Multipoint-Detection Strain Sensor with a Single Electrode Using Optical Ultrasound Generated by Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
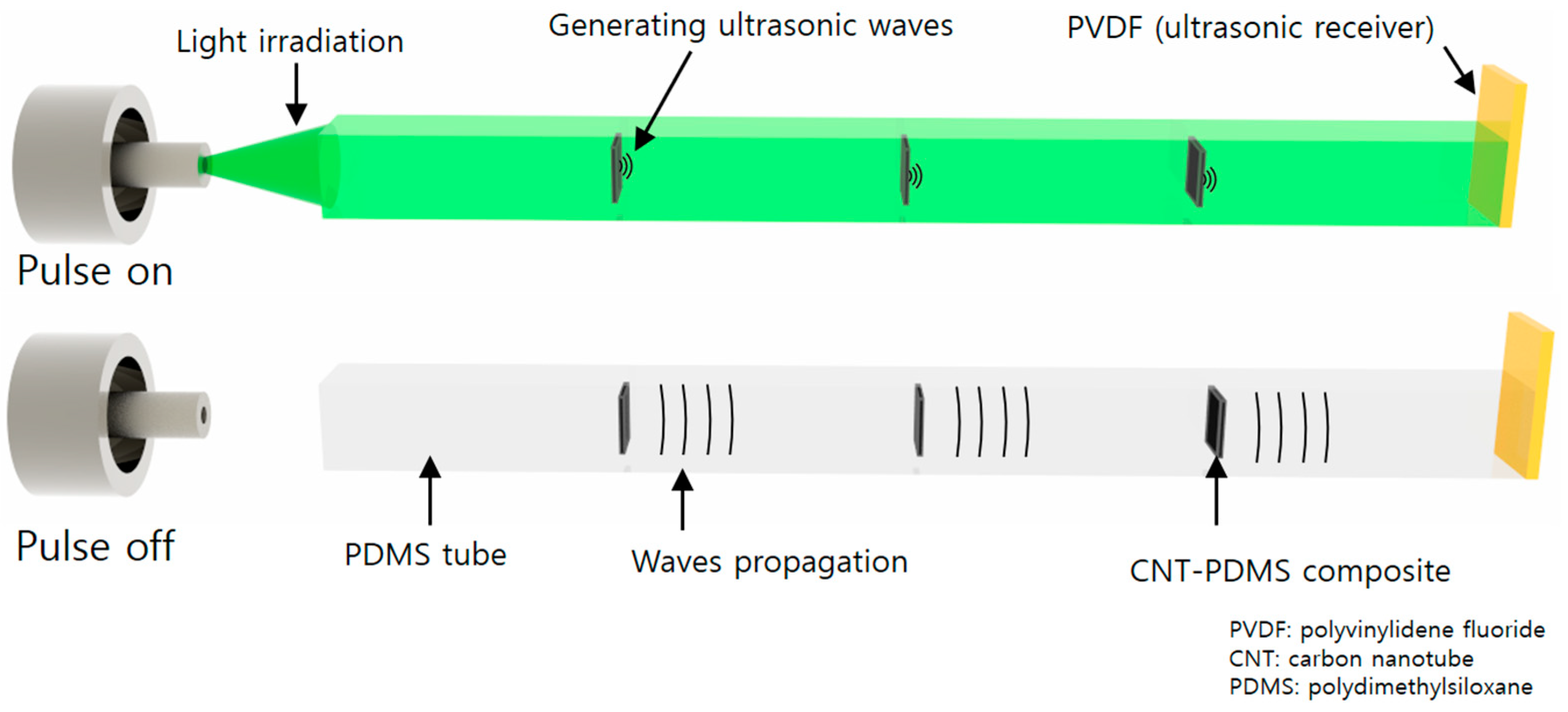
2.1. Operational Principle of the Photoacoustic Strain Sensor
2.2. Fabrication of the Tube-Type CNT Strain Sensor
2.3. Fabrication and Characterization of the CNT–PDMS Composite
2.4. Experimental Setup
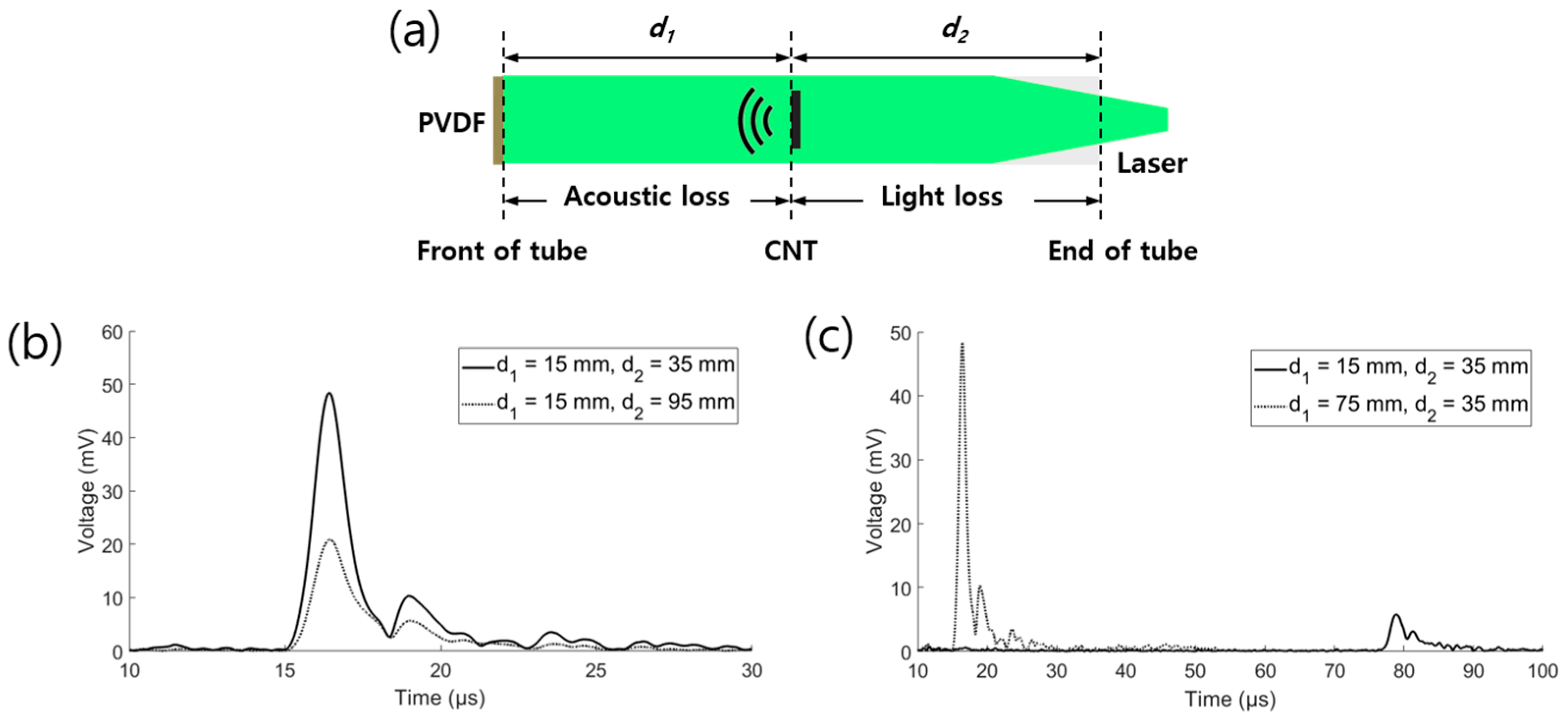
2.5. Empirical Analysis of the Energy Loss
3. Results and Discussion
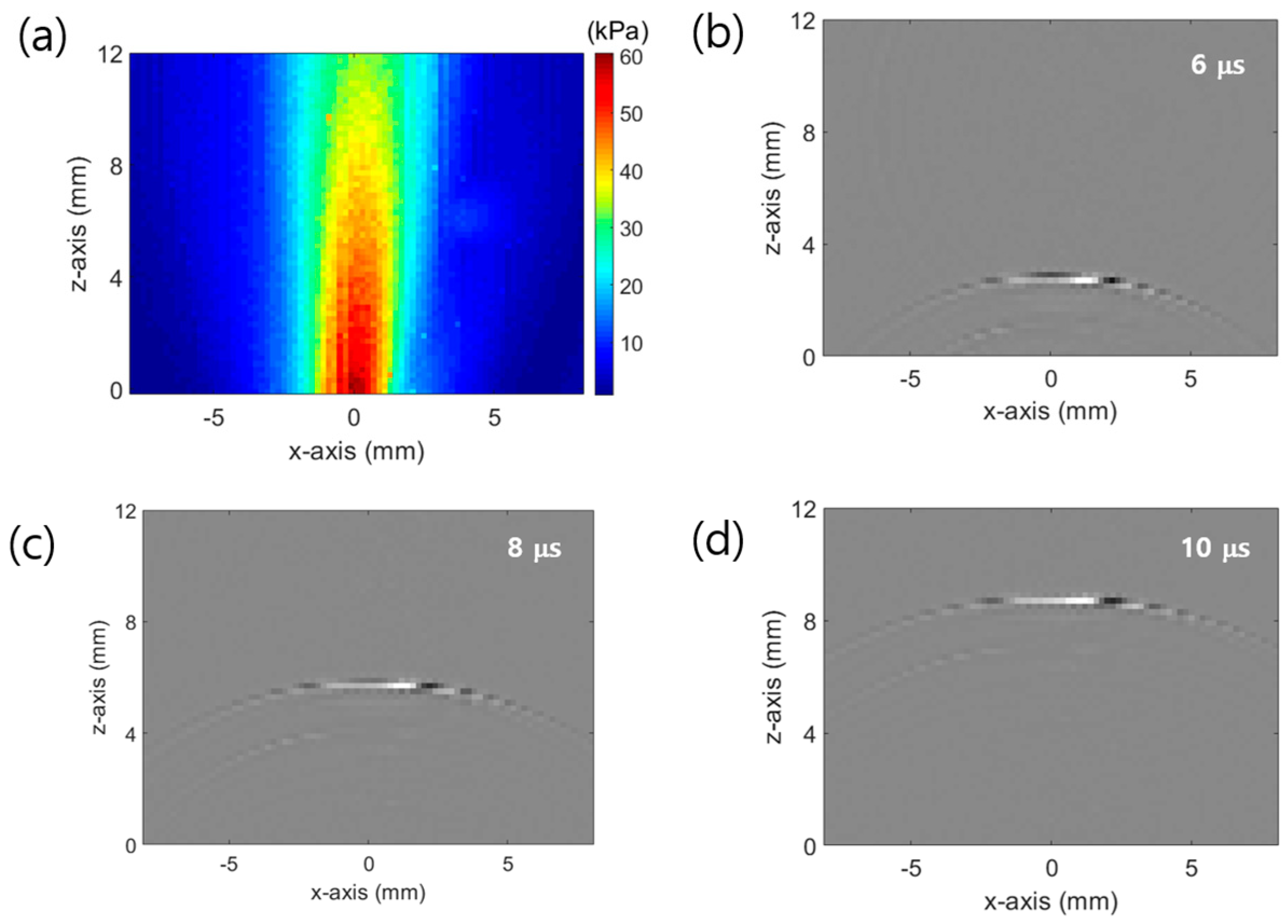
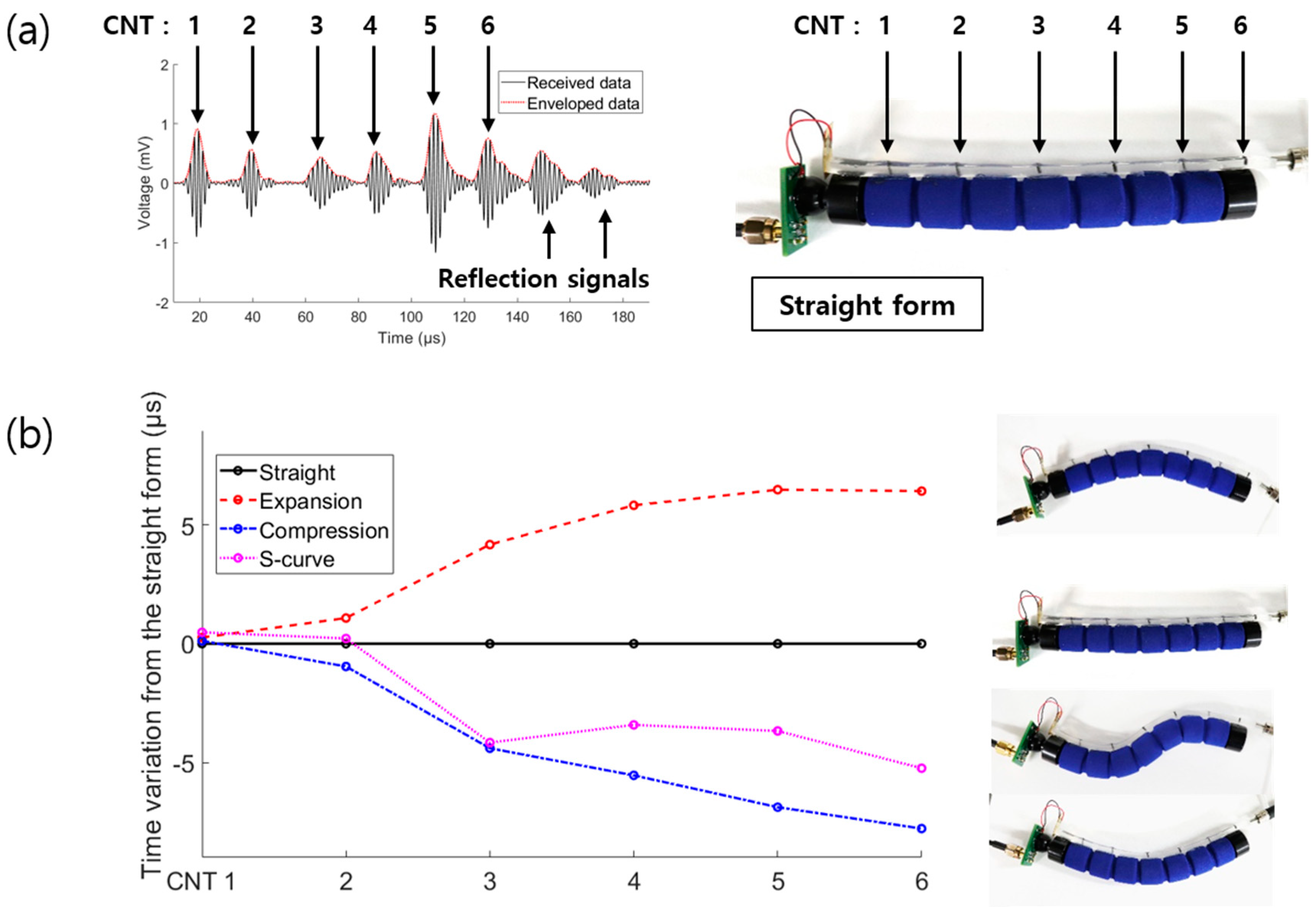
3.1. Multipoint Expansion Experiment
3.2. Glove Bending Experiment
3.3. Flexible Phantom Bending Experiment
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Lorussi, F.; Tognetti, A.; Tesconi, M.; Pastacaldi, P.; De Rossi, D. Strain sensing fabric for hand posture and gesture monitoring. Stud. Health Technol. Inf. 2004, 108, 266–270. [Google Scholar] [CrossRef]
- Carbonaro, N.; Mura, G.D.; Lorussi, F.; Paradiso, R.; De Rossi, D.; Tognetti, A. Exploiting wearable goniometer technology for motion sensing gloves. IEEE J. Biomed. Health Inf. 2014, 18, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hua, Q.; Yu, R.; Zhang, Y.; Pan, C. Flexible, stretchable and wearable multifunctional sensor array as artificial electronic skin for static and dynamic strain mapping. Adv. Electron. Mater. 2015, 1, 1500142. [Google Scholar] [CrossRef]
- Chuang, W.C.; Lin, H.T.; Chen, W.L. Strengthening of back muscles using a module of flexible strain sensors. Sensors 2015, 15, 3975–3987. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, M.; Mei, T.; McCoul, D.; Qin, S.; Zhao, Z.; Zhao, J. Wearable stretch sensors for motion measurement of the wrist joint based on dielectric elastomers. Sensors 2017, 17, 2708. [Google Scholar] [CrossRef] [PubMed]
- Mattmann, C.; Clemens, F.; Tröster, G. Sensor for measuring strain in textile. Sensors 2008, 8, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Seyedin, S.; Zhang, P.; Naebe, M.; Qin, S.; Chen, J.; Wang, X.; Razal, J.M. Textile strain sensors: a review of the fabrication technologies, performance evaluation and applications. Mater. Horiz. 2019, 6, 219–249. [Google Scholar] [CrossRef]
- Park, Y.L.; Chen, B.R.; Pérez-Arancibia, N.O.; Young, D.; Stirling, L.; Wood, R.J.; Goldfield, E.C.; Nagpal, R. Design and control of a bio-inspired soft wearable robotic device for ankle-foot rehabilitation. Bioinspiration Biomim. 2014, 9, 016007. [Google Scholar] [CrossRef]
- Ding, Y.; Galiana, I.; Asbeck, A.T.; Marco, S.; De Rossi, M.; Bae, J.; Santos, R.T.; Araujo, V.L.; Lee, S.; Holt, K.G. Biomechanical and physiological evaluation of multi-joint assistance with soft exosuits. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 25, 119–130. [Google Scholar] [CrossRef]
- Takayama, T.; Takeshima, H.; Hori, T.; Omata, T. A twisted bundled tube locomotive device proposed for in-pipe mobile robot. IEEE/ASME Trans. Mechatron. 2015, 20, 2915–2923. [Google Scholar] [CrossRef]
- Ranzani, T.; Gerboni, G.; Cianchetti, M.; Menciassi, A. A bioinspired soft manipulator for minimally invasive surgery. Bioinspiration Biomim. 2015, 10, 035008. [Google Scholar] [CrossRef] [PubMed]
- Burgner-Kahrs, J.; Rucker, D.C.; Choset, H. Continuum robots for medical applications: a survey. IEEE Trans. Rob. 2015, 31, 1261–1280. [Google Scholar] [CrossRef]
- Qiu, A.; Li, P.; Yang, Z.; Yao, Y.; Lee, I.; Ma, J. A path beyond metal and silicon: polymer/nanomaterial composites for stretchable strain sensors. Adv. Funct. Mater. 2019, 29, 1806306. [Google Scholar] [CrossRef]
- Park, Y.L.; Chen, B.R.; Wood, R.J. Design and fabrication of soft artificial skin using embedded microchannels and liquid conductors. IEEE Sens. J. 2012, 12, 2711–2718. [Google Scholar] [CrossRef]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-dimensional nanonetworks for giant stretchability in dielectrics and conductors. Nat. Commun. 2012, 3, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Wang, F.X.; Li, J.J.; Peng, H.D.; Yan, J.H.; Pan, G.B. Wearable wide-range strain sensors based on ionic liquids and monitoring of human activities. Sensors 2017, 17, 2621. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, T.; Kim, D.; Park, Y.L.; Jo, S. Use of deep learning for characterization of microfluidic soft sensors. IEEE Robot. Autom. Lett. 2018, 3, 873–880. [Google Scholar] [CrossRef]
- Zhu, B.; Niu, Z.; Wang, H.; Leow, W.R.; Wang, H.; Li, Y.; Zheng, L.; Wei, J.; Huo, F.; Chen, X. Microstructured graphene arrays for highly sensitive flexible tactile sensors. Small 2014, 10, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.V.H.H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A highly elastic, capacitive strain gauge based on percolating nanotube networks. Nano Lett. 2012, 12, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shao, J.; Chen, S.; Chen, X.; Tian, H.; Li, X.; Wang, L.; Wang, D.; Lu, B. Flexible Capacitive Pressure Sensor Enhanced by Tilted Micropillar Arrays. ACS Appl. Mater. Interfaces 2019, 11, 17796–17803. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.; Lee, J.I.; Kim, M.O.; Chung, T.; Oh, Y.; Lim, S.C.; Park, J.; Kim, J. Development of a flexible three-axis tactile sensor based on screen-printed carbon nanotube-polymer composite. J. Micromech. Microeng. 2014, 24, 075012. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Yu, W.; Ziaie, B. Highly stretchable and sensitive unidirectional strain sensor via laser carbonization. ACS Appl. Mater. Interfaces 2015, 7, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, Z.; Pan, Z.J. Flexible strain sensors fabricated using carbon-based nanomaterials: a review. Curr. Opin. Solid-State Mater. Sci. 2018, 22, 213–228. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Raju, A.P.A.; Lewis, A.; Derby, B.; Young, R.J.; Kinloch, I.A.; Zan, R.; Novoselov, K.S. Wide-area strain sensors based upon graphene-polymer composite coatings probed by Raman spectroscopy. Adv. Funct. Mater. 2014, 24, 2865–2874. [Google Scholar] [CrossRef]
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sougrat, R.; Zhang, Y.Z.; Kim, H.; Anjum, D.H.; Lee, K.H.; Alshareef, H.N.; Jiang, Q. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [Green Version]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef]
- Pegan, J.D.; Zhang, J.; Chu, M.; Nguyen, T.; Park, S.J.; Paul, A.; Kim, J.; Bachman, M.; Khine, M. Skin-mountable stretch sensor for wearable health monitoring. Nanoscale 2016, 8, 17295–17303. [Google Scholar] [CrossRef]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly stretchable piezoresistive graphene-nanocellulose nanopaper for strain sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Zhong, J.; Zhong, Q.; Hu, Q.; Wu, N.; Li, W.; Wang, B.; Hu, B.; Zhou, J. Stretchable self-powered fiber-based strain sensor. Adv. Funct. Mater. 2015, 25, 1798–1803. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, D.; Cho, H.; Park, I.; Kim, J. Soft nanocomposite based multi-point, multi-directional strain mapping sensor using anisotropic electrical impedance tomography. Sci. Rep. 2017, 7, 39837. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lou, Z.; Chen, D.; Jiang, K.; Shen, G. Polymer-enhanced highly stretchable conductive fiber strain sensor used for electronic data gloves. Adv. Mater. Technol. 2016, 1, 1600136. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kim, Y.H.; Jo, H.G.; Pyun, J.Y.; Kwon, S.W.; Park, K.K. Single-shot waterless low-profile photoacoustic system: near-field volumetric imaging in vivo for blood vessels based on capacitive micromachined ultrasonic transducer (CMUT). Sensors 2019, 19, 995. [Google Scholar] [CrossRef]
- Won Baac, H.; Ok, J.G.; Park, H.J.; Ling, T.; Chen, S.L.; Hart, A.J.; Guo, L.J. Carbon nanotube composite optoacoustic transmitters for strong and high frequency ultrasound generation. Appl. Phys. Lett. 2010, 97, 234104. [Google Scholar] [CrossRef] [Green Version]
- Colchester, R.J.; Mosse, C.A.; Bhachu, D.S.; Bear, J.C.; Carmalt, C.J.; Parkin, I.P.; Treeby, B.E.; Papakonstantinou, I.; Desjardins, A.E. Laser-generated ultrasound with optical fibres using functionalised carbon nanotube composite coatings. Appl. Phys. Lett. 2014, 104, 173502. [Google Scholar] [CrossRef] [Green Version]
- Noimark, S.; Colchester, R.J.; Blackburn, B.J.; Zhang, E.Z.; Alles, E.J.; Ourselin, S.; Beard, P.C.; Papakonstantinou, I.; Parkin, I.P.; Desjardins, A.E. Carbon-nanotube–PDMS composite coatings on optical fibers for all-optical ultrasound imaging. Adv. Funct. Mater. 2016, 26, 8390–8396. [Google Scholar] [CrossRef]
- Poduval, R.K.; Noimark, S.; Colchester, R.J.; Macdonald, T.J.; Parkin, I.P.; Desjardins, A.E.; Papakonstantinou, I. Optical fiber ultrasound transmitter with electrospun carbon nanotube-polymer composite. Appl. Phys. Lett. 2017, 110, 223701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lan, X.; Lei, S.; Ou-Yang, J.; Yang, X.; Zhu, B. Effects of carbon nanotube thermal conductivity on optoacoustic transducer performance. Carbon 2019, 145, 112–118. [Google Scholar] [CrossRef]
- Chang, W.Y.; Huang, W.; Kim, J.; Li, S.; Jiang, X. Candle soot nanoparticles-polydimethylsiloxane composites for laser ultrasound transducers. Appl. Phys. Lett. 2015, 107, 161903. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Tian, Y.; Zou, X.; Silva, V.; Chery, A.; Wang, X. High-efficiency optical ultrasound generation using one-pot synthesized polydimethylsiloxane-gold nanoparticle nanocomposite. J. Opt. Soc. Am. B 2012, 29, 2016–2020. [Google Scholar] [CrossRef]
- Rao, K.S.; Chaudhary, A.K. Raman and time-resolved pulsed photoacoustic spectroscopy of solid trinitrotoluene in graphite mixture: For identification of double resonant optical phonon signatures. Opt. Laser Technol. 2019, 109, 149–156. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Let. 2000, 84, 4613–4616. [Google Scholar] [CrossRef]
- Noimark, S.; Colchester, R.J.; Poduval, R.K.; Maneas, E.; Alles, E.J.; Zhao, T.; Zhang, E.Z.; Ashworth, M.; Tsolaki, E.; Chester, A.H. Polydimethylsiloxane composites for optical ultrasound generation and multimodality imaging. Adv. Funct. Mater. 2018, 28, 1704919. [Google Scholar] [CrossRef]
- Choi, W.Y.; Park, K.K. Array type miniaturized ultrasonic sensors to detect urban sinkholes. Measurement 2019, 141, 371–379. [Google Scholar] [CrossRef]




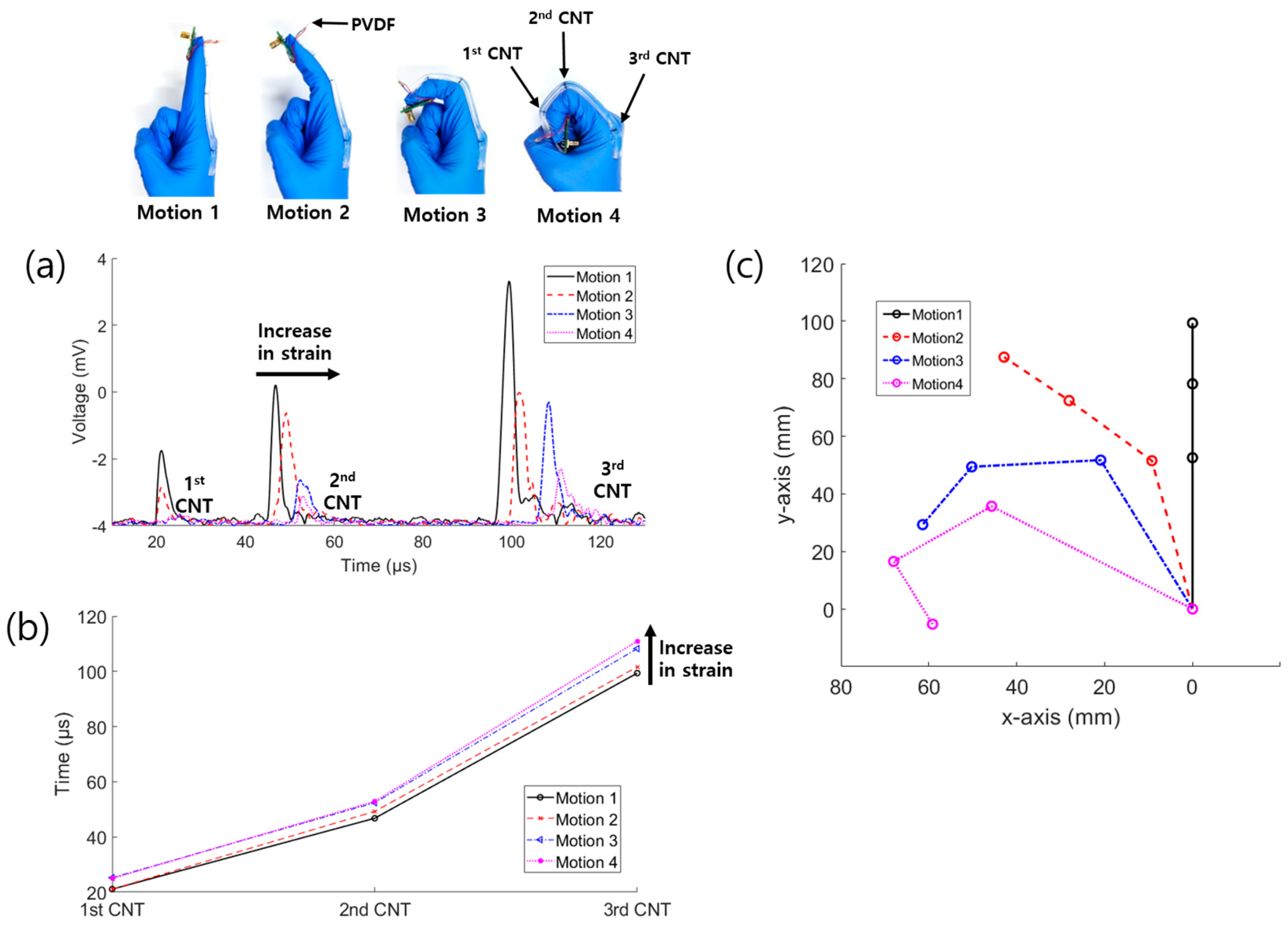
| Time Variation | Motion 2 | Motion 3 | Motion 4 |
|---|---|---|---|
| 1st CNT | 0.08 μs | 4.14 μs | 3.86 μs |
| 2nd CNT | 2.48 μs | 5.64 μs | 6.12 μs |
| 3rd CNT | 2.26 μs | 8.86 μs | 11.56 μs |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.Y.; Jo, H.G.; Kwon, S.W.; Kim, Y.H.; Pyun, J.Y.; Park, K.K. Multipoint-Detection Strain Sensor with a Single Electrode Using Optical Ultrasound Generated by Carbon Nanotubes. Sensors 2019, 19, 3877. https://doi.org/10.3390/s19183877
Choi WY, Jo HG, Kwon SW, Kim YH, Pyun JY, Park KK. Multipoint-Detection Strain Sensor with a Single Electrode Using Optical Ultrasound Generated by Carbon Nanotubes. Sensors. 2019; 19(18):3877. https://doi.org/10.3390/s19183877
Chicago/Turabian StyleChoi, Won Young, Hyeong Geun Jo, Soo Won Kwon, Young Hun Kim, Joo Young Pyun, and Kwan Kyu Park. 2019. "Multipoint-Detection Strain Sensor with a Single Electrode Using Optical Ultrasound Generated by Carbon Nanotubes" Sensors 19, no. 18: 3877. https://doi.org/10.3390/s19183877





