Institut de Recherches sur la Catalyse et l’Environnement de Lyon, IRCELYON, UMR 5256, Université Claude Bernard-Lyon 1, Bat Chevreul, 43 Bd du 11 Novembre 1918, 69622 Villeurbanne, France
Catalysts 2018, 8(7), 265; https://doi.org/10.3390/catal8070265 - 29 Jun 2018
Cited by 9 | Viewed by 3739
Abstract
The two first surface elementary steps of a gas/solid catalytic reaction are the adsorption/desorption at least one of the reactants leading to its adsorption equilibrium which can be or not disturbed by the others surface elementary steps leading to the products. The variety
[...] Read more.
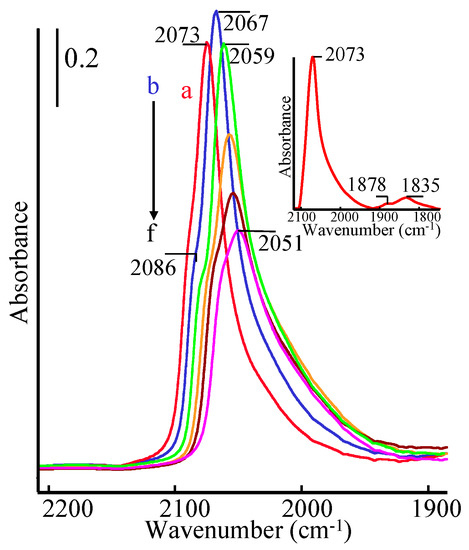
The two first surface elementary steps of a gas/solid catalytic reaction are the adsorption/desorption at least one of the reactants leading to its adsorption equilibrium which can be or not disturbed by the others surface elementary steps leading to the products. The variety of the sites of a conventional catalyst may lead to the formation of different coadsorbed species such as linear, bridged and threefold coordinated species for the adsorption of CO on supported metal particles. The aim of the present article is to summarize works performed in the last twenty years for the development and applications of an analytical method named Adsorption Equilibrium InfraRed spectroscopy (AEIR) for the measurement of the individual heats of adsorption of coadsorbed species and for the validation of mathematical expressions for their adsorption coefficients and adsorption models. The method uses the evolution of the IR bands characteristic of each of coadsorbed species during the increase in the adsorption temperature in isobaric conditions. The presentation shows that the versatility of AEIR leads to net advantages as compared to others conventional methods particularly in the context of the microkinetic approach of catalytic reactions.
Full article
(This article belongs to the Special Issue Multiscale and Innovative Kinetic Approaches in Heterogeneous Catalysis)
▼
Show Figures