Excellent Fireproof Characteristics and High Thermal Stability of Rice Husk-Filled Polyurethane with Halogen-Free Flame Retardant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PU-RHs with and without FRs
2.3. Characterization Equipment
2.3.1. Spectroscopic Analysis:
2.3.2. Field emission scanning electron microscope
2.3.3. Thermogravimetric Analysis
2.3.4. Flame-Retardant Test
2.3.5. Mechanical Test
2.3.6. Density Measurement
2.3.7. Measurement of Sorption Isotherms
3. Results and discussion
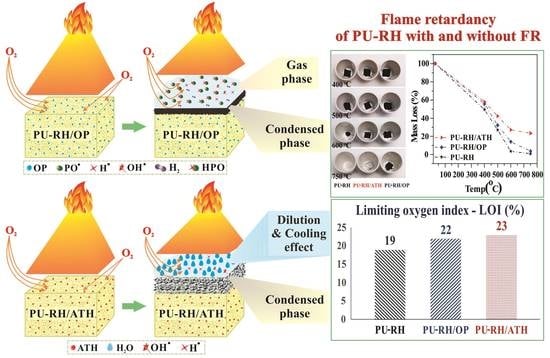
3.1. Fire-Retardant Performances
3.2. Thermal Decomposition Properties
3.3. Fire-Retardant Mode of Action
3.4. Physical and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Konig, A.; Kroke, E. Flame retardancy working mechanism of methyl-DOPO and MPPP in flexible polyurethane foam. Fire Mater. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Zatorski, W.; Brzozowski, Z.K.; Kolbrecki, A. New developments in chemical modification of fire-safe rigid polyurethane foams. Polym. Degrad. Stab. 2008, 93, 2071–2076. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Kulesza, K.; Pielichowski, K. Thermal decomposition of bisphenol A-based polyether urethanes blown with pentane: Part II—Influence of the novel NaH2PO4/NaHSO4 flame retardant system. J. Anal. Appl. Pyrolysis 2006, 76, 249–253. [Google Scholar] [CrossRef]
- Patrick, J.; Sottos, N.; White, S. Microvascular based self-healing polymeric foam. Polymer 2012, 53, 4231–4240. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schirald, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Mosiewicki, M.A.; Yoshida, M.I.; Silva, M.C.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash I: Synthesis and physical chemical characterization. Polym. Test. 2013, 32, 438–445. [Google Scholar] [CrossRef]
- Herrmann, A.S.; Nickel, J.; Riedel, U. Construction materials based upon biologically renewable resources from components to finished parts. Polym. Degrad. Stab. 1998, 59, 251–261. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohyd. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Kozlowsk, R.; Mieleniak, B. New trends in the utilization of byproducts of fibre crops residue in pulp and paper industry, building engineering, automotive industry and interior furnishing. In Proceedings of the Third International Symposium on Natural Polymers and Composites, São Pedro, Brazil, 14–17 May 2000; Mattoso, L., Leao, A., Frollini, E., Eds.; Embrapa Instrumentação Agropecuária: São Pedro, Brazil, 2000; pp. 504–510. [Google Scholar]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Padkho, N. A new design recycle agricultural waste materials for profitable use rice straw and maize husk in wall. Procedia Eng. 2012, 32, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Battegazzore, D.; Alongi, J.; Frache, A. Poly(lactic acid)-based composites containing natural fillers: Thermal, mechanical and barrier properties. J. Polym. Environ. 2014, 22, 88–98. [Google Scholar] [CrossRef]
- Faludi, G.; Hári, J.; Móczó, J.; Pukánszky, B. Fiber association and network formation PLA/lignocellulosic fiber composites. Compos. Sci. Technol. 2013, 77, 67–73. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Wood-fiber-reinforced poly(lactic acid) composites: Evaluation of the physicomechanical and morphology properties. J. Appl. Polym. Sci. 2006, 102, 4856–4869. [Google Scholar] [CrossRef]
- Bogren, K.M.; Gamstedt, E.K.; Neagu, R.C.; AÅkerholm, M.; LindstroÖm, M. Dynamic-mechanical properties of wood-fiber reinforced polylactide: Experimental characterization and micromechanical modeling. J. Thermoplast. Compos. Mater. 2006, 19, 613–637. [Google Scholar] [CrossRef]
- Freivalde, L.; Kukle, S.; Andzs, M.; Buksans, E.; Gravitis, J. Flammablity of raw insulation materials made of hemp. Compos. Part B Eng. 2014, 67, 510–514. [Google Scholar] [CrossRef]
- Palumbo, M.; Formosa, J.; Lacasta, A.M. Thermal degradation and fire behavior of thermal insulation materials based on food crop by-products. Constr. Build. Mater. 2015, 79, 34–39. [Google Scholar] [CrossRef]
- Gallo, E.; Sanchez-Olivares, G.; Schartel, B. Flame retardancy of starch-based biocomposites—Aluminum hydroxide coconut fiber synergy. Polimery 2013, 58, 395–403. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.Y.; Ji, X.; Li, Z.M.; Tang, J.H. Synthesis and characterization of expandable graphite–poly(methyl methacrylate) composite particles and their application to flame retardation of rigid polyurethane foams. Polym. Degrad. Stab. 2009, 94, 971–979. [Google Scholar] [CrossRef]
- Duquesne, S.; LeBras, M.; Bourbigot, S.; Delobel, R.; Vezin, H.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Expandable graphite: A fire retardant additive for polyurethane coatings. Fire Mater. 2003, 27, 103–117. [Google Scholar] [CrossRef]
- Chen, X.X.; Li, J.F.; Ming, G. Thermal degradation and flame retardant mechanism of the rigid polyurethane foam including functionalized graphene oxide. Polymers 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Kirpluks, M.; Cabulis, U.; Zeltins, V.; Stiebra, L.; Avots, A. Rigid polyurethane foam thermal insulation protected with mineral intumescent mat. Autex Res. J. 2014, 14, 259–269. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Gudarzi, M.M. Intumescent flame retardant polyurethane/reduced grapheme oxide composites with improved mechanical, thermal, and barrier properties. J. Mater. Sci. 2014, 49, 243–254. [Google Scholar] [CrossRef]
- Acuña, P.; Li, Z.; Santiago-Calvo, M.; Villafañe, F.; Rodríguez-Perez, M.A.; Wang, D.Y. Influence of the characteristics of expandable graphite on the morphology, thermal properties, fire behaviour and compression performance of a rigid polyurethane foam. Polymers 2019, 11, 168. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.J.; Li, L.J. Flame Retardant behavior of ternary synergistic systems in rigid polyurethane foams. Polymers 2019, 11, 207. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron–grapheme and melamine salt of pentaerythritol phosphate on flame retardant thermoplastic polyurethane. Polym. Adv. Technol. 2016, 27, 1508–1516. [Google Scholar] [CrossRef]
- Battegazzore, D.; Alongi, J.; Duraccio, D.; Frache, A. Reuse and valorisation of hemp fibres and rice husk particles for fire resistant fibre boards and particle boards. J. Polym. Environ. 2018, 26, 3731–3744. [Google Scholar] [CrossRef]
- Evans, W.; Isaac, D.; Suddell, B.; Crosky, A. Natural fibres and their composites: A global perspective. In Proceedings of the Risø International Symposium on Materials Science, Roskilde, Denmark, 2–5 September 2002; Lilholt, H., Madsen, B., Toftegaard, H.L., Cendre, E., Megins, M., Mikkelsen, L.P., Sørensen, B.F., Eds.; Risø National Laboratory: Roskilde, Denmark, 2002; pp. 1–14. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- ISO. Hygrothermal Performance of Building Materials and Products-Determination of Hygroscopic Sorption Properties; ISO12571:2000(E); ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Roels, S.; Talukdar, P.; James, C.; Simonson, C.J. Reliability of material data measurements for hygroscopic buffering. Int. J. Heat Mass Transf. 2010, 53, 5355–5363. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zillig, W.; Künzel, H.M.; Zhang, X. Evaluation of moisture sorption models and modified Mualem model for prediction of desorption isotherm for wood materials. Build. Environ. 2015, 92, 387–395. [Google Scholar] [CrossRef]
- Pan, H.F.; Shen, Q.; Zhang, Z.N.; Yu, B.H.; Lu, Y.S. MoS2-filled coating on flexible polyurethane foam via layer-by-layer assembly technique: flame-retardant and smoke suppression properties. J. Mater. Sci. 2018, 53, 9340–9349. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the modes of action of phosphorus-based flame retardants in polymeric systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schartel, B. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine cyanurate in glass-fibre-reinforced poly(1,4-butyleneterephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Langfeld, K.; Wilke, A.; Sut, A.; Greiser, A.; Ulmer, B.; Andrievici, V.; Limbach, P.; Bastian, M.; Schartel, B. Halogen-free fire retardant styrene-ethylene-butylene-styrene-based thermo plastic elastomers using synergistic aluminum diethylphosphinate–based combinations. J. Fire Sci. 2015, 33, 157–177. [Google Scholar] [CrossRef]
- Hoang, D.Q.; Nguyen, T.H.; An, H.Y.; Kim, J.H. Organo-phosphorus flame retardants for unsaturated polyester derived from recycled poly(ethylene terephthalate). Macromol. Res. 2016, 24, 537–546. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On the performance and mechanism of brominated and halogen free flame retardants in formulations of glass fibre reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2014, 104, 71–86. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly(1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Rao, W.H.; Liao, W.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Flame-retardant and smoke-suppressant flexible polyurethane foams based on reactive phosphorus-containing polyol and expandable graphite. J. Hazard. Mater. 2018, 360, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, H.Y.; Yu, B.; Shi, Y.Q.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y.M. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Xu, Y.J.; Wang, J.; Tan, Y.; Qi, M.; Chen, L.; Wang, Y.Z. A novel and feasible approach for one-pack flame-retardant epoxy resin with long pot life and fast curing. Chem. Eng. J. 2018, 337, 30–39. [Google Scholar] [CrossRef]
- Song, L.; He, Q.L.; Hu, Y.; Chen, H.; Liu, L. Study on thermal degradation and combustion behaviors of PC/POSS hybrids. Polym. Degrad. Stab. 2008, 93, 627–639. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G.X. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Song, L.; Lu, H.D.; Wang, Z.Z. Flame retardancy and thermal degradation of intumescent flame retardant starch-based biodegradable composites. Ind. Eng. Chem. Res. 2009, 48, 3150–3157. [Google Scholar] [CrossRef]
- Shih, P.Y.; Yung, S.W.; Chin, T.S. Thermal and corrosion behavior of P2O5-Na2O-CuO glasses. J. Non-Cryst. Solid. 1998, 224, 143–152. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Data for Use in X-ray Photoelectron Spectroscopy; Muilenberg, G.E., Ed.; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1979. [Google Scholar]
- Fredriksson, M.; Thybring, E.E. Scanning or desorption isotherms? Characterising sorption hysteresis of wood. Cellulose 2018, 25, 4477–4485. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirt, P.; Kanchanda, P.; Sathonsaowaphak, A.; Cao, H.T. Sulfate resistance of blended cements containing fly ash and rice husk ash. Constr. Build. Mater. 2007, 21, 1356–1361. [Google Scholar] [CrossRef]
- Zhao, Q.; Tao, J.; Yam, R.C.M.; Mok, A.C.K.; Li, R.K.Y.; Song, C. Biodegradation behavior of polycaprolactone/rice husk ecocomposites in simulated soil medium. Polym. Degrad. Stab. 2008, 93, 1571–1576. [Google Scholar] [CrossRef]










| Samples | PU–RH*/FR (wt/wt) | ATH (php) | OP (php) |
|---|---|---|---|
| PU–RH | 100.0/0.0 | - | - |
| PU–RH/ATH100 | 73.0/27.0 | 100 | - |
| PU–RH/ATH125 | 68.0/32.0 | 125 | - |
| PU–RH/OP15 | 95.0/5.0 | - | 15 |
| PU–RH/OP20 | 93.0/7.0 | - | 20 |
| Samples | PU–RH/FR(wt/wt) | LOI(%) | UL94 HB | UL94 V | ||
|---|---|---|---|---|---|---|
| t1/t2a | Dripping | Rating | ||||
| PU–RH | 100.0/0.0 | 19 | Fail | No rating | ||
| PU–RH/ATH100 | 73.0/27.0 | - | HB | 8/7 | No | V-1 |
| PU–RH/ATH125 | 68.0/32.0 | 23 | HB | 1/1 | No | V-0 |
| PU–RH/OP15 | 95.0/5.0 | - | 77 mm/min (Fail) | 5/1 | No | * |
| PU–RH/OP20 | 93.0/7.0 | 22 | HB | 2/1 | No | V-0 |
| Samples | PU–RH/FR (wt/wt) | T5 (°C) | T50 (°C) | Residue at 550 °C (%) | Residue at 750 °C (%) |
|---|---|---|---|---|---|
| PU–RH | 100/0 | 234 | 364 | 35.0 | 1.3 |
| PU–RH/ATH125 | 68/32 | 266 | 537 | 48.7 | 22.2 |
| PU–RH/OP20 | 93/7 | 274 | 496 | 46.1 | 17.0 |
| Samples | C1s (%) | O1s (%) | Al2p (%) | P2p (%) |
|---|---|---|---|---|
| PU–RH | 81.66 | 16.71 | - | - |
| PU–RH/ATH125 | 15.73 | 59.71 | 25.1 | - |
| PU–RH/OP20 | 26.42 | 50.55 | 9.93 | 13.11 |
| Samples | PU–RH/FR (wt/wt) | Apparent Density (kg/m3) | Compressive Strength (kPa) |
|---|---|---|---|
| PU–RH | 100/0 | 57.02 0.53 | 219.96 11.80 |
| PU–RH/ATH125 | 68/32 | 84.94 0.67 | 164.96 17.42 |
| PU–RH/OP20 | 93/7 | 58.62 1.07 | 152.91 4.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, H.T.Q.; Nguyen, B.T.; Pham, L.H.; Pham, C.T.; Do, T.V.V.; Hoang, C.N.; Nguyen, N.N.; Kim, J.; Hoang, D. Excellent Fireproof Characteristics and High Thermal Stability of Rice Husk-Filled Polyurethane with Halogen-Free Flame Retardant. Polymers 2019, 11, 1587. https://doi.org/10.3390/polym11101587
Phan HTQ, Nguyen BT, Pham LH, Pham CT, Do TVV, Hoang CN, Nguyen NN, Kim J, Hoang D. Excellent Fireproof Characteristics and High Thermal Stability of Rice Husk-Filled Polyurethane with Halogen-Free Flame Retardant. Polymers. 2019; 11(10):1587. https://doi.org/10.3390/polym11101587
Chicago/Turabian StylePhan, Huong T.Q., Binh T. Nguyen, Lam H. Pham, Chi T. Pham, Thi Vi Vi Do, Cuong N. Hoang, Nguyen Ngan Nguyen, Jinhwan Kim, and DongQuy Hoang. 2019. "Excellent Fireproof Characteristics and High Thermal Stability of Rice Husk-Filled Polyurethane with Halogen-Free Flame Retardant" Polymers 11, no. 10: 1587. https://doi.org/10.3390/polym11101587