Studying Electrotaxis in Microfluidic Devices
Abstract
:1. Introduction
2. Electrotaxis in Traditional Devices
3. Electrotaxis in Microfluidic Devices
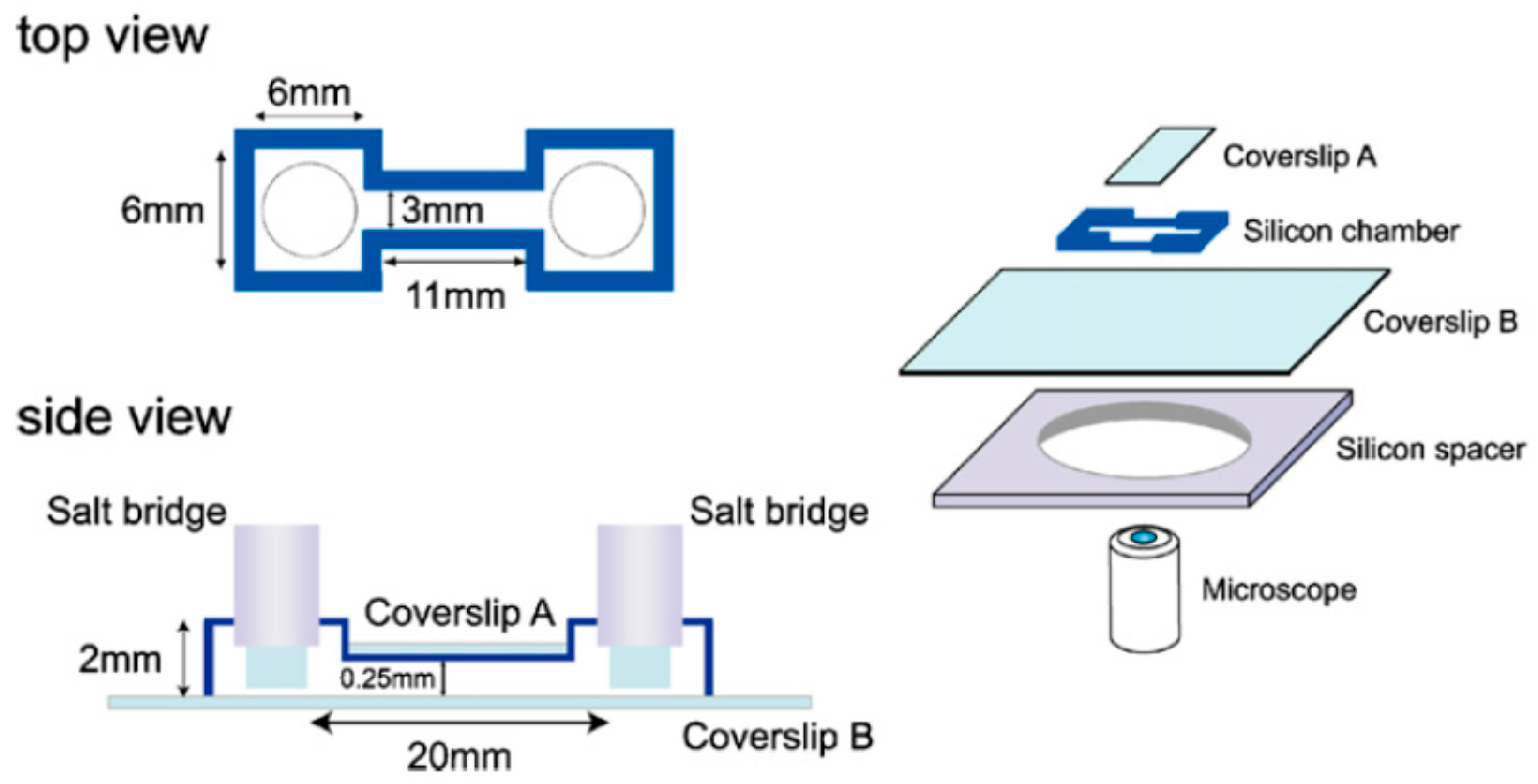
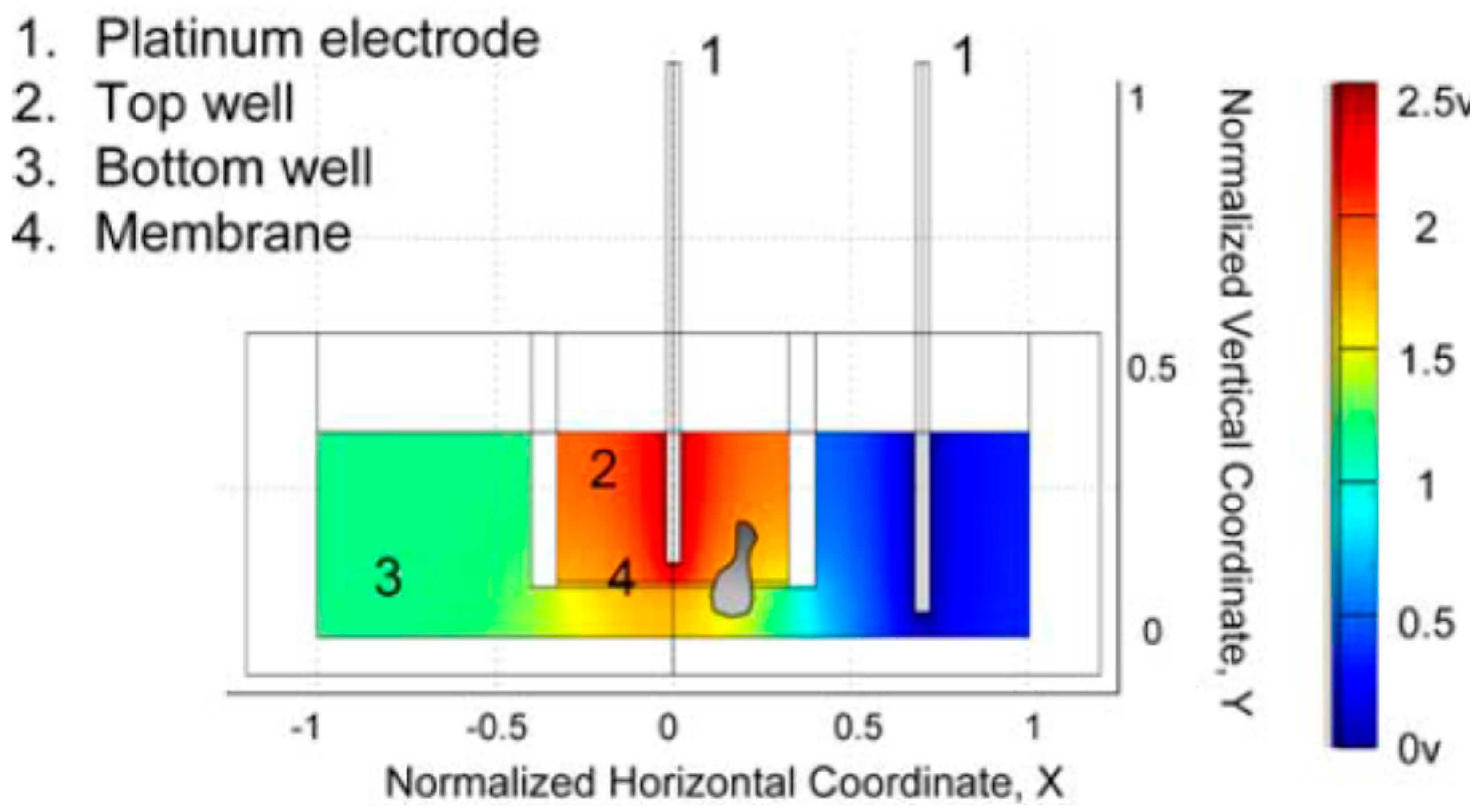
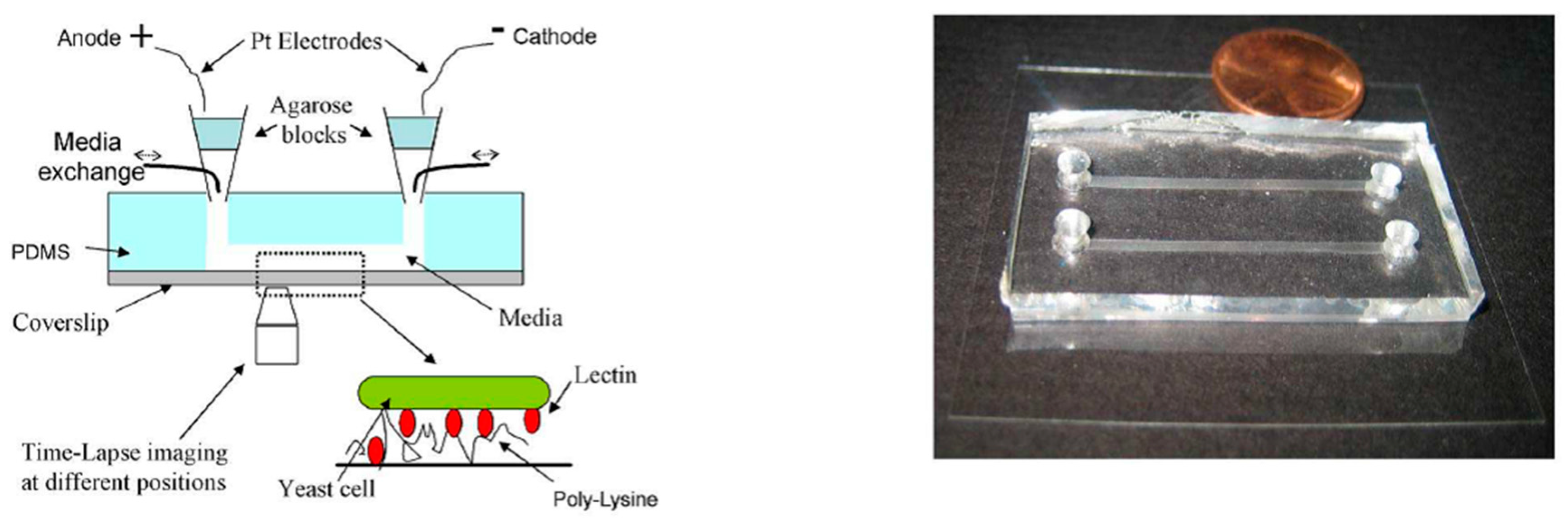
3.1. PDMS-Based Microfluidic Devices
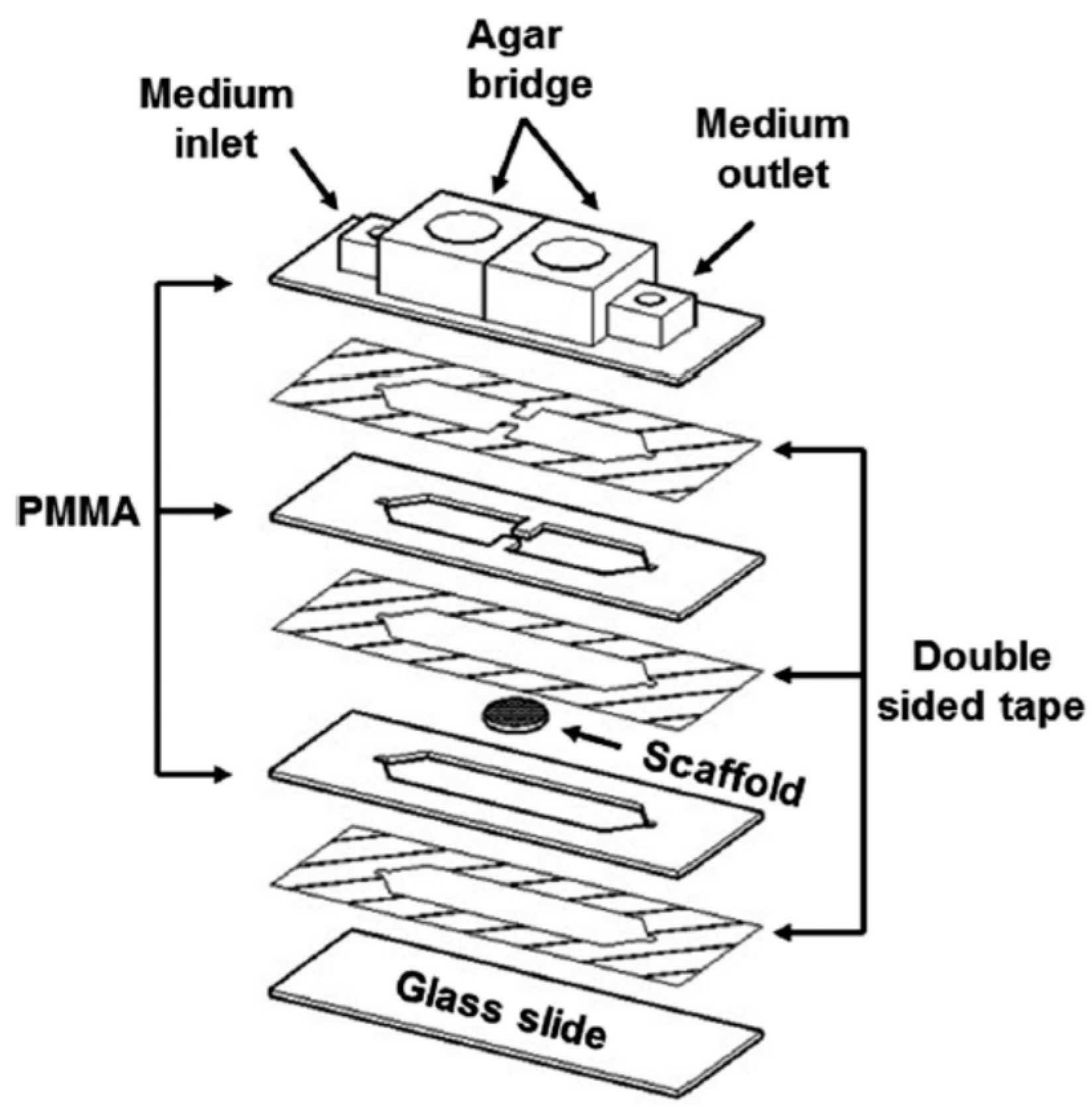
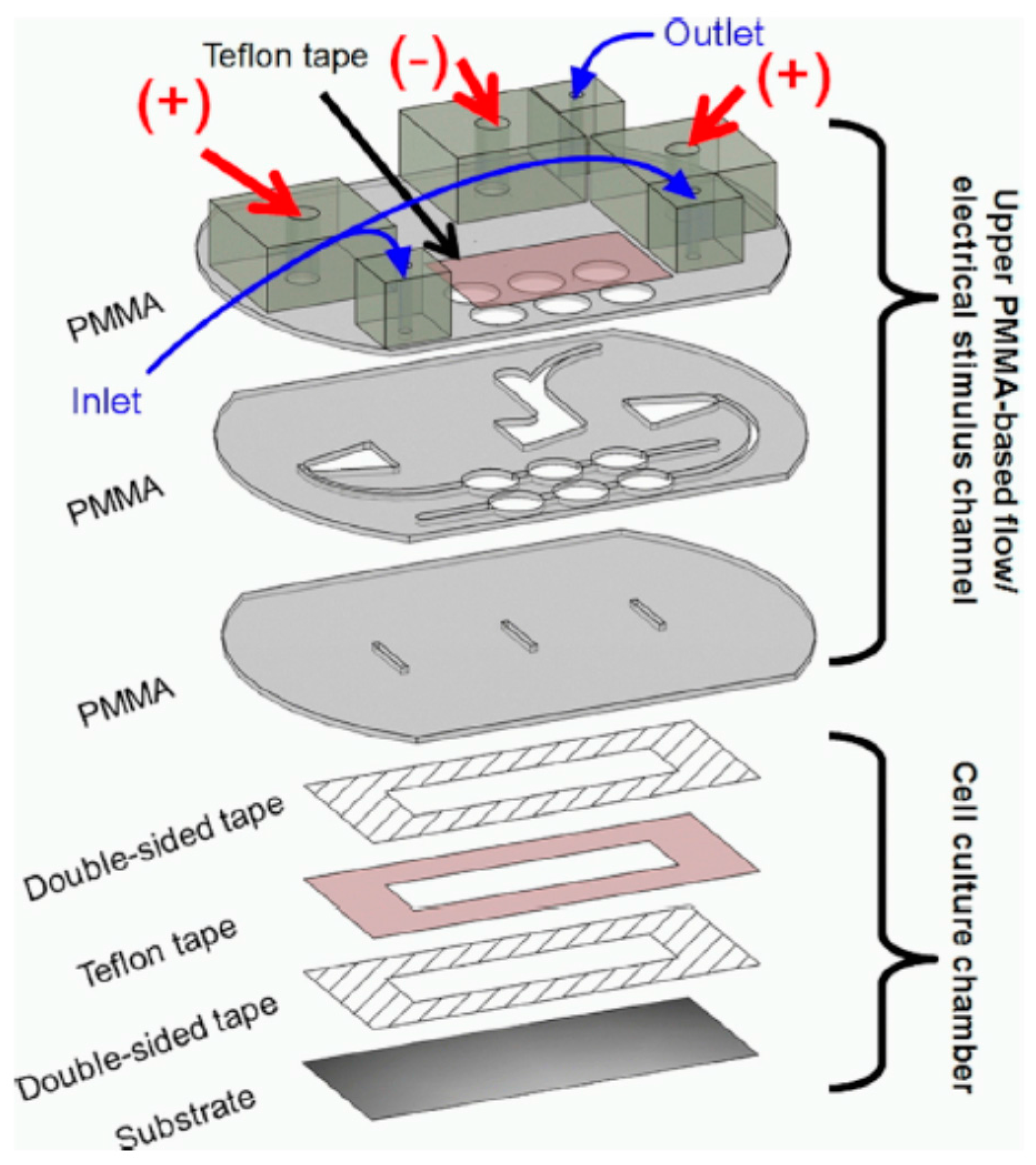
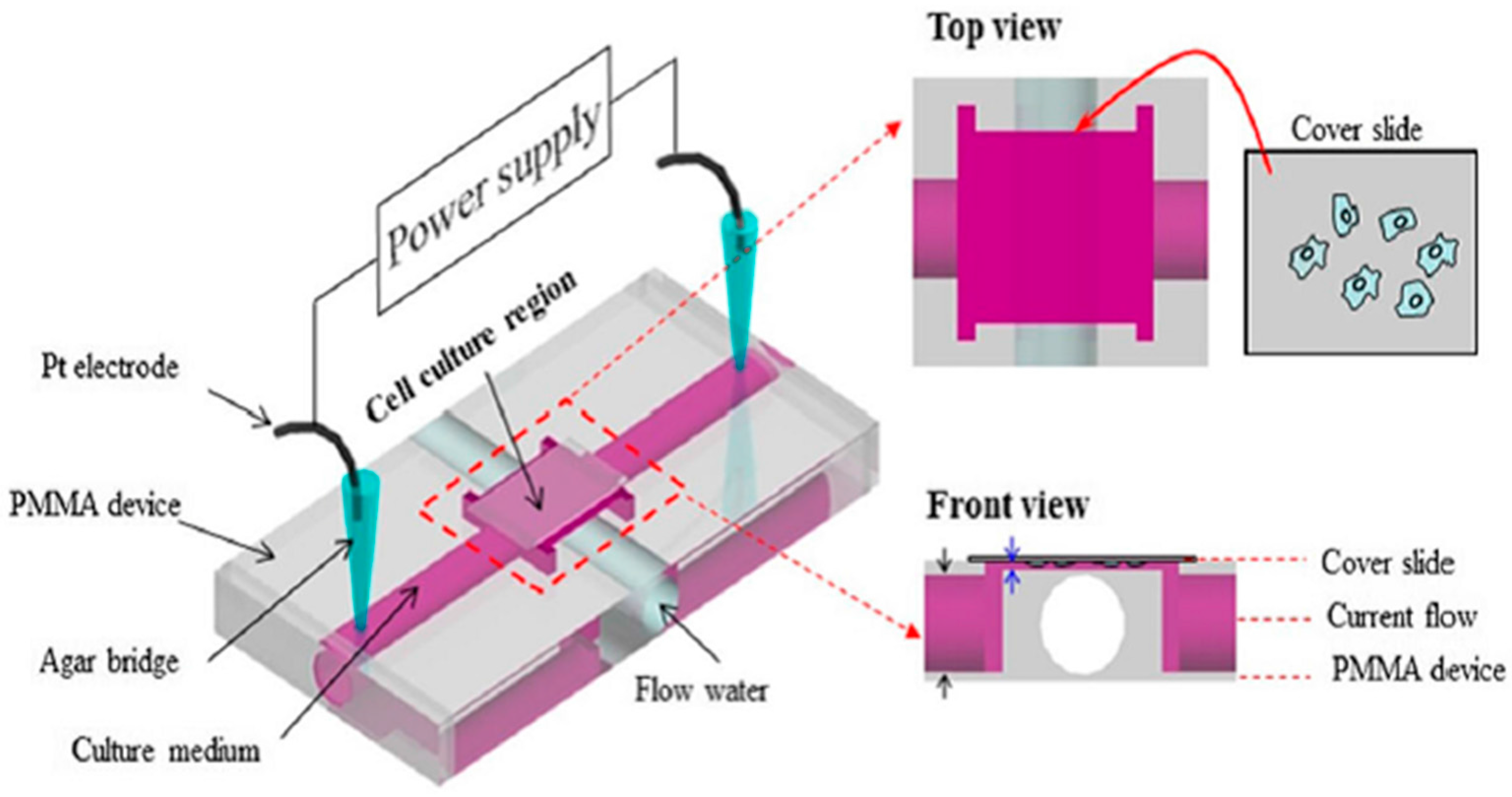
3.2. PMMA-Based Microfluidic Devices
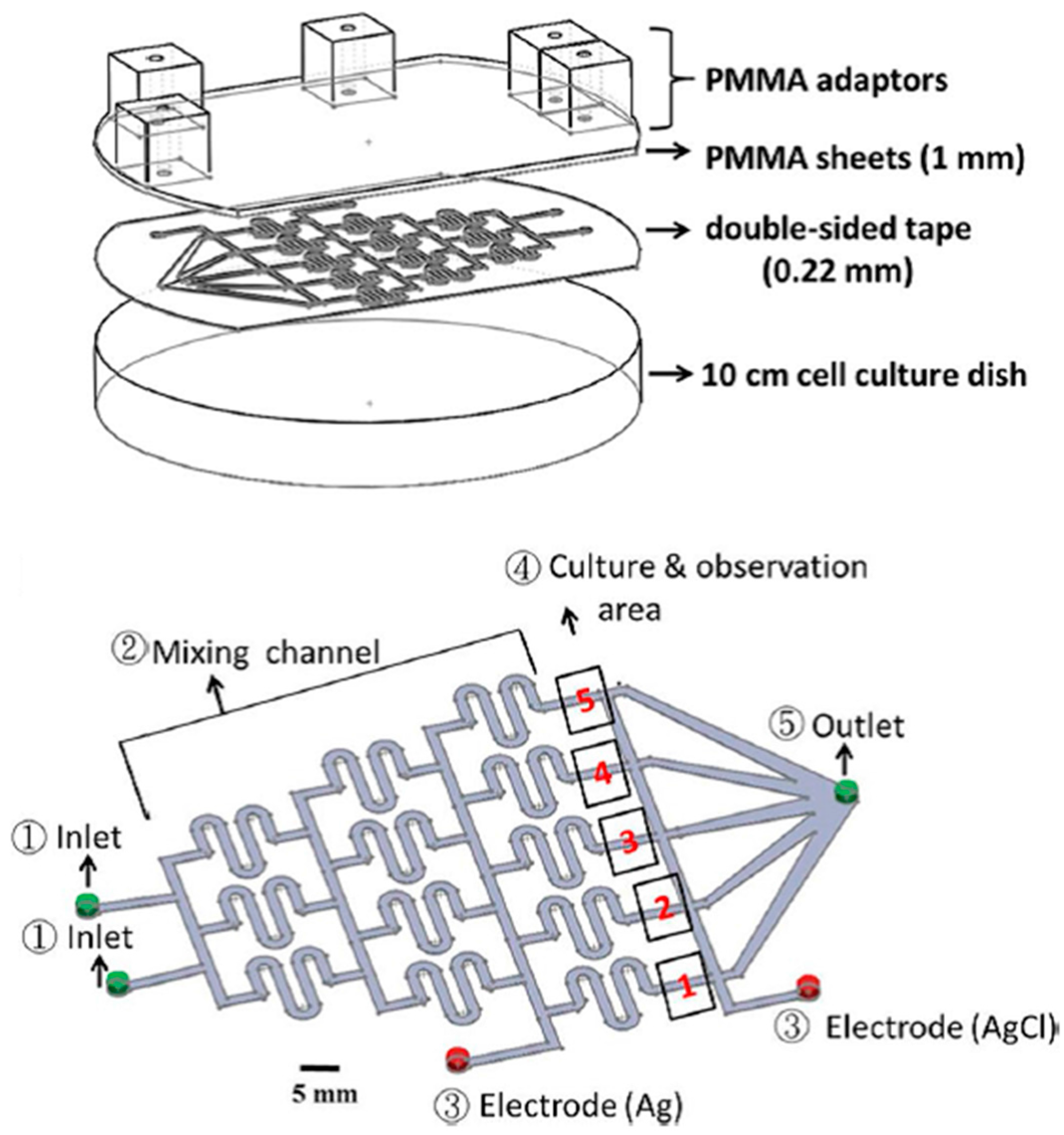
4. Combination of Electrotaxis and Chemical Stimuli
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Costa, G.; Harrington, K.I.; Lovegrove, H.E.; Page, D.J.; Chakravartula, S.; Bentley, K.; Herbert, S.P. Asymmetric division coordinates collective cell migration in angiogenesis. Nat. Cell Biol. 2016, 18, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Graupera, M.; Guillermet-Guibert, J.; Foukas, L.C.; Phng, L.K.; Cain, R.J.; Salpekar, A.; Pearce, W.; Meek, S.; Millan, J.; Cutillas, P.R.; et al. Angiogenesis selectively requires the p110 alpha isoform of PI3K to control endothelial cell migration. Nature 2008, 453, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Teng, S.F.; Zhang, Y.Y.; Zhang, W.G.; Zhang, X.W.; Xu, K.; Yao, H.S.; Yao, J.; Wang, H.L.; Liang, X.W.; et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget 2017, 8, 47052–47063. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zhang, B.; Lin, Y.; Yang, Y.; Liu, X.Q.; Lu, F. Breast cancer metastasis suppressor 1 inhibits SDF-1 alpha-induced migration of non-small cell lung cancer by decreasing CXCR4 expression. Cancer Lett. 2008, 269, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, S.A.; Wu, Z.P.; Han, Z.T.; Yan, W.J.; Liu, T.L.; Wei, H.F.; Song, D.W.; Zhou, W.; Yang, X.H.; et al. XCR1 promotes cell growth and migration and is correlated with bone metastasis in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2015, 464, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.R.; Zheng, X.B.; Zheng, Y.X. Lamin-B1 contributes to the proper timing of epicardial cell migration and function during embryonic heart development. Mol. Biol. Cell 2016, 27, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, A.; Belyaeva, V.; Siekhaus, D.E. Drosophila immune cell migration and adhesion during embryonic development and larval immune responses. Curr. Opin. Cell Biol. 2015, 36, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sakai, M.; Tabu, K.; Wang, L.; Tsunematsu, R.; Tsuda, M.; Sawa, H.; Nagashima, K.; Nishihara, H.; Hatakeyama, S.; et al. Human synovial sarcoma proto-oncogene Syt is essential for early embryonic development through the regulation of cell migration. Lab. Investig. 2009, 89, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Nardini, J.T.; Chapnick, D.A.; Liu, X.D.; Bortz, D.M. Modeling keratinocyte wound healing dynamics: Cell-cell adhesion promotes sustained collective migration. J. Theor. Biol. 2016, 400, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Yang, Y.L.; Zhang, D.D.; Wong, P.K. Advances in Wound-Healing Assays for Probing Collective Cell Migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Arciero, J.C.; Mi, Q.; Branca, M.F.; Hackam, D.J.; Swigon, D. Continuum Model of Collective Cell Migration in Wound Healing and Colony Expansion. Biophys. J. 2011, 100, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xu, X.H.; Hereld, D. Chemotaxis, chemokine receptors and human disease. Cytokine 2008, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.Y.; Heit, B.; Kubes, P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc. Res. 2010, 86, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hazewindus, M.; Haenen, G.R.M.M.; Weseler, A.R.; Bast, A. Protection against Chemotaxis in the Anti-Inflammatory Effect of Bioactives from Tomato Ketchup. PLoS ONE 2014, 9, e114387. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; De Ros, S.C.; Yodoi, J.J.; Holmgren, A.; Ghezzi, P.; Herzenberg, L.A.; Herzenberg, L.A. Chronic elevation of plasma thioredoxin: Inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc. Natl. Acad. Sci. USA 2001, 98, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Y.; Sun, Y.H.; Min, Y.G.; Chi, J.G.; Kim, C.K. Chemotaxis of blood neutrophils from patients with primary ciliary dyskinesia. J. Korean Med. Sci. 2003, 18, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Roussos, E.T.; Condeelis, J.S.; Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer 2011, 11, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Lu, E.Y.; Pan, H.J.; Lee, C.H. Directional migration of cancer cells induced by a blue light intensity gradient. Biomed. Opt. Express 2015, 6, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Tur-Kaspa, I.; Gakamsky, A.; Giojalas, L.C.; Breitbart, H.; Eisenbach, M. Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract. Nat. Med. 2003, 9, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Sheehan, P.E.; Perry, L.L.; O’Connor, K.; Csonka, L.N.; Applegate, B.M.; Whitman, L.J. Quantifying the magnetic advantage in magnetotaxis. Biophys. J. 2006, 91, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.G.; Choi, Y.S.; Alonso-Latorre, B.; del Alamo, J.C.; Engler, A.J. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol. J. 2013, 8, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, B.C.; DiMilla, P.A.; Walker, M.; Kim, S.; Wong, J.Y. Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength. Biophys. J. 2009, 97, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Lachowski, D.; Cortes, E.; Pink, D.; Chronopoulos, A.; Karim, S.A.; Morton, J.P.; Hernandez, A.E.D. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Ford, A.J.; Rajagopalan, P. Opposing Rigidity-Protein Gradients Reverse Fibroblast Durotaxis. ACS Biomater. Sci. Eng. 2015, 1, 621–631. [Google Scholar] [CrossRef]
- Song, S.; Kim, M.; Shin, J.H. Upstream mechanotaxis behavior of endothelial cells. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2106–2110. [Google Scholar]
- Sun, Y.S.; Peng, S.W.; Lin, K.H.; Cheng, J.Y. Electrotaxis of lung cancer cells in ordered three-dimensional scaffolds. Biomicrofluidics 2012, 6, 014102. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Liu, J.; Zhang, X.Z.; Zhang, L.; Jiang, J.Y.; Nolta, J.; Zhao, M. Guided Migration of Neural Stem Cells Derived from Human Embryonic Stem Cells by an Electric Field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Baldessari, F.; Gyenge, C.C.; Sato, T.; Chambers, R.D.; Santiago, J.G.; Butcher, E.C. Lymphocyte electrotaxis in vitro and in vivo. J. Immunol. 2008, 181, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Pu, J.; Forrester, J.V.; McCaig, C.D. Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB J. 2002, 16, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Reid, B.; Cao, L.; Zhao, M. Electrotaxis and wound healing: Experimental methods to study electric fields as a directional signal for cell migration. Methods Mol. Biol. 2009, 571, 77–97. [Google Scholar] [PubMed]
- Nuccitelli, R.; Nuccitelli, P.; Li, C.; Narsing, S.; Pariser, D.M.; Lui, K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Repair Regen. 2011, 19, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Lu, H.H.; Hung, C.T.; Nicoll, S.B.; Bulinski, J.C. Effects of applied DC electric field on ligament fibroblast migration and wound healing. Connect. Tissue Res. 2007, 48, 188–197. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 34117. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Hou, H.S.; Sun, Y.S.; Cheng, J.Y.; Lo, K.Y. Correlation between cell migration and reactive oxygen species under electric field stimulation. Biomicrofluidics 2015, 9, 054120. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive Oxygen Species (Ros)—A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Huo, Y.A.; Qiu, W.Y.; Pan, Q.; Yao, Y.F.; Xing, K.Y.; Lou, M.F. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp. Eye Res. 2009, 89, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Aykin-Burns, N.; Cloud, C.; Cavazos, L.S.; Bickenbach, J.R.; Spitz, D.R. The Role of Oxygen and Reactive Oxygen Species (ROS) in Wound Healing. Free Radic. Biol. Med. 2008, 45, S96. [Google Scholar]
- Braddock, M.; Campbell, C.J.; Zuder, D. Current therapies for wound healing: Electrical stimulation, biological therapeutics, and the potential for gene therapy. Int. J. Dermatol. 1999, 38, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Hunckler, J.; de Mel, A. A current affair: Electrotherapy in wound healing. J. Multidiscip. Healthc. 2017, 10, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Balakatounis, K.C.; Angoules, A.G. Low-intensity electrical stimulation in wound healing: Review of the efficacy of externally applied currents resembling the current of injury. Eplasty 2008, 8, e28. [Google Scholar] [PubMed]
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Reger, S.I.; Hyodo, A.; Negami, S.; Kambic, H.E.; Sahgal, V. Experimental wound healing with electrical stimulation. Artif. Organs 1999, 23, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Asgari-Moghadam, Z.; Maroufi, M.; Rezaie, F.S.; Bayat, M.; Rakhshan, M. Experimental wound healing using microamperage electrical stimulation in rabbits. J. Rehabil. Res. Dev. 2006, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Gu, Y.; Pu, J.; Reid, B.; Zhao, Z.Q.; Zhao, M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007, 2, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Korohoda, W.; Mycielska, M.; Janda, E.; Madeja, Z. Immediate and long-term galvanotactic responses of Amoeba proteus to dc electric fields. Cell Motil. Cytoskelet. 2000, 45, 10–26. [Google Scholar] [CrossRef]
- Sato, M.J.; Ueda, M.; Takagi, H.; Watanabe, T.M.; Yanagida, T.; Ueda, M. Input-output relationship in galvanotactic response of Dictyostelium cells. Biosystems 2007, 88, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Costa, L.; Terekhov, A.; Jowhar, D.; Hofmeister, W.; Janetopoulos, C. On-Chip Open Microfluidic Devices for Chemotaxis Studies. Microsc. Microanal. 2012, 18, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Wu, M.M. Microfluidics for Mammalian Cell Chemotaxis. Ann. Biomed. Eng. 2012, 40, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Allen, S.G.; Ingram, P.N.; Buckanovich, R.; Merajver, S.D.; Yoon, E. Single-cell Migration Chip for Chemotaxis-Based Microfluidic Selection of Heterogeneous Cell Populations. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Ludi, H.; Widmer, H.M. Planar Chips Technology for Miniaturization and Integration of Separation Techniques into Monitoring Systems—Capillary Electrophoresis on a Chip. J. Chromatogr. 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Harrison, D.J.; Manz, A.; Fan, Z.H.; Ludi, H.; Widmer, H.M. Capillary Electrophoresis and Sample Injection Systems Integrated on a Planar Glass Chip. Anal. Chem. 1992, 64, 1926–1932. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. Gas-Chromatographic Air Analyzer Fabricated on a Silicon-Wafer. IEEE Trans. Electron. Dev. 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.Y.; Chen, G. Fabrication, modification, and application of department of analytical poly(methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, Y.H.; Chen, G. Fabrication of poly(methyl methacrylate) microfluidic chips by redox-initiated polymerization. Electrophoresis 2007, 28, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Muck, A.; Wang, J.; Jacobs, M.; Chen, G.; Chatrathi, M.P.; Jurka, V.; Vyborny, Z.; Spillman, S.D.; Sridharan, G.; Schoning, M.J. Fabrication of poly(methyl methacrylate) microfluidic chips by atmospheric molding. Anal. Chem. 2004, 76, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Du, X.G.; Guan, Y.X.; Wang, F.R.; Fang, Z.L. Fabrication of poly(methyl methacrylate) (PMMA) microfluidic chips by a simple hot embossing method. Chem. J. Chin. Univ. 2003, 24, 1962–1966. [Google Scholar]
- Djamgoz, M.B.A.; Mycielska, M.; Madeja, Z.; Fraser, S.P.; Korohoda, W. Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltagegated Na+ channel activity. J. Cell Sci. 2001, 114 Pt 14, 2697–2705. [Google Scholar] [PubMed]
- Gamboa, O.L.; Pu, J.; Townend, J.; Forrester, J.V.; Zhao, M.; McCaig, C.; Lois, N. Electrical estimulation of retinal pigment epithelial cells. Exp. Eye Res. 2010, 91, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hammerick, K.E.; Longaker, M.T.; Prinz, F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010, 397, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.P.; Wang, F.; Guo, A.H.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab Chip 2010, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Minc, N.; Chang, F. Electrical Control of Cell Polarization in the Fission Yeast Schizosaccharomyces pombe. Curr. Biol. 2010, 20, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nandagopal, S.; Wu, D.; Romanuik, S.F.; Paul, K.; Thomson, D.J.; Lin, F. Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 2011, 11, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.W.; Zhu, K.; Zhang, Y.; Zhu, Z.J.; Xu, Z.P.; Zhao, M.; Pan, T.R. ElectroTaxis-on-a-Chip (ETC): An integrated quantitative high-throughput screening platform for electrical field-directed cell migration. Lab Chip 2014, 14, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Xu, T.; Zou, H.; Chen, X.M.; Sun, D.; Yang, M.S. Cell migration microfluidics for electrotaxis-based heterogeneity study of lung cancer cells. Biosens. Bioelectron. 2017, 89, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yang, J.; Yang, M.S. Dose-dependent cell-based assays in V-shaped microfluidic channels. Lab Chip 2006, 6, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gupta, B.; Selvaganapathy, P.R. An automated microfluidic system for screening Caenorhabditis elegans behaviors using electrotaxis. Biomicrofluidics 2016, 10, 014117. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Parashar, A.; Gibson, R.; Robertson, A.P.; Martin, R.J.; Pandey, S. A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab Chip 2011, 11, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, R.; Ge, A.; Hu, L.; Wang, S.; Feng, X.; Du, W.; Liu, B.F. Highly efficient microfluidic sorting device for synchronizing developmental stages of C. elegans based on deflecting electrotaxis. Lab Chip 2015, 15, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, L.; Zhang, M.; Lin, F. Microfluidic device for studying cell migration in single or co-existing chemical gradients and electric fields. Biomicrofluidics 2012, 6, 024121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Arkonac, D.E.; Chao, P.H.; Vunjak-Novakovic, G. Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, C.Y.; Li, D.; Zheng, X.L.; Tang, J.B.; Bu, J.Y.; Sun, H.; Yang, Z.K.; Sun, W.J.; Yu, X.G. Calcium Ion Flow Permeates Cells through SOCs to Promote Cathode-Directed Galvanotaxis. PLoS ONE 2015, 10, e0139865. [Google Scholar] [CrossRef] [PubMed]
- Lalli, M.L.; Asthagiri, A.R. Collective Migration Exhibits Greater Sensitivity But Slower Dynamics of Alignment to Applied Electric Fields. Cell. Mol. Bioeng. 2015, 8, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Cheng, J.Y.; Yen, M.H.; Young, T.H. Electrotaxis of lung cancer cells in a multiple-electric-field chip. Biosens. Bioelectron. 2009, 24, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Yen, M.H.; Kuo, C.T.; Young, T.H. A transparent cell-culture microchamber with a variably controlled concentration gradient generator and flow field rectifier. Biomicrofluidics 2008, 2, 024105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Wei, C.W.; Hsu, K.H.; Young, T.H. Direct-write laser micromachining and universal surface modification of PMMA for device development. Sens. Actuator B Chem. 2004, 99, 186–196. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Yen, M.H.; Hsu, W.C.; Jhang, J.H.; Young, T.H. ITO patterning by a low power Q-switched green laser and its use in the fabrication of a transparent flow meter. J. Micromech. Microeng. 2007, 17, 2316–2323. [Google Scholar] [CrossRef]
- Huang, C.W.; Chen, H.Y.; Yen, M.H.; Chen, J.J.W.; Young, T.H.; Cheng, J.Y. Gene Expression of Human Lung Cancer Cell Line CL1–5 in Response to a Direct Current Electric Field. PLoS ONE 2011, 6, e25928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.F.; Peng, S.W.; Wu, C.Y.; Chang, H.F.; Cheng, J.Y. Electrotaxis of oral squamous cell carcinoma cells in a multiple-electric-field chip with uniform flow field. Biomicrofluidics 2012, 6, 034116. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109 Pt 4, 713–726. [Google Scholar] [PubMed]
- Potter, C.M.F.; Lundberg, M.H.; Harrington, L.S.; Warboys, C.M.; Warner, T.D.; Berson, R.E.; Moshkov, A.V.; Gorelik, J.; Weinberg, P.D.; Mitchell, J.A. Role of Shear Stress in Endothelial Cell Morphology and Expression of Cyclooxygenase Isoforms. Arterioscler. Thromb. Vasc. 2011, 31, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.; Tambe, D.; Hardin, C.C.; Krishnan, R.; Fredberg, J.J. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol. Cell Physiol. 2015, 308, C657–C664. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Huang, C.W.; Chang, H.F.; Chen, J.J.W.; Lee, C.H.; Cheng, J.Y. Evaluation of EGFR and RTK Signaling in the Electrotaxis of Lung Adenocarcinoma Cells under Direct-Current Electric Field Stimulation. PLoS ONE 2013, 8, e73418. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Kao, Y.C.; Chi, P.Y.; Huang, C.W.; Lin, J.Y.; Chou, C.F.; Cheng, J.Y.; Lee, C.H. Asymmetric cancer-cell filopodium growth induced by electric-fields in a microfluidic culture chip. Lab Chip 2011, 11, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Mong, H.Y.; Lin, W.C. Noninterferometric wide-field optical profilometry with nanometer depth resolution. Opt. Lett. 2002, 27, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Xu, T.; Chen, X.M.; Lin, S.; Cho, M.; Sun, D.; Yang, M.S. Effects of direct current electric fields on lung cancer cell electrotaxis in a PMMA-based microfluidic device. Anal. Bioanal. Chem. 2017, 409, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Hsieh, M.H.; Liu, C.C.; Pan, H.J.; Liao, W.Y.; Cheng, J.Y.; Kuo, P.L.; Lee, C.H. Modulating chemotaxis of lung cancer cells by using electric fields in a microfluidic device. Biomicrofluidics 2014, 8, 024107. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.S.; Tsai, H.F.; Chiu, H.T.; Cheng, J.Y. Simultaneous chemical and electrical stimulation on lung cancer cells using a multichannel-dual-electric-field chip. Biomicrofluidics 2014, 8, 052007. [Google Scholar] [CrossRef]
- Lo, K.Y.; Wu, S.Y.; Sun, Y.S. A microfluidic device for studying the production of reactive oxygen species and the migration in lung cancer cells under single or coexisting chemical/electrical stimulation. Microfluid. Nanofluid. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Hou, H.S.; Chang, H.F.; Cheng, J.Y. Electrotaxis Studies of Lung Cancer Cells Using a Multichannel Dual-Electric-Field Microfluidic Chip. J. Vis. Exp. 2015, e53340. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.Y.; Sun, Y.S.; Hou, H.S.; Wu, S.Y.; Zhu, Y.; Cheng, J.Y.; Lo, K.Y. Designing Microfluidic Devices for Studying Cellular Responses Under Single or Coexisting Chemical/Electrical/Shear Stress Stimuli. J. Vis. Exp. 2016, e54397. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Lee, C.H.; Chen, B.C. Imaging live cells at high spatiotemporal resolution for lab-on-a-chip applications. Lab Chip 2016, 16, 2014–2024. [Google Scholar] [CrossRef] [PubMed]


| Advantages | Drawbacks | References | ||
|---|---|---|---|---|
| Conventional Devices | Dish-based or coverslip-based |
|
| [47,49,62,63,64] |
| Transwell-based |
|
| [29] | |
| Microfluidic Devices | PDMS-based |
|
| [66,67,68,69,70,72,73,74,75,76,77,78] |
| PMMA-based |
|
| [27,29,35,79,83,84,88,89,91] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-S. Studying Electrotaxis in Microfluidic Devices. Sensors 2017, 17, 2048. https://doi.org/10.3390/s17092048
Sun Y-S. Studying Electrotaxis in Microfluidic Devices. Sensors. 2017; 17(9):2048. https://doi.org/10.3390/s17092048
Chicago/Turabian StyleSun, Yung-Shin. 2017. "Studying Electrotaxis in Microfluidic Devices" Sensors 17, no. 9: 2048. https://doi.org/10.3390/s17092048





