, J.K.; Rosenberg, R.E.; Anderson, C.; Samango-Sprouse, C. Immunization beliefs and practices among autism families. In Presented at International Meeting for Autism Research, Philadelphia, PA, USA, 21 May 2010.
- Meldgaard, M.K.; Hviid, A.; Vestergaard, M.; Schendel, D.; Wohlfahrt, J.; Thorsen, P.; Olsen, J.; Melbye, M. A population-based study of measles, mumps, and rubella vaccination and autism. N. Engl. J. Med. 2002, 347, 1477–1482. [Google Scholar]
- Campion, E.W. Suspicions about the safety of vaccines. N. Engl. J. Med. 2002, 347, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- DeLong, G. A positive association found between autism prevalence and childhood vaccination uptake across the U.S. population. J. Toxicol. Env. Health A 2011, 74, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Davidson, R.; Mascitelli, L. Might cholesterol sulfate deficiency contribute to the development of autistic spectrum disorder? Med. Hypotheses 2012, 8, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Fuda, H.; Yanai, H.; Lee, Y.; Fukushige, T.; Kanzaki, T.; Strott, C.A. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. J. Invest. Dermatol. 2004, 122, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla-Bernardina, B.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Coloso, R.M.; Garcia, R.A.; Banks, M.F. Cysteine concentration regulates cysteine metabolism to glutathione, sulfate and taurine in rat hepatocytes. J. Nutr. 1992, 122, 420–427. [Google Scholar] [PubMed]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.A.; Nata, R.; Geier, M.R. Biomarkers of environmental toxicity and susceptibility in autism. J. Neurol. Sci. 2009, 280, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.A.; Geier, M.R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem. Res. 2009, 34, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Horan, F.E.; Hirsch, F.G.; Wood, L.A.; Wright, I.S. Surface effects on blood-clotting components as determined by zeta-potentials. J. Clin. Invest. 1950, 29, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M.; Seneff, S. The Initial Common Pathway of Inflammation, Disease, and Sudden Death. Entropy 2012, 14, 1399–1442. [Google Scholar] [CrossRef]
- Dai, G.; Chou, N.; He, L.; Gyamfi, M.A.; Mendy, A.J.; Slitt, A.L.; Klaassen, C.D.; Wan, Y.-J.Y. Retinoid X receptor alpha regulates the expression of glutathione S-transferase genes and modulates acetaminophen-glutathione conjugation in mouse liver. Mol. Pharmacol. 2005, 68, 1590–1596. [Google Scholar] [PubMed]
- Coughtrie, M.W.; Bamforth, K.J.; Sharp, S.; Jones, A.L.; Borthwick, E.B.; Barker, E.V.; Roberts, R.C.; Hume, R.; Burchell, A. Sulfation of endogenous compounds and Xenobiotics: Interactions and function in health and disease. Chem. Biol. Interact. 1994, 92, 247–256. [Google Scholar] [CrossRef]
- Schnell, R.C.; Park, K.S.; Davies, M.H.; Merrick, B.A.; Weir, S.W. Protective effects of selenium on acetaminophen-induced hepatotoxicity in the rat. Toxicol. Appl. Pharmacol. 1988, 95, 1–11. [Google Scholar] [CrossRef]
- Damodaran, M.; Priya, L.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar]
- Becker, K.G.; Schultz, S.T. Similarities in features of autism and asthma and a possible link to acetaminophen use. Med. Hypotheses 2010, 74, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.G. Autism, asthma, inflammation, and the hygiene hypothesis. Med. Hypotheses 2007, 69, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Magalhäes, E.S.; Pinto-Mariz, F.; Bastos-Pinto, S.; Pontes, A.T.; Prado, E.A.; de Azevedo, L.C. Immune allergic response in Asperger syndrome. J. Neuroimmunol. 2009, 216, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Hanyu, O.; Fuda, H.; Strott, C.A. Novel role of cholesterol sulfate in gene regulation during skin development. FASEB J. 2008, 22, 782. [Google Scholar]
- Presland, R.B. Function of filaggrin and caspase-14 in formation and maintenance of the epithelial barrier. Dermatol. Sinica 2009, 27, 1–14. [Google Scholar]
- Palmer, C.N.; Ismail, T.; Lee, S.P.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Smith, F.J.; McLean, W.H.; Mukhopadhyay, S. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J. Allergy Clin. Immunol. 2007, 120, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Schuttelaar, M.L.A.; Kerkhof, M.; Jonkman, M.F.; Koppelman, G.H.; Brunekreef, B.; de Jongste, J.C.; Wijga, A.; McLean, W.H.I.; Postma, D.S. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy 2009, 64, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Roma, M.G.; Bernal, C.A.; de Lujan Alvarez, M.; Carrillo, M.C. Biliary secretory function in rats chronically intoxicated with aluminum. Toxicol. Sci. 2004, 79, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Sjövall, J. Bile acids: analysis in biological fluids and tissues. J. Lipid Res. 2010, 51, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Lee, Y.-T.; Hsieh, H.-S.; Hwang, D.-F. Effect of taurine on toxicity of aluminum in rats. E Spen Eur. E J. Clin. Nutr. Metab. 2009, 4, e187–e192. [Google Scholar] [CrossRef]
- Siri, K.; Lyons, T. Cutting-Edge Therapies for Autism 2011–2012; Skyhorse Publishing: New York, NY, USA, 2011; p. 74. [Google Scholar]
- Adams, J.B.; Bara, M.; Geis, E.; Mitchell, J.; Ingram, J.; Hensley, A.; Zappia, I.; Newmark, S.; Gehn, E.; Rubin, R.A.; et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: Part A-Medical results. BMC Pharmacol. Toxicol. 2009, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Bara, M.; Geis, E.; Mitchell, J.; Ingram, J.; Hensley, A.; Zappia, I.; Newmark, S.; Gehn, E.; Rubin, R.A.; et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: Part B-Behavioral results. BMC Clin. Pharmacol. 2009, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J. Autism and vitamin D. Med. Hypotheses. 2008, 70, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, J.C.; March, J.L.; Häner, M.; Aebi, U. Effect of aluminum and other multivalent cations on neurofilaments in vitro: An electron microscopic study. J. Struct. Biol. Mar. 1990, 103, 2–12. [Google Scholar] [CrossRef]
- Joshi, J.G. Neurochemical hypothesis: participation by aluminum in producing critical mass of colocalized errors in brain leads to neurological disease. Comp. Biochem. Physiol. C 1991, 100, 103–105. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B. Hypothesis: Neuro-inflammation, blood-brain barrier, seizures and autism. J. Neuroinflamm. 2011, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Ault, K.A.; Giuliano, A.R.; Wheeler, C.M.; Koutsky, L.A.; Malm, C.; Lehtinen, M.; et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005, 6, 271–278. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; de Carvalho, N.S.; et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Verstraeten, T.; Descamps, D.; David, M.P.; Zahaf, T.; Hardt, K.; Izurieta, P.; Dubin, G.; Breuer, T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008, 26, 6630–6638. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.K.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Redhead, K.; Quinlan, G.J.; Das, R.G.; Gutteridge, J.M. Aluminium-adjuvanted vaccines transiently increase aluminium levels in murine brain tissue. Pharmacol. Toxicol. 1992, 70, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.L.; Hintz, S.R.; Mackenzie, N.I.; Kerner, J.A., Jr. Aluminum exposure from pediatric parenteral nutrition: meeting the new FDA regulation. J. Parenter. Enteral. Nutr. 2008, 32, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L. Aluminum and Alzheimer's disease: after a century of controversy, is there a plausible link? J. Alzheimers Dis. 2011, 23, 567–598. [Google Scholar] [PubMed]
- MacLeod, M.K.L.; McKee, A.S.; David, A.; Wang, J.; Mason, R.; Kapplera, J.W.; Marrack, P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7914–7919. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’-autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Siesjö, P.; Eriksson, H. The immunobiology of aluminium adjuvants: How do they really work? Trends Immunol. 2010, 31, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wittayanukulluk, A.; Jiang, D.; Regnier, F.E.; Hem, S.L. Effect of microenvironment pH of aluminum hydroxide adjuvant on the chemical stability of adsorbed antigen. Vaccine 2004, 22, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Blaxill, M.F.; Redwood, L.; Bernard, S. Thimerosal and autism? A plausible hypothesis that should not be dismissed. Med. Hypotheses 2004, 62, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Thimerosal in vaccines: A joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR Morb. Mortal Wkly. Rep. 1999, 48, 563–565. [Google Scholar]
- Bohrer, D.; do Nascimento, P.C.; Binotto, R.; Becker, E. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part III: Interaction container-chemicals during the heating for sterilisation. J. Trace Elem. Med. Biol. 2003, 17, 107–115. [Google Scholar] [CrossRef]
- Cuthbertson, B.; McBay, W.E.; Welch, A.G.; Perry, R.J.; Foster, P.R. Aluminium and human albumin solutions. Brit. Med. J. 1987, 295, 1062. [Google Scholar] [CrossRef]
- Zatta, P.; Alfrey, A.C. Aluminium Toxicity in Infants' Health and Disease; World Scientific Publishers: Singapore, 1997; p. 192. [Google Scholar]
- Wills, M.R.; Savory, J. Water content of aluminum, dialysis dementia, and osteomalacia. Environ. Health Persp. 1985, 63, 141–147. [Google Scholar] [CrossRef]
- Pogue, A.I.; Li, Y.Y.; Cui, J.-G.; Zhao, Y.; Kruck, T.P.A.; Percy, M.E.; Tarr, M.A.; Lukiw, W.J. Characterization of an NF-jB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J. Inorg. Biochem. 2009, 103, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Jones, B.M.; Bhattacharjee, S.; Percy, M.E.; Zhao, Y.; Lukiw, W.J. Metal-sulfate induced generation of ROS in human brain cells: Detection using an isomeric mixture of 5- and 6-carboxy-2′,7′-dichlorofluoresce in diacetate (carboxy-DCFDA) as a cell permeant tracer. Int. J. Mol. Sci. 2012, 13, 9615–9626. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Spinetti, P.R.; Tedeschi, A. Water dynamics at the root of metamorphosis in living organisms. Water 2010, 2, 566–586. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Magnetobiology: the kT paradox and possible solutions. Electromagn. Biol. Med. 2007, 26, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Aimo, L.; Oteiza, P.I. Aluminium and lead: molecular mechanisms of brain toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.Y. Calmodulin plays a pivotal role in cellular regulation. Science 1980, 207, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bü̈sselberg, D.; Platt, B.; Haas, H.L.; Carpenter, D.O. Voltage gated calcium channel currents of rat dorsal root ganglion (DRG) cells are blocked by A13+. Brain Res. 1993, 622, 163–168. [Google Scholar] [CrossRef]
- Siegel, N.; Haug, A. Aluminum interaction with calmodulin. Evidence for altered structure and function from optical and enzymatic studies. Biochim. Biophys. Acta 1983, 744, 36–45. [Google Scholar] [CrossRef]
- Lemire, J.; Appanna, V.D. Aluminum toxicity and astrocyte dysfunction: A metabolic link to neurological disorders. J. Inorg. Biochem. 2011, 105, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Mailloux, R.; Puiseux-Dao, S.; Appanna, V.D. Aluminum-induced defective mitochondrial metabolism perturbs cytoskeletal dynamics in human astrocytoma cells. J. Neurosci. Res. 2009, 87, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Janssen, B.; Buys, E.; Sips, P.; Brouckaert, P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J. Clin. Invest. 2006, 116, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, H.C.; Poe, M.; Gerig, G.; Smith, R.G.; Provenzale, J.; Ross, A.; Gilmore, J.; Piven, J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch. Gen. Psychiatry 2005, 62, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E. Brain development in autism: Early overgrowth followed by premature arrest of growth. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Miñana, M.-D.; Montoliu, C.; Saez, R.; Corbalán, R.; Manzo, L.; Felipo, V. Prenatal exposure to aluminum reduces expression of neuronal nitric oxide synthase and of soluble guanylate cyclase and impairs glutamatergic neurotransmission in rat cerebellum. J. Neurochem. 1999, 73, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of Serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar] [PubMed]
- Rezwan, K.; Meier, L.P.; Rezwan, M.; Vörös, J.; Textor, M.; Gauckler, L.J. Bovine serum albumin adsorption onto colloidal Al2O3 particles: A new model based on Zeta potential and UV-Vis measurements. Langmuir 2004, 20, 10055–10061. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Nordberg, G.F.; Sager, P.R. Reproductive and developmental toxicity of metals. Scand. J. Work Environ. Health 1985, 11, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Burbacher, T.M.; Shen, D.D.; Liberato, N.; Grant, K.S.; Cernichiari, E.; Clarkson, T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ. Health Perspect. 2005, 113, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Chian, D.; Lipkin, W.I. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol. Psychiatr. 2004, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Chaitua, N. Effects of divalent metals on the isolated rat glomerulus. Toxicology 1990, 61, 119–133. [Google Scholar] [CrossRef]
- Wakefield, A.J. MMR vaccination and autism. Lancet 1999, 354, 949–950. [Google Scholar] [CrossRef]
- Madsen, K.M.; Lauritsen, M.B.; Pedersen, C.B.; Thorsen, P.; Plesner, A.M.; Andersen, P.H.; Mortensen, P.B. Thimerosal and the occurrence of autism: Negative ecological evidence from Danish population-based data. Pediatrics 2003, 112, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. Overview of vaccine adjuvants: present and future. Vaccine 2002, 20, S7–S12. [Google Scholar] [CrossRef]
- Eickhoff, T.C.; Myers, M. Workshop summary Aluminum in vaccines. Vaccine 2002, 20, S1–S4. [Google Scholar] [CrossRef]
- Gallagher, O.M.; Goodman, M.S. Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997–2002. J. Toxicol. Environ. Health A 2010, 73, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Zinka, B.; Rauch, E.; Buettner, A.; Ruëff, F.; Penning, R. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine 2006, 24, 5779–5780. [Google Scholar] [CrossRef] [PubMed]
- Von Kries, R.; Toschke, A.M.; Strassburger, K.; Kundi, M.; Kalies, H.; Nennstiel, U.; Jorch, G.; Rosenbauer, J.; Giani, G. Sudden and unexpected deaths after the administration of hexavalent vaccines (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, Haemophilius influenzae type b): Is there a signal? Eur. J. Pediatr. 2005, 164, 61–69. [Google Scholar]
- Traversa, G.; Spila-Alegiani, S.; Bianchi, C.; degli Atti, M.C.; Frova, L.; Massari, M.; Raschetti, R.; Salmaso, S.; Scalia Tomba, G. Sudden unexpected deaths and vaccinations during the first two years of life in Italy: A case series study. PLoS One 2011, 6, e16363. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, R.; Hecker, H.; Poethko-Müller, C.; Schlaud, M.; Vennemann, M.; Whitaker, H.J.; Farrington, C.P. A modified self-controlled case series method to examine association between multidose vaccinations and death. Stat. Med. 2011, 30, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.; Gold, M.S.; Kemp, A.S.; McIntyre, P.B.; Burgess, M.A.; Campbell-Lloyd, S. Anaphylaxis following quadrivalent human papillomavirus vaccination. Can. Med. Assoc. J. 2008, 179, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pourcyrous, M.; Korones, S.B.; Kristopher, L.A.; Bada, H.S. Primary immunization of premature infants with gestational age < 35 weeks: Cardiorespiratory complications and C-reactive protein responses associated with administration of single and multiple separate vaccines simultaneously. J. Pediatr. 2007, 151, 167–171. [Google Scholar] [PubMed]
- Goldman, G.S.; Miller, N.Z. Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990-2010. Hum. Exp. Toxicol. 2012, 31, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.R.; Geier, D.A. Hepatitis B vaccination safety. Ann. Pharmacother. 2002, 36, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Swarbrick, L.; Gherardi, R.K.; Authier, F.-J. A role for the body burden of aluminium in vaccine-associated macrophagic myofasciitis and chronic fatigue syndrome. Med. Hypotheses 2009, 72, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T. Accurate methods for the statistics of surprise and coincidence. Comp. Ling. 1993, 19, 61–74. [Google Scholar]
- Liu, J.; Li, A.; Seneff, S. Automatic drug side effect discovery from online patient-submitted reviews: Focus on statin drugs. In Proceedings of First International Conference on Advances in Information Mining and Management (I.M.M.M.), Barcelona, Spain, 23–29 October, 2011.
- Afzal, N.; Murch, S.; Thirrupathy, K.; Berger, L.; Fagbemi, A.; Heuschkel, R. Constipation with acquired megarectum in children with autism. Pediatrics 2003, 112, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Gillott, A.; Furniss, F.; Walter, A. Anxiety in high-functioning children with autism. Autism 2001, 5, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.V.; Mandell, W. Consequence of low birth weight. Dev. Psychol. 1970, 3, 363–383. [Google Scholar] [CrossRef]
- Lin, B.; Kubushiro, K.; Akiba, Y.; Cui, Y.; Tsukazaki, K.; Nozawa, S.; Iwamori, M. Alteration of acidic lipids in human sera during the course of pregnancy: Characteristic increase in the concentration of cholesterol sulfate. J. Chromatogr. B 1997, 704, 99–104. [Google Scholar] [CrossRef]
- Harumi, J.; Sun, S.; Le, H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. Neuroimmunology 2001, 120, 170–179. [Google Scholar]
- Schultz, S.T.; Klonoff-Cohen, H.S.; Wingard, D.L.; Akshoomoff, N.A.; Macera, C.A.; Ji, M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder: The results of a parent survey. Autism 2008, 12, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A. Acetaminophen may mediate oxidative stress and neurotoxicity in autism. Med. Hypotheses 2012, 78, 351–351. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Desilva, M.; Gu, T.T.; Qiang, M.; Whang, K. Effects of the Analgesic Acetaminophen (Paracetamol) and its para-Aminophenol Metabolite on Viability of Mouse-Cultured Cortical Neurons. Basic Clin. Pharmacol. Toxicol. 2011, 110, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation deficit in ‘low-functioning’ autistic children: A pilot study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Thinktwice Global Vaccine Institute. Available online: www.thinktwice.com (accessed on 30 July 2012).
- Centers for Disease Control. Recommended immunization schedules for persons aged 0–18 years―United States, 2010. MMWR Morb. Mortal Wkly. Rep. 2009, 58, 1–4. [Google Scholar]
- Centers for Disease Control. Preventing pneumococcal disease among infants and young children. MMWR Morb. Mortal Wkly. Rep. 2000, 49, 1–38. [Google Scholar]
- Haley, B.E. Mercury toxicity: Genetic susceptibility and synergistic effects. Medical Veritas 2005, 2, 535–542. [Google Scholar] [CrossRef]
- National Vaccine Information Center, Hepatitis B vaccine: The untold story. Avaiable online: http://www.nvic.org/nvic-archives/newsletter/untoldstory.aspx (accessed on 10 October 2012).
- Scott, H.D.; Thacher-Renshaw, A.; Rosenbaum, S.E.; Waters, W.J., Jr.; Green, M.; Andrews, L.G.; Faich, G.A. Physician reporting of adverse drug reactions. Results of the Rhode Island Adverse Drug Reaction Reporting Project. J. Am. Med. Assoc. 1990, 263, 1785–1788. [Google Scholar] [CrossRef]
- Tomljenovic, L.; Shaw, C.A. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 2012, 21, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fido, A.; Al-Saad, S. Toxic trace elements in the hair of children with autism. Autism. 2005, 9, 290–298. [Google Scholar] [PubMed]
- Holmes, A.S.; Blaxill, M.F.; Haley, B.E. Reduced levels of mercury in first baby haircuts of autistic children. Int. J. Toxicol. 2003, 22, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Holloway, C.E.; George, F.; Quig, D. Analyses of toxic metals and essential minerals in the hair of arizona children with autism and associated conditions, and their mothers. Biol. Trace Elem. Res. 2006, 110, 193–208. [Google Scholar] [CrossRef]
- Rowland, I.; Davies, M.; Evans, J. Tissue content of mercury in rats given methylmercury chloride orally: Influence of intestinal flora. Arch. Environ. Health 1980, 35, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Seneff, S. A dialogue system for accessing drug reviews. In Proceedings of Automatic Speech Recognition and Understanding Workshop (ASRU), Waikoloa, HI, USA, December 2011; pp. 324–329.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Seneff, S.; Davidson, R.M.; Liu, J. Empirical Data Confirm Autism Symptoms Related to Aluminum and Acetaminophen Exposure. Entropy 2012, 14, 2227-2253. https://doi.org/10.3390/e14112227
Seneff S, Davidson RM, Liu J. Empirical Data Confirm Autism Symptoms Related to Aluminum and Acetaminophen Exposure. Entropy. 2012; 14(11):2227-2253. https://doi.org/10.3390/e14112227
Chicago/Turabian StyleSeneff, Stephanie, Robert M. Davidson, and Jingjing Liu. 2012. "Empirical Data Confirm Autism Symptoms Related to Aluminum and Acetaminophen Exposure" Entropy 14, no. 11: 2227-2253. https://doi.org/10.3390/e14112227
APA StyleSeneff, S., Davidson, R. M., & Liu, J. (2012). Empirical Data Confirm Autism Symptoms Related to Aluminum and Acetaminophen Exposure. Entropy, 14(11), 2227-2253. https://doi.org/10.3390/e14112227





