Molecules 2018, 23(1), 18; https://doi.org/10.3390/molecules23010018 - 22 Dec 2017
Cited by 8 | Viewed by 4585
Abstract
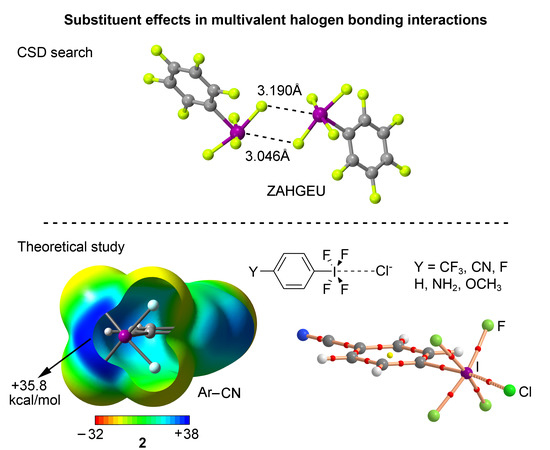
In this manuscript, we combined ab initio calculations (RI-MP2/def2-TZVPD level of theory) and a search in the CSD (Cambridge Structural Database) to analyze the influence of aromatic substitution in charge-assisted multivalent halogen bonding complexes. We used a series of benzene substituted iodine derivatives
[...] Read more.
In this manuscript, we combined ab initio calculations (RI-MP2/def2-TZVPD level of theory) and a search in the CSD (Cambridge Structural Database) to analyze the influence of aromatic substitution in charge-assisted multivalent halogen bonding complexes. We used a series of benzene substituted iodine derivatives C6H4(IF4)Y (Y = H, NH2, OCH3, F, CN, and CF3) as Lewis acids and used Cl− as electron rich interacting atoms. We have represented the Hammett’s plot and observed a good regression coefficient (interaction energies vs. Hammett’s σ parameter). Additionally, we demonstrated the direct correlation between the Hammett’s σ parameter and the value of molecular electrostatic potential measured at the I atom on the extension of the C–I bond. Furthermore, we have carried out AIM (atoms in molecules) and NBO (natural bonding orbital) analyses to further describe and characterize the interactions described herein. Finally, we have carried out a search in the CSD (Cambridge Structural Database) and found several X-ray structures where these interactions are present, thus giving reliability to the results derived from the calculations.
Full article
(This article belongs to the Special Issue Halogen Bonds and Beyond)
►
Show Figures