Application of Hyperspectral Imaging to Underwater Habitat Mapping, Southern Adriatic Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Underwater Hyperspectral Imager (UHI)
2.3. Data Acquisition
2.4. High Resolution Camera Image Data Processing
2.5. UHI Data Processing
2.5.1. Radiometric Processing
2.5.2. Georeferencing
2.5.3. Reflectance Processing
2.6. UHI Spectral Supervised Classification
2.6.1. Spectral Angle Mapper (SAM)
2.6.2. SAM Classification Accuracy
3. Results
3.1. Spectral Library for CWC Site
3.2. Spectral Library for the Coralligenous Site
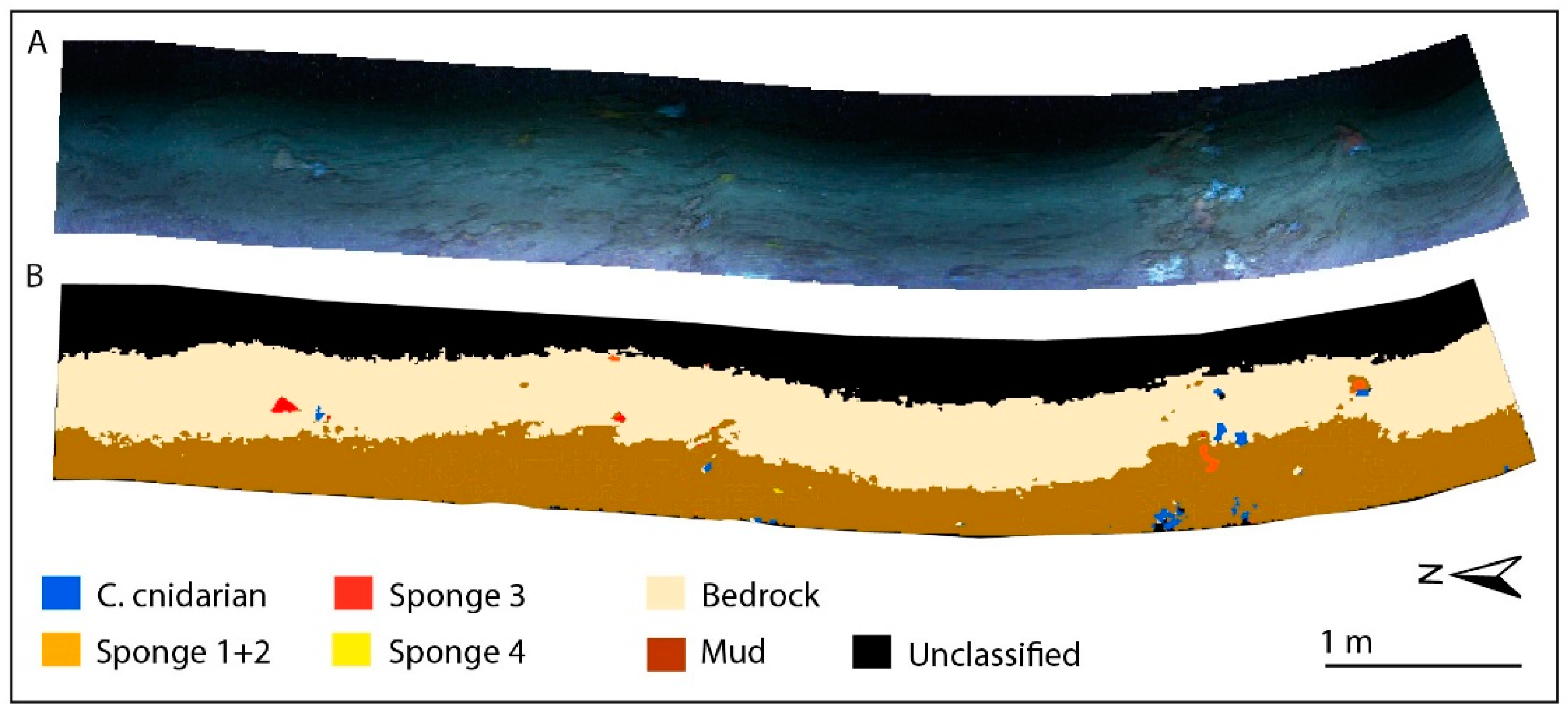
3.3. Supervised Classification Results for CWC Site
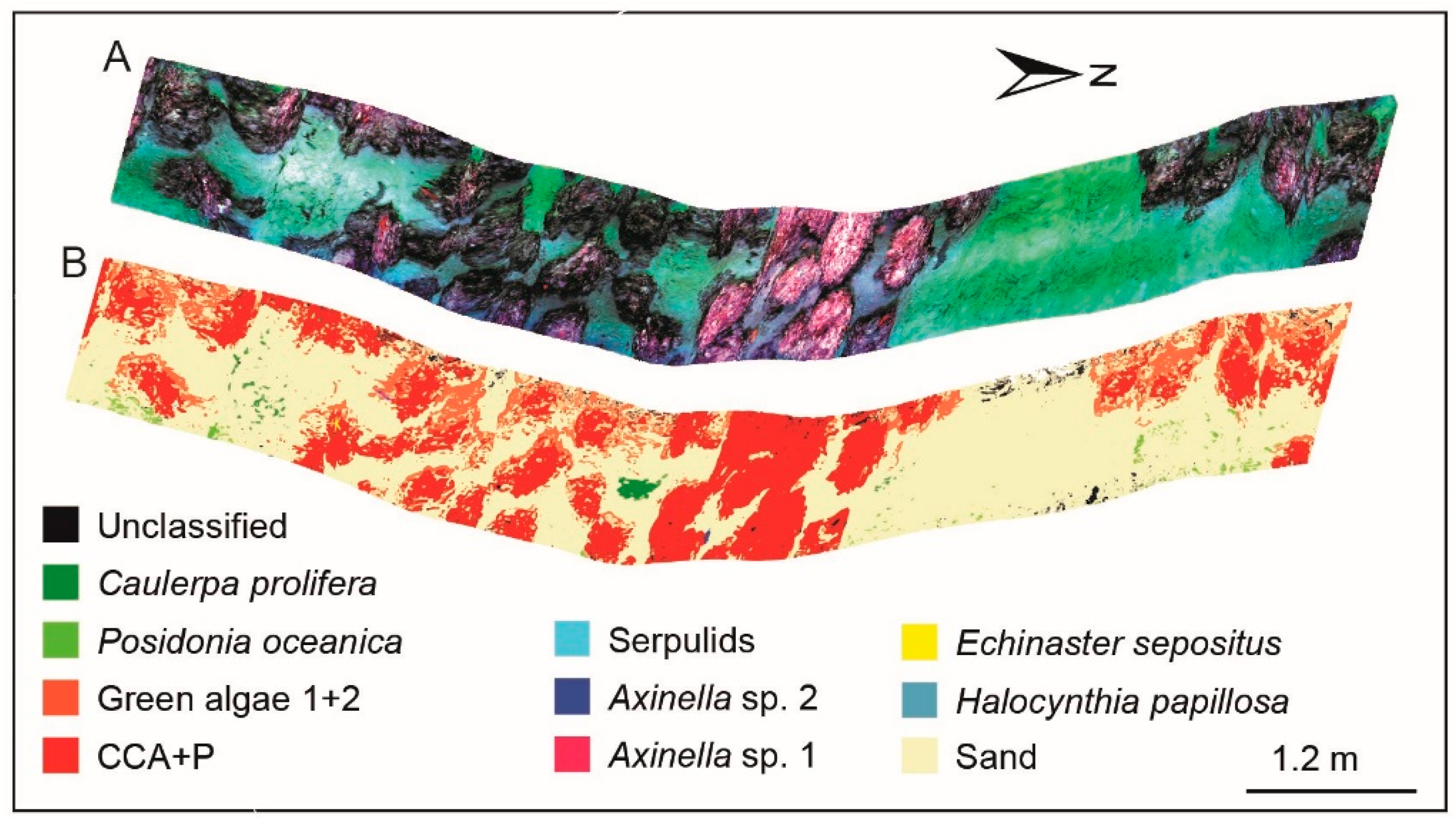
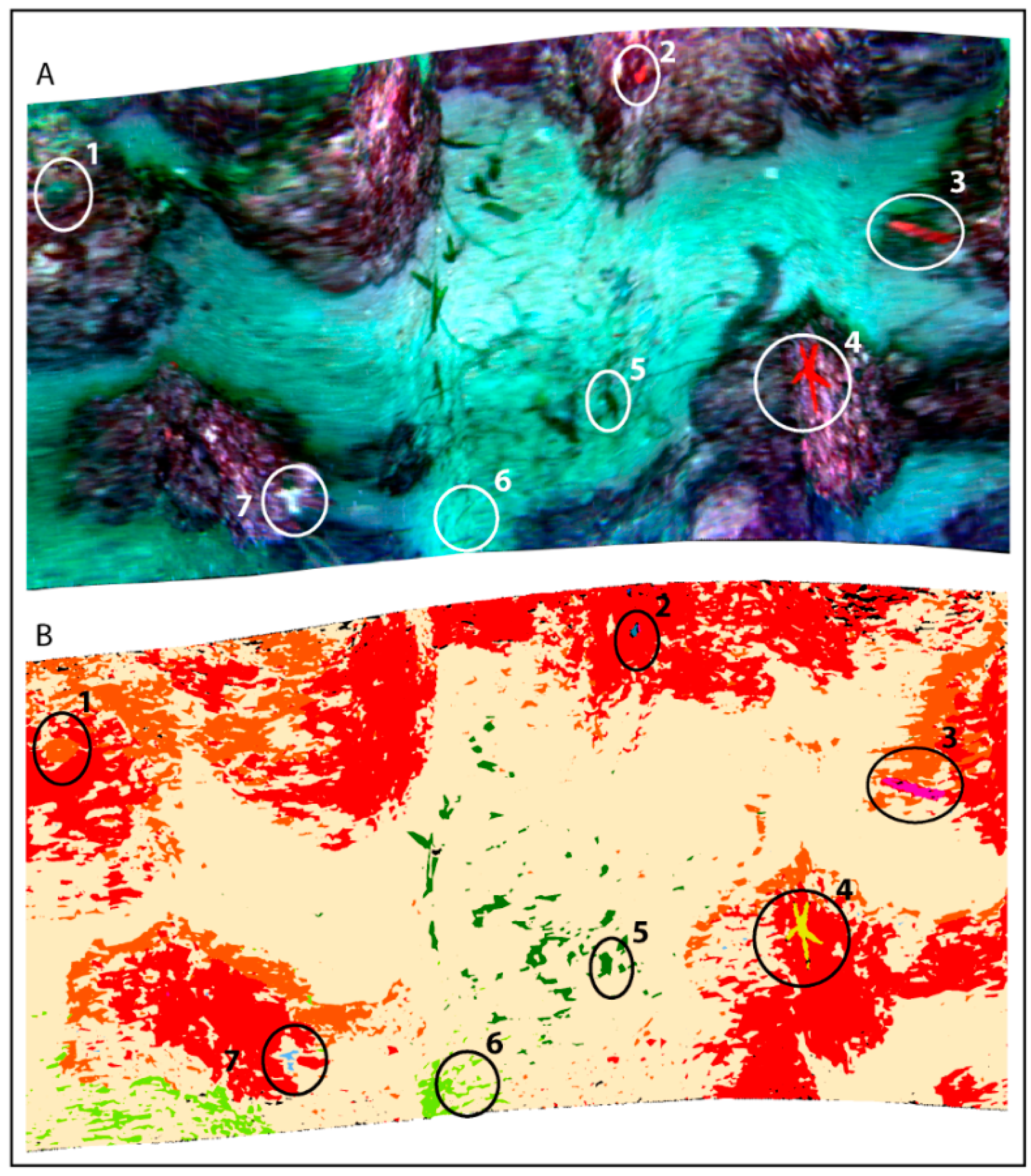
3.4. Supervised Classification Results for Coralligenous Site
3.5. Classification accuracy for CWC and Coralligenous Sites
4. Discussion
4.1. Evaluation of the Acquisition Set-up and Suggestion of Best Practice for Data Collection
- an ROV ensuring a constant heading and altitude above the seafloor and suitable to host the UHI and other devices (e.g., RGB camera, lamps);
- an efficient positioning system for the ROV and the UHI itself, able to provide accurate and adequately dense navigation data;
- an appropriate lamp system to illuminate the surveyed area uniformly in function of water depth and sunlight;
- an RGB camera mounted vertically alike the UHI camera, to record concomitantly the seafloor for the OOI identification;
- an advanced background knowledge of the target area.
4.2. Evaluation of Spectral Libraries for Seafloor Mapping
4.3. SAM Classification
4.4. Evaluation of the UHI for Seabed Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, C.J.; Smith, S.J.; Lawton, P.; Andersond, J.T. Benthic habitat mapping: A review of progress towards improved understanding of the spatial ecology of the seafloor using acoustic techniques. Estuar. Coast. Shelf Sci. 2011, 92, 502–520. [Google Scholar] [CrossRef]
- Angeletti, L.; Bargain, A.; Foglini, F.; Grande, V.; Prampolini, M.; Taviani, M. Cold-water coral multiscale habitat mapping: Methodologies and perspectives. In Mediterranean Cold-Water Corals: Past, Present and Future, Coral Reefs of the World; Orejas, C., Jiménez, C., Eds.; Springer International Publishing: Berlin, Germany, 2019; Volume 9. [Google Scholar]
- Lim, A.; Wheeler, A.J.; Arnaubec, A. High-resolution facies zonation within a cold-water coral mound: The case of the Piddington Mound, Porcupine Seabight, NE Atlantic. Mar. Geol. 2017, 390, 120–130. [Google Scholar] [CrossRef]
- Berg, T.; Fürhaupter, K.; Teixeira, H.; Uusitalo, L.; Zampoukas, N. The Marine Strategy Framework Directive and the ecosystem-based approach–pitfalls and solutions. Mar. Pollut. Bull. 2015, 96, 18–28. [Google Scholar] [CrossRef]
- Johnsen, G.; Volent, Z.; Dierssen, H.; Pettersen, R.; Ardelan, M.V.; Søreide, F.; Fearns, P.; Ludvigsen, M.; Moline, M. Underwater hyperspectral imagery to create biogeochemical maps of seafloor properties. In Subsea Optics and Imaging, 1st ed.; Watson, J., Zielinski, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 508–540. [Google Scholar]
- Dumke, I.; Nornes, S.M.; Purser, A.; Marcon, Y.; Ludvigsen, M.; Ellefmo, S.L.; Johnsen, G.; Søreide, F. First hyperspectral imaging survey of the deep seafloor: High-resolution mapping of manganese nodules. Remote Sens. Environ. 2018, 209, 19–30. [Google Scholar] [CrossRef]
- Dumke, I.; Purser, A.; Marcon, Y.; Nornes, S.M.; Johnsen, G.; Ludvigsen, M.; Søreide, F. Underwater hyperspectral imaging as an in situ taxonomic tool for deep-sea megafauna. Sci. Rep. 2018, 8, 12860. [Google Scholar] [CrossRef] [PubMed]
- Klonowski, W.M.; Fearns, P.R.C.S.; Lynch, M.J. Retrieving key benthic cover types and bathymetry from hyperspectral imagery. JARS 2007, 1, 011505. [Google Scholar] [CrossRef]
- Volent, Z.; Johnsen, G.; Sigernes, F. Kelp forest mapping by use of airborne hyperspectral imager. J. Appl. Remote Sens. 2007, 1, 011505. [Google Scholar] [CrossRef]
- Fearns, P.R.C.; Klonowski, W.; Babcock, R.C.; England, P.; Phillips, J. Shallow water substrate mapping using hyperspectral remote sensing. Cont. Shelf Res. 2011, 31, 1249–1259. [Google Scholar] [CrossRef]
- Chang, C.; Member, S.; Du, Q. Estimation of Number of Spectrally Distinct Signal Sources in Hyperspectral Imagery. IEEE Trans. Geosci. Remote Sens. 2004, 42, 608–619. [Google Scholar] [CrossRef] [Green Version]
- Dickey, T.; Lewis, M.; Chang, G. Optical oceanography: Recent advances and future directions using global remote sensing and in situ observations. Rev. Geophys. 2006, 44, 1–39. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Randolph, K. Remote Sensing of Ocean Color. In Earth System Monitoring; Orcutt, J., Ed.; Springer: New York, NY, USA, 2013; pp. 439–472. ISBN 978-1-4614-5683-4. [Google Scholar]
- Hochberg, E.J.; Atkinson, M.J. Spectral discrimination of coral reef benthic communities. Coral Reefs 2000, 19, 164–171. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J.; Andréfouët, S. Spectral reflectance of coral reef bottom-types worldwide and implications for coral reef remote sensing. Remote Sens. Environ. 2003, 85, 159–173. [Google Scholar] [CrossRef]
- Kutser, T.; Miller, I.; Jupp, D.L.B. Mapping coral reef benthic substrates using hyperspectral space-borne images and spectral libraries. Estuar. Coast. Shelf Sci. 2006, 70, 449–460. [Google Scholar] [CrossRef]
- Petit, T.; Bajjouk, T.; Mouquet, P.; Rochette, S.; Vozel, B.; Delacourt, C. Hyperspectral remote sensing of coral reefs by semi-analytical model inversion—Comparison of different inversion setups. Remote Sens. Environ. 2017, 190, 348–365. [Google Scholar] [CrossRef]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping seagrass species, cover and biomass in shallow waters: An assessment of satellite multi-spectral and airborne hyper-spectral imaging systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Dierssen, H.M. Overview of hyperspectral remote sensing for mapping marine benthic habitats from airborne and underwater sensors. In Imaging Spectrometry XVIII, Proceedings of SPIE- International Society for Optics and Photonics, San Diego, CA, USA, 26–28 August 2013; Mouroulis, P., Ed.; 2013; Volume 8870. [Google Scholar]
- Dierssen, H.M.; Chlus, A.; Russell, B. Hyperspectral discrimination of floating mats of seagrass wrack and the macroalgae Sargassum in coastal waters of Greater Florida Bay using airborne remote sensing. Remote Sens. Environ. 2015, 167, 247–258. [Google Scholar] [CrossRef]
- Chennu, A.; Färber, P.; Volkenborn, N.; Al-Najjar, M.A.A.; Janssen, F.; de Beer, D.; Polerecky, L. Hyperspectral imaging of the microscale distribution and dynamics of microphytobenthos in intertidal sediments. Limnol. Oceanogr. Methods 2013, 11, 511–528. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, R.; Johnsen, G.; Bruheim, P.; Andreassen, T. Development of hyperspectral imaging as a bio-optical taxonomic tool for pigmented marine organisms. Org. Divers. Evol. 2014, 14, 237–246. [Google Scholar] [CrossRef]
- Ludvigsen, M.; GJohnsen, G.; Sørensen, A.J.; Lågstad, P.A.; Ødegård, Ø. Scientific operations combining ROV and AUV in the Trondheim Fjord. Mar. Technol. Soc. J. 2014, 48, 59–71. [Google Scholar] [CrossRef]
- Tegdan, J.; Ekehaug, S.; Hansen, I.M.; Sandvik Aas, L.M.; Steen, K.J.; Pettersen, R.; Beuchel, F.; Camus, L. Underwater hyperspectral imaging for environmental mapping and monitoring of seabed habitats. In Proceedings of the OCEANS 2015, Genova, Italy, 18–21 May 2015; pp. 1–6. [Google Scholar]
- Johnsen, G.; Ludvigsen, M.; Sørensen, A.; Sandvik Aas, L.M. The use of underwater hyperspectral imaging deployed on remotely operated vehicles—Methods and applications. IFAC 2016, 49, 476–481. [Google Scholar] [CrossRef]
- Mogstad, A.A.; Johnsen, G. Spectral characteristics of coralline algae: A multi-instrumental approach, with emphasis on underwater hyperspectral imaging. Appl. Opt. 2017, 56, 9957. [Google Scholar] [CrossRef]
- Ødegård, Ø.; Mogstad, A.A.; Johnsen, G.; Sørensen, A.J.; Ludvigsen, M. Underwater hyperspectral imaging: A new tool for marine archaeology. Appl. Opt. 2018, 57, 3214. [Google Scholar] [CrossRef]
- Sture, Ø.; Ludvigsen, M.; Søreide, F.; Sandvik Aas, L.M. Autonomous underwater vehicles as a platform for underwater hyperspectral imaging. In Proceedings of the OCEANS 2017, Aberdeen, UK, 19–22 June 2017; pp. 1–8. [Google Scholar]
- Cochrane, S.K.J.; Ekehaug, S.; Refit, E.C.; Hansen, I.M.; Sandvik Aas, L.M. Detection of deposited drill cuttings on the sea floor—A comparison between underwater hyperspectral imagery and the human eye. Mar. Pollut. Bull. 2019. in review. [Google Scholar]
- Ødegård, Ø.; Sørensen, A.J.; Hansen, R.E.; Ludvigsen, M. A new method for underwater archaeological surveing using sensors and unmanned platforms. IFAC-PapersOnLine 2016, 49, 486–493. [Google Scholar]
- Letnes, P.A.; Hansen, I.M.; Sandvik Aas, L.M.; Eide, I.; Pettersen, R.; Tassara, L. Underwater hyperspectral classification of deep sea corals exposed to 2-methylnaphthalene. PLoS ONE 2019, 14, e0209960. [Google Scholar] [CrossRef]
- Foglini, F.; Angeletti, L.; Bracchi, V.A.; Chimienti, G.; Grande, V.; Hansen, I.M.; Meroni, A.N.; Marchese, F.; Mercorella, A.; Prampolini, M.; et al. Underwater Hyperspectral Imaging for seafloor and benthic habitat mapping. In Proceedings of the 2018 IEEE International Workshop on Metrology for the sea (MetroSea 2018), Bari, Italy, 8–10 October 2018; pp. 201–205. [Google Scholar]
- Gavazzi, G.M.; Madricardo, F.; Janowski, L.; Kruss, A.; Blondel, P.; Sigovini, M.; Foglini, F. Evaluation of seabed mapping methods for fine-scale classification of extremely shallow benthic habitats—Application to the Venice Lagoon, Italy. Estuar. Coast. Shelf Sci. 2016, 170, 45–60. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- Freiwald, A.; Beuck, L.; Rüggeberg, A.; Taviani, M.; Hebbeln, D.; R/V Meteor Cruise M70-1 Participants. The white coral community in the Central Mediterranean Sea Revealed by ROV Surveys. Oceanography 2009, 22, 58–74. [Google Scholar] [CrossRef]
- Angeletti, L.; Taviani, M.; Canese, S.; Foglini, F.; Mastrototaro, F.; Argnani, A.; Trincardi, F.; Bakran-Petricioli, T.; Ceregato, A.; Chimienti, G.; et al. New deep-water cnidarian sites in the southern Adriatic Sea. Mediterr. Mar. Sci. 2014, 15, 263–273. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Cardone, F.; Carlucci, R.; Carluccio, A.; Chimienti, G.; Corriero, G.; Longo, C.; Maiorano, P.; Mastrototaro, F.; et al. Macro-and megafauna recorded in the submarine Bari Canyon (southern Adriatic, Mediterranean Sea) using different tools. Mediterr. Mar. Sci. 2015, 16, 180–196. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Beuck, L.; Campiani, E.; Canese, S.; Foglini, F.; Freiwald, A.; Montagna, P.; Trincardi, F. Reprint of ‘On and off the beaten track: Megafaunal sessile life and Adriatic cascading processes’. Mar. Geol. 2016, 375, 146–160. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Cardone, F.; Montagna, P.; Danovaro, R. A unique and threatened deep water coral-bivalve biotope new to the Mediterranean Sea offshore the Naples megalopolis. Sci. Rep. 2019, 9, 3411. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, G.; Bo, M.; Taviani, M.; Mastrototaro, F. Occurrence and Biogeography of Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future, Coral Reefs of the World; Orejas, C., Jiménez, C., Eds.; Springer International Publishing: Berlin, Germany, 2019; Volume 9. (in press) [Google Scholar]
- Foglini, F.; Angeletti, L.; Campiani, E.; Correggiari, A.; Grande, V.; Leidi, E.; Madricardo, F.; Mercorella, A.; Remia, R.; Taviani, M. Habitat mapping in the Adriatic (Mediterranean Sea) from coastal areas to deep sea: Approaches and methodologies for assessing seafloor habitat for sustainable and integrated sea management strategy. In Proceedings of the GeoHab 2015, Salvador, Brazil, 3–8 May 2015. [Google Scholar]
- Sanfilippo, R.; Vertino, A.; Rosso, A.; Beuck, L.; Freiwald, A.; Taviani, M. Serpula aggregates and their role in deep-sea coral communities in the southern Adriatic Sea. Facies 2013, 59, 663–677. [Google Scholar] [CrossRef]
- Bargain, A.; Foglini, F.; Pairaud, I.; Bonaldo, D.; Carniel, S.; Angeletti, L.; Taviani, M.; Rochette, S.; Fabri, M.C. Predictive habitat modeling in two Mediterranean canyons including hydrodynamic variables. Prog. Oceanogr. 2018, 169, 151–168. [Google Scholar] [CrossRef]
- Addamo, A.M.; Vertino, A.; Stolarski, J.; García-Jiménez, R.; Taviani, M.; Machordom, A. Merging scleractinian genera: The overwhelming genetic similarity between solitary Desmophyllum and colonial Lophelia. BMC Evol. Biol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Bracchi, V.; Savini, A.; Marchese, F.; Palamara, S.; Basso, D.; Corselli, C. Coralligenous habitat in the Mediterranean Sea: A geomorphological description from remote data. Ital. J. Geosci. 2015, 134, 32–40. [Google Scholar] [CrossRef]
- Bracchi, V.A.; Basso, D.; Marchese, F.; Corselli, C.; Savini, A. Coralligenous morphotypes on subhorizontal substrate: A new categorization. Cont. Shelf Res. 2017, 144, 10–20. [Google Scholar] [CrossRef]
- Laborel, J. Le concrétionnement algal ‘coralligène’ et son importance geomorphologique en Méditerranée. Recueil des travaux Station Marine d’Endoume 1961, 23, 37–60. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la mer Méditerranée. Recent Trav. De La Stn. Mar. De Endoume 1964, 31, 1–137. [Google Scholar]
- Bellan-Santini, D.; Lacaze, J.C.; Poizat, C. Les biocénoses marines et littorales de Méditerranée, synthèse, menaces et perspectives. Collection Patrimoines Naturels. Muséum National d’Histoire Naturelle 1994, 19, 1–246. [Google Scholar]
- Bressan, G.; Babbini, I.; Ghirardelli, L.; Basso, D. Bio-costruzione e bio-distruzione di corallinales nel Mar Mediterraneo. Biol. Mar. Mediterr. 2001, 8, 131–174. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Piazzi, L.; Gennaro, P.; Balata, D. Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Poll. Bull. 2012, 64, 2623–2629. [Google Scholar] [CrossRef]
- Chimienti, G.; Stithou, M.; Mura, I.D.; Mastrototaro, F.; D’Onghia, G.; Tursi, A.; Izzi, C.; Fraschetti, S. An explorative assessment of the importance of Mediterranean Coralligenous habitat to local economy: The case of recreational diving. J. Environ. Account. Manag. 2017, 5, 315–325. [Google Scholar] [CrossRef]
- Sarà, M. Research on Benthic Fauna of Southern Adriatic Italian Coast: Final Scientific Report; Office of Naval Research: Washington, DC, USA, 1968; pp. 1–53. [Google Scholar]
- Sarà, M. Un biotopo da proteggere: Il coralligeno pugliese. In Proceedings of the Atti del I Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 21–25 April 1971; pp. 145–151. [Google Scholar]
- Angeletti, L.; Prampolini, M.; Foglini, F.; Grande, V.; Taviani, M. Cold-water coral habitat in the Bari Canyon System, Southern Adriatic Sea (Mediterranean Sea). In Seafloor Geomorphology as Benthic Habitat, 2nd ed.; Harris, P., Baker, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. (in press) [Google Scholar]
- Johnsen, G.; Volent, Z.; Sakshaug, E.; Sigernes, F.; Pettersson, L. Remote sensing in the Barents Sea. In Ecosystem Barents Sea; Sakshaug, E., Johnsen, G.H., Kovacs, K.M., Eds.; Fagbokforlaget: Bergen, Norway, 2009; Volume 2, pp. 139–166. [Google Scholar]
- Dukan, F.; Ludvigsen, M.; Sorensen, A.J. Dynamic positioning system for a small size ROV with experimental results. In Proceedings of the IEEE OCEANS, Santander, Spain, 6–9 June 2011; pp. 1–10. [Google Scholar] [CrossRef]
- Williams, D.J.; Shah, M. A fast algorithm for active contours and curvature estimation. CVGIP Image Under. 1992, 55, 14–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 18 May 2019).
- Kruse, F.A.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The Spectral Image Processing System (SIPS) Interactive Visualization and Analysis of Imaging Spectrometer Data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Crósta, A.P.; Sabine, C.; Taranik, J.V. Hydrothermal Alteration Mapping at Bodie, California, Using AVIRIS Hyperspectral Data. Remote Sens. Environ. 1998, 64, 309–319. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Lu, X.; Wang, Z.; Chen, C.; He, H. Classification of Urban Hyperspectral Remote Sensing Imagery Based on Optimized Spectral Angle Mapping. J. Indian Soc. Remote Sens. 2019, 47, 289–294. [Google Scholar] [CrossRef]
- Boland, G.S.; Etnoyer, P.J.; Fisher, C.R.; Hickerson, E.L. State of deep-sea coral and sponge ecosystems of the Gulf of Mexico Region: Texas to the Florida Straits. In The State of Deep-Sea Coral and Sponge Ecosystems of the United States; Hourigan, T.F., Etnoyer, P.J., Cairns, S.D., Eds.; NOAA Tech. Memo. NMFS-OHC-4; Silver Spring: Silver, MD, USA, 2017; pp. 321–378. [Google Scholar]
- Freiwald, A. Geobiology of Lophelia pertusa (Scleractinia) Reefs in the North Atlantic. Unpublished Habilitation Thesis, Bremen University, Bremen, Germany, 1998. [Google Scholar]
- Freiwald, A.; Fosså, J.H.; Grehan, A.; Koslow, T.; Roberts, J.M. Cold-water coral reefs—Out of sight—No longer out of mind. In UNEP-WCMC Biodiversity; UNEP Coral Reef Unit: Cambridge, UK, 2004; Volume 22, pp. 1–84. [Google Scholar]
- Santín, A.; Grynó, J.; Ambroso, S.; Uriz, M.J.; Dominguez-Carrió, C.; Gili, J.M. Distribution patterns and demographic trends of demosponges at the Menorca Channel (Northwestern Mediterranean Sea). Prog. Oceanogr. 2019, 173, 9–25. [Google Scholar] [CrossRef]
- Bell, J.J.; Barnes, D.K.A. Sponge morphological diversity: A qualitative predictor of species diversity? Aquat. Conserv. 2001, 11, 109–121. [Google Scholar] [CrossRef]
- Calcinai, B.; Moratti, V.; Martinelli, M.; Bavestrello, G.; Taviani, M. Uncommon sponges associated with deep coral bank and maerl habitats in the Strait of Sicily (Mediterranean Sea). Ital. J. Zool. 2013, 80, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Rueda, J.L.; Urra, J.; Aguilar, R.; Angeletti, L.; Bo, M.; García-Ruiz, C.; González-Duarte, M.; López, E.; Madurell, T.; Maldonado, M.; et al. Cold-water coral associated fauna in the Mediterranean Sea and adjacent areas. In Mediterranean Cold-Water Corals: Past, Present and Future, Coral Reefs of the World; Orejas, C., Jiménez, C., Eds.; Springer International Publishing: Berlin, Germany, 2019; Volume 9. [Google Scholar]




| Benthic Class | Colonial Cnidarian | Sponge | Mud | Bedrock | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 1 | 2 |
| Taxonomy | M. oculata/D. pertusum | Hexactinellida sp. | Demospongiae sp. 1 | Demospongiae sp. 2 | Demospongiae sp. 3 | |||||
| Threshold | 0.035 | 0.025 | 0.08 | 0.08 | 0.15 | 0.045 | 0.035 | 0.08 | 0.08 | 0.08 |
| Benthic Class | CCA+P | Green Algae | Seagrass | Associated Organism | Sand | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | 1 | 2 | 3 | 1 | 2 | 3 | 1 | Sponge 1 | Sponge 2 | Serpulids | Red Starfish | Red Ascidia | 1 |
| Taxonomy | C. bursa/F. petiolata | C. prolifera | P. oceanica | Axinella sp. 1 | Axinella sp. 2 | E. sepositus | H. papillosa | ||||||
| Threshold | 0.2 | 0.02 | 0.07 | 0.08 | 0.08 | 0.065 | 0.045 | 0.08 | 0.07 | 0.03 | 0.18 | 0.06 | 0.2 |
| Overall Accuracy 84.38% | ||||||
|---|---|---|---|---|---|---|
| Mud | Sponge | Bedrock | C. cnidarian | TOT | UA | |
| Mud | 7 | 0 | 2 | 1 | 10 | 67.78 |
| Sponge | 0 | 7 | 0 | 0 | 7 | 100.00 |
| Bedrock | 1 | 1 | 6 | 0 | 8 | 66.67 |
| C. cnidarian | 0 | 0 | 0 | 7 | 7 | 100.00 |
| TOT | 8 | 8 | 8 | 8 | 32 | |
| PA | 87.50 | 87.50 | 75.00 | 87.5 | ||
| Overall Accuracy 72% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serpulids | E. sepositus | H. papillosa | Axinella sp.1 | Axinella sp.2 | CCA+P | Green Algae 3 | Sand | P. oceanica | Green Algae 1+2 | TOT | UA | |
| Serpulids | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 100.0 |
| E. sepositus | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 100.0 |
| H. papillosa | 0 | 0 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 88.9 |
| Axinella sp.1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 100.0 |
| Axinella sp.2 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 8 | 100.0 |
| CCA+P | 1 | 0 | 0 | 1 | 0 | 8 | 0 | 1 | 0 | 0 | 11 | 61.5 |
| Green algae 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 100.0 |
| Sand | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 4 | 1 | 15 | 25.0 |
| P. oceanica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 100.0 |
| Green algae 1+2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 7 | 9 | 70.0 |
| TOT | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 80 | |
| PA | 87.5 | 100 | 100 | 75 | 100 | 100 | 37.5 | 62.5 | 50 | 87.5 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglini, F.; Grande, V.; Marchese, F.; Bracchi, V.A.; Prampolini, M.; Angeletti, L.; Castellan, G.; Chimienti, G.; Hansen, I.M.; Gudmundsen, M.; et al. Application of Hyperspectral Imaging to Underwater Habitat Mapping, Southern Adriatic Sea. Sensors 2019, 19, 2261. https://doi.org/10.3390/s19102261
Foglini F, Grande V, Marchese F, Bracchi VA, Prampolini M, Angeletti L, Castellan G, Chimienti G, Hansen IM, Gudmundsen M, et al. Application of Hyperspectral Imaging to Underwater Habitat Mapping, Southern Adriatic Sea. Sensors. 2019; 19(10):2261. https://doi.org/10.3390/s19102261
Chicago/Turabian StyleFoglini, Federica, Valentina Grande, Fabio Marchese, Valentina A. Bracchi, Mariacristina Prampolini, Lorenzo Angeletti, Giorgio Castellan, Giovanni Chimienti, Ingrid M. Hansen, Magne Gudmundsen, and et al. 2019. "Application of Hyperspectral Imaging to Underwater Habitat Mapping, Southern Adriatic Sea" Sensors 19, no. 10: 2261. https://doi.org/10.3390/s19102261











