Associations between Dietary Fiber Intake in Infancy and Cardiometabolic Health at School Age: The Generation R Study
Abstract
:1. Introduction
2. Subjects and Methods
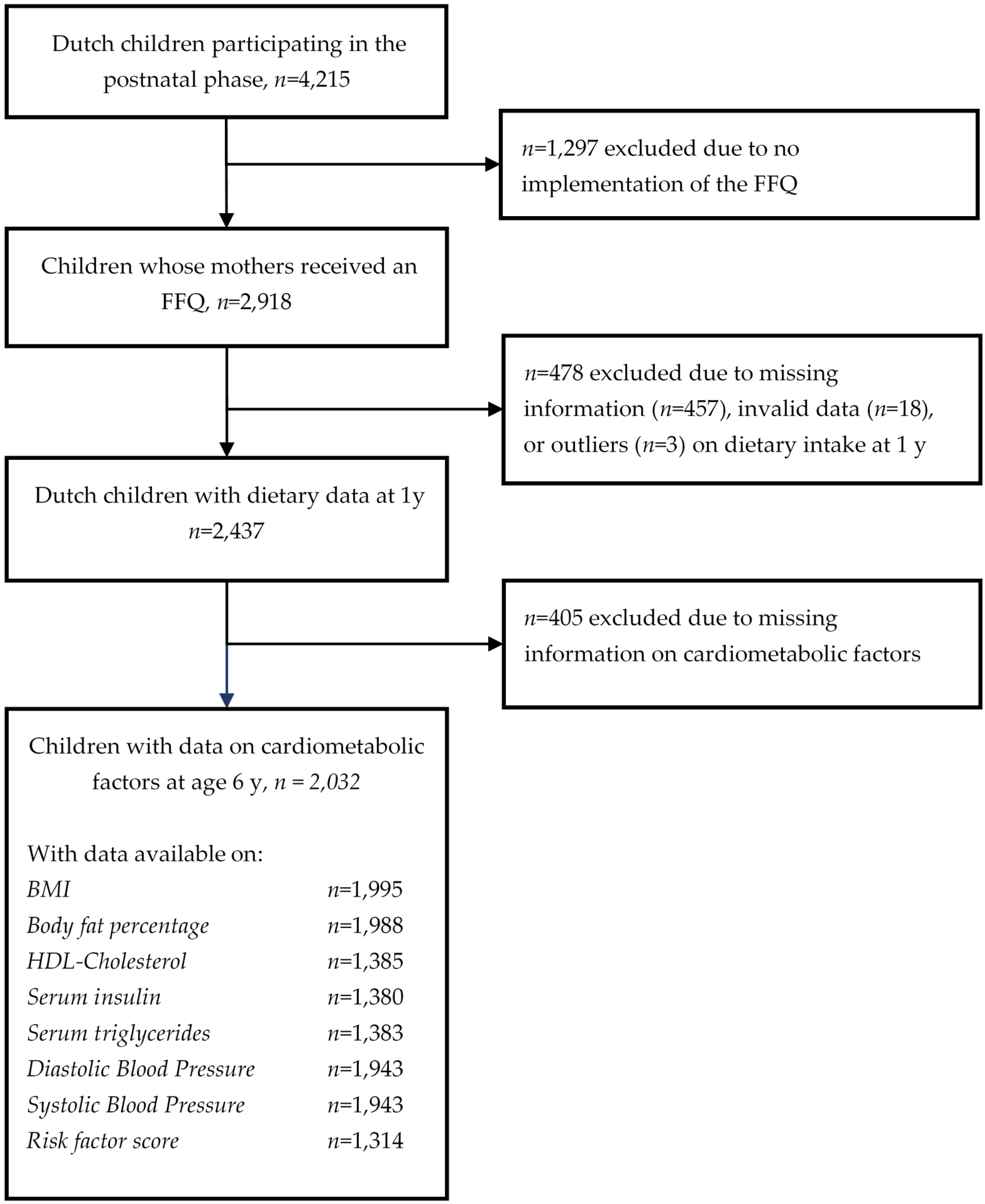
2.1. Study Design and Subjects
2.2. Dietary Intake Assessment
2.3. Cardiometabolic Health Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Associations between DF Intake and Cardiometabolic Health
3.3. Associations between DF Intake from Different Sources and Cardiometabolic Health
3.4. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BF% | Body Fat Percentage |
| BMI | Body Mass Index |
| DBP | Diastolic Blood Pressure |
| DF | Dietary Fiber |
| FFQ | Food Frequency Questionnaire |
| HDL-C | High Density Lipoprotein Cholesterol |
| LDL-C | Low Density Lipoprotein Cholesterol |
| SBP | Systolic Blood Pressure |
| SDS | Standard Deviation Score |
References
- Grooms, K.N.; Ommerborn, M.J.; Pham, D.Q.; Djousse, L.; Clark, C.R. Dietary fiber intake and cardiometabolic risks among us adults, nhanes 1999–2010. Am. J. Med. 2013, 126, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Pereira, M.A.; Kroenke, C.H.; Hilner, J.E.; Van Horn, L.; Slattery, M.L.; Jacobs, D.R., Jr. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA 1999, 282, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics 1998, 101, 518–525. [Google Scholar] [PubMed]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Glueck, C.J.; Wang, P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: The princeton-lipid research clinics follow-up study. Metabolism 2012, 61, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Glueck, C.J.; Woo, J.G.; Wang, P. Risk factors for cardiovascular disease and type 2 diabetes retained from childhood to adulthood predict adult outcomes: The princeton lrc follow-up study. Int. J. Pediatr. Endocrinol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Karaolis-Danckert, N.; Libuda, L.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relation of dietary glycemic index, glycemic load, and fiber and whole-grain intakes during puberty to the concurrent development of percent body fat and body mass index. Am. J. Epidemiol. 2009, 169, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ruottinen, S.; Lagstrom, H.K.; Niinikoski, H.; Ronnemaa, T.; Saarinen, M.; Pahkala, K.A.; Hakanen, M.; Viikari, J.S.; Simell, O. Dietary fiber does not displace energy but is associated with decreased serum cholesterol concentrations in healthy children. Am. J. Clin. Nutr. 2010, 91, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Brauchla, M.; Juan, W.; Story, J.; Kranz, S. Sources of dietary fiber and the association of fiber intake with childhood obesity risk (in 2–18 year olds) and diabetes risk of adolescents 12–18 year olds: Nhanes 2003–2006. J. Nutr. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Cheng, G.; Gunther, A.L.; Liese, A.D.; Remer, T.; Karaolis-Danckert, N. Relation of dietary glycemic index, glycemic load, added sugar intake, or fiber intake to the development of body composition between ages 2 and 7 y. Am. J. Clin. Nutr. 2008, 88, 755–762. [Google Scholar] [PubMed]
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Kelly, L.A.; Lane, C.J.; Byrd-Williams, C.E.; Toledo-Corral, C.M.; Roberts, C.K.; Spruijt-Metz, D.; Weigensberg, M.J.; et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight latino youth. Am. J. Clin. Nutr. 2007, 86, 1331–1338. [Google Scholar] [PubMed]
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Toledo-Corral, C.M.; Goran, M.I. Inverse relation between dietary fiber intake and visceral adiposity in overweight latino youth. Am. J. Clin. Nutr. 2009, 90, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huybrechts, I.; Vereecken, C.; Mouratidou, T.; Valtuena, J.; Kersting, M.; Gonzalez-Gross, M.; Bolca, S.; Warnberg, J.; Cuenca-Garcia, M.; et al. Dietary fiber intake and its association with indicators of adiposity and serum biomarkers in european adolescents: The helena study. Eur. J. Nutr. 2015, 54, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Jenner, D.A.; English, D.R.; Vandongen, R.; Beilin, L.J.; Armstrong, B.K.; Miller, M.R.; Dunbar, D. Diet and blood pressure in 9-year-old australian children. Am. J. Clin. Nutr. 1988, 47, 1052–1059. [Google Scholar]
- Rinaldi, A.E.; de Oliveira, E.P.; Moreto, F.; Gabriel, G.F.; Corrente, J.E.; Burini, R.C. Dietary intake and blood lipid profile in overweight and obese schoolchildren. BMC Res. Notes 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; van den Hooven, E.H.; Tielemans, M.J.; Hofman, A.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; Franco, O.H. Protein intake in early childhood and cardiometabolic health at school age: The generation R study. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.A.; Parrett, A.M. Dietary fibre in infancy and childhood. Proc. Nutr. Soc. 2003, 62, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Brauchla, M.; Slavin, J.L.; Miller, K.B. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv. Nutr. 2012, 3, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Xie, C.; Garcia, A.L. Dietary fibre and health in children and adolescents. Proc. Nutr. Soc. 2015, 74, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.J.; Agostoni, C.; Axelsson, I.; Edwards, C.A.; Goulet, O.; Hernell, O.; Koletzko, B.; Lafeber, H.N.; Micheli, J.L.; Michaelsen, K.F.; et al. Nondigestible carbohydrates in the diets of infants and young children: A commentary by the espghan committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.A.; Higbee, D.R.; Donahoo, W.T.; Brown, I.L.; Bell, M.L.; Bessesen, D.H. Resistant starch consumption promotes lipid oxidation. Nutr. Metab. 2004, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Jaddoe, V.W.; van Duijn, C.M.; Franco, O.H.; van der Heijden, A.J.; van Iizendoorn, M.H.; de Jongste, J.C.; van der Lugt, A.; Mackenbach, J.P.; Moll, H.A.; Raat, H.; et al. The generation r study: Design and cohort update 2012. Eur. J. Epidemiol. 2012, 27, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Kiefte-de Jong, J.C.; de Vries, J.H.; Bleeker, S.E.; Jaddoe, V.W.; Hofman, A.; Raat, H.; Moll, H.A. Socio-demographic and lifestyle determinants of ‘western-like’ and ‘health conscious’ dietary patterns in toddlers. Br. J. Nutr. 2013, 109, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Kiefte-de Jong, J.C.; Geelen, A.; Villamor, E.; Moll, H.A.; de Jongste, J.C.; Raat, H.; Hofman, A.; Jaddoe, V.W.; Franco, O.H.; et al. The development of a diet quality score for preschool children and its validation and determinants in the generation R study. J. Nutr. 2015, 145, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, S.; Jansen-van der Vliet, M.; Brants, H.A.M.; van der Heijden, L.J.M.; Hulshof, K.F.A.M.; Langius, J.A.E.; van Oosten, H.M.; Pruissen-Boskaljon, J.C. Nevo-Table 2006. Dutch Food Composition Table 2006; The NEVO Foundation and the Dutch Nutrition Centre: The Hague, The Netherlands, 2006. [Google Scholar]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.V.; Erler, N.S.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; van den Hooven, E.H.; Franco, O.H.; Voortman, T. Dietary intake of protein in early childhood is associated with growth. J. Nutr. 2016, in press. [Google Scholar]
- Voortman, T.; Braun, K.V.; Kiefte-de Jong, J.C.; Jaddoe, V.W.; Franco, O.H.; van den Hooven, E.H. Protein intake in early childhood and body composition at the age of 6 years: The generation r study. Int. J. Obes. 2016, 40, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, C.J.; Kooijman, M.N.; van Duijn, C.M.; Franco, O.H.; de Jongste, J.C.; Klaver, C.C.; Mackenbach, J.P.; Moll, H.A.; Raat, H.; Rings, E.H.; et al. The generation R study: Biobank update 2015. Eur. J. Epidemiol. 2014, 29, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.N.; Tz Sung, R.Y.; Leung, L.C. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press. Monit. 2006, 11, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE Publications Ltd.: London, UK, 2013; pp. 176–180. [Google Scholar]
- Eisenmann, J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- Statistics Netherlands. Dutch Standard Classification of Education 2003 (Standaard Onderwijsindeling 2003); Statistics Netherlands (Centraal Bureau voor de Statistiek): Den Haag/Heerlen, The Netherlands, 2003.
- Niklasson, A.; Ericson, A.; Fryer, J.G.; Karlberg, J.; Lawrence, C.; Karlberg, P. An update of the swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr. Scand. 1991, 80, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kocevska, D.; Voortman, T.; Dashti, H.S.; van den Hooven, E.H.; Ghassabian, A.; Rijlaarsdam, J.; Schneider, N.; Feskens, E.J.; Jaddoe, V.W.; Tiemeier, H.; et al. Macronutrient intakes in infancy are associated with sleep duration in toddlerhood. J. Nutr. 2016, 146, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [PubMed]
- Hastie, T.J. Generalized Additive Models. Chapter 7 of Statistical Models in S; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992. [Google Scholar]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- Health Council of The Netherlands. Guideline for Dietary Fiber Intake; Health Council of The Netherlands: The Hague, The Netherlands, 2006; pp. 1–90.
- Carlson, J.J.; Eisenmann, J.C.; Norman, G.J.; Ortiz, K.A.; Young, P.C. Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in us adolescents. J. Am. Diet. Assoc. 2011, 111, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.W. Food fibre as an obstacle to energy intake. Lancet 1973, 2, 1418–1421. [Google Scholar] [CrossRef]
- Fernandez, M.L. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr. Opin. Lipidol. 2001, 12, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Willis, H.J.; Eldridge, A.L.; Beiseigel, J.; Thomas, W.; Slavin, J.L. Greater satiety response with resistant starch and corn bran in human subjects. Nutr. Res. 2009, 29, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effect of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, D.; Naugler, C. Fasting time and lipid levels in a community-based population: A cross-sectional study. Arch. Intern. Med. 2012, 172, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Hancox, R.J.; Landhuis, C.E. Correlation between measures of insulin resistance in fasting and non-fasting blood. Diabetol. Metab. Syndr. 2011, 3, 23. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD, Median (IQR), or n (%) | |
|---|---|
| Infancy Characteristics | |
| Gestational age at birth (weeks) | 40.1 (39.3–41.1) |
| Birth weight (g) | 3499 ± 563 |
| Girls (n) | 1031 (50.7%) |
| Receiving breastfeeding | |
| ● Never | 272 (13.3%) |
| ● Partial in the first 4 months | 1154 (56.8%) |
| ● Exclusively in the first 4 months | 606 (29.9%) |
| Timing of introduction of fruits and vegetables | |
| ● <4 months | 162 (7.9%) |
| ● 4–6 months | 1716 (84.4%) |
| ● ≥6 months | 154 (7.7%) |
| Characteristics at Dietary Assessment | |
| Age (months) | 12.9 (12.6–13.9) |
| Dietary fiber (DF) intake (g/day) | 15.0 ± 4.3 |
| ● DF from cereals | 8.0 (6.2–10.0) |
| ● DF from potatoes | 1.1 (0.4–1.9) |
| ● DF from fruit and vegetables | 4.7 (3.2–6.2) |
| ● DF from legumes | 0.2 (0.0–0.6) |
| Energy intake (kcal/day) | 1267 (1070–1491) |
| Receiving any dietary supplements | 973 (47.9%) |
| Characteristics at Cardiometabolic Health Assessment | |
| Age (year) | 5.9 (5.8–6.1) |
| Height (cm) | 118 (115–122) |
| Weight (kg) | 21.8 (20.2–23.8) |
| BMI (kg/m2) (n = 1995) | 15.6 (15.0–16.5) |
| Body fat percentage (n = 1988) | 23.1 (20.4–26.4) |
| Serum HDL-cholesterol (mmol/L) (n = 1385) | 1.33 ± 0.30 |
| Serum insulin (pmol/L) (n = 1380) | 114 (63.8–183.6) |
| Serum triglycerides (mmol/L) (n = 1383) | 0.98 (0.72–1.29) |
| Diastolic blood pressure (mmHg) (n = 1943) | 60 ± 6 |
| Systolic blood pressure (mmHg) (n = 1943) | 102 ± 8 |
| Physical activity (h/day) | 1.60 (1.00–2.43) |
| Screen time (h/day) | 1.14 (0.75–1.71) |
| Seldom or no smoking in household | 1829 (90.0%) |
| Parental Characteristics | |
| Maternal age at enrolment (year) | 32.3 (29.7–34.6) |
| Maternal BMI at enrolment (kg/m2) | 23.3 (21.7–25.8) |
| Household income ≥ €2,200 per month | 1586 (78.0%) |
| Educational level parents | |
| ● No higher education | 421 (20.7%) |
| ● One parent higher education | 495 (24.4%) |
| ● Both parents higher education | 1116 (54.9%) |
| Maternal hypercholesterolemia, diabetes mellitus, or hypertension | 93 (4.6%) |
| Smoking during pregnancy | |
| ● Never | 1606 (79.0%) |
| ● Until pregnancy was known | 213 (10.5%) |
| ● Continued | 213 (10.5%) |
| Alcohol consumption during pregnancy | |
| ● Never | 627 (30.9%) |
| ● Until pregnancy was known | 364 (17.9%) |
| ● Continued | 1041 (51.2%) |
| Use of folic acid supplements during pregnancy | |
| ● Start periconceptional | 1245 (61.3%) |
| ● Start in first 10 weeks of pregnancy | 599 (29.4%) |
| ● No | 188 (9.3%) |
| Gestational hypertension, preeclampsia, or gestational diabetes mellitus | 183 (9.1%) |
| DF Intake (per 1 g/day) | Energy-Adjusted DF Intake (per 1 g/day) 1 | |||
|---|---|---|---|---|
| Crude Model 2 | Covariate-Adjusted Model 3 | Crude Model 2 | Covariate-Adjusted Model 3 | |
| Cardiometabolic risk factor score | −0.014 * | −0.022 * | −0.023 * | −0.022 * |
| n = 1314 | (−0.024, −0.003) | (−0.037, −0.006) | (−0.038, −0.007) | (−0.038, −0.006) |
| BF% (SDS) | −0.005 | −0.005 | −0.004 | −0.003 |
| n = 1988 | (−0.014, 0.004) | (−0.017, 0.007) | (−0.016, 0.008) | (−0.015, 0.010) |
| HDL-C (SDS) | 0.018 * | 0.027 * | 0.023 * | 0.026 *,4 |
| n = 1385 | (0.012, 0.024) | (0.011, 0.044) | (0.007, 0.039) | (0.009, 0.043) |
| Insulin (SDS) | 0.001 | 0.002 | −0.005 | −0.003 |
| n = 1380 | (−0.005, 0.007) | (−0.014, 0.019) | (−0.013, 0.003) | (−0.020, 0.015) |
| Triglycerides (SDS) | −0.008 | −0.019 * | −0.015 | −0.018 *,4 |
| n = 1383 | (−0.020, 0.004) | (−0.035, −0.003) | (−0.031, 0.001) | (−0.036, −0.002) |
| DBP (SDS) | −0.006 | −0.006 | −0.004 | −0.003 |
| n = 1943 | (−0.015, 0.004) | (−0.019, 0.008) | (−0.017, 0.009) | (−0.017, 0.011) |
| SBP (SDS) | −0.001 | −0.007 | −0.009 | −0.009 |
| n = 1943 | (−0.006, 0.004) | (−0.020, 0.007) | (−0.022, 0.005) | (−0.023, 0.005) |
| Cereals (per 1 g/day) | Potatoes (per 1 g/day) | Fruit & Vegetables (per 1 g/day) | Legumes (per 1 g/day) | |||||
|---|---|---|---|---|---|---|---|---|
| DF Intake | Energy-Adjusted DF Intake 1 | DF Intake | Energy-Adjusted DF Intake 1 | DF Intake 1 | Energy-Adjusted DF Intake 1 | DF Intake | Energy-Adjusted DF Intake 1 | |
| Cardiometabolic risk factor score (SDS) | −0.006 | −0.004 | −0.050 * | −0.051 * | −0.009 | −0.009 | −0.032 | −0.03 |
| n = 1311 | (−0.026, 0.014) | (−0.026, 0.019) | (−0.093, −0.008) | (−0.096, −0.009) | (−0.034, 0.017) | (−0.034, 0.016) | (−0.111, 0.048) | (−0.110, 0.049) |
| BF% (SDS) | −0.009 | −0.005 | 0 | −0.001 | 0.006 | 0.006 | −0.001 | 0 |
| n = 1984 | (−0.024, 0.005) | (−0.022, 0.011) | (−0.032, 0.032) | (−0.033, 0.032) | (−0.012, 0.025) | (−0.013, 0.025) | (−0.061, 0.059) | (−0.059, 0.060) |
| HDL-C (SDS) | 0.008 | 0.002 | 0.028 * | 0.033 | 0.027 * | 0.028 * | 0.035 | 0.033 |
| n = 1383 | (−0.013, 0.029) | (−0.021, 0.024) | (0.016, 0.072) | (−0.012, 0.077) | (0.000, 0.054) | (0.002, 0.054) | (−0.047, 0.117) | (−0.048, 0.115) |
| Insulin (SDS) | 0.001 | −0.008 | −0.018 | −0.01 | 0.012 | 0.013 | −0.015 | −0.016 |
| n = 1378 | (−0.019, 0.022) | (−0.030, 0.015) | (−0.055, 0.033) | (−0.053, 0.034) | (−0.015, 0.039) | (−0.014, 0.040) | (−0.097, 0.067) | (−0.098, 0.066) |
| Triglycerides (SDS) | 0.011 | −0.007 | −0.075 * | −0.076 * | −0.02 | −0.021 | −0.015 | −0.014 |
| n = 1381 | (−0.010, 0.032) | (−0.015, 0.000) | (−0.120, −0.030) | (−0.121, −0.032) | (−0.048, 0.007) | (−0.050, 0.006) | (−0.098, 0.068) | (−0.098, 0.069) |
| DBP (SDS) | −0.007 | −0.003 | −0.001 | −0.0034 | −0.004 | −0.005 | −0.016 | 0.017 |
| n = 1939 | (−0.024, 0.009) | (−0.021, 0.016) | (−0.038, 0.035) | (−0.040, 0.033) | (−0.025, 0.017) | (−0.026, 0.016) | (−0.052, 0.084) | (−0.051, 0.085) |
| SBP (SDS) | −0.009 | −0.012 | 0.004 | 0.004 | 0.001 | 0.002 | −0.008 | −0.008 |
| n = 1939 | (−0.026, 0.009) | (−0.032, 0.008) | (−0.034, 0.041) | (−0.033, 0.042) | (−0.021, 0.023) | (−0.021, 0.024) | (−0.028, 0.012) | (−0.028, 0.012) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Gijssel, R.M.A.; Braun, K.V.E.; Kiefte-de Jong, J.C.; Jaddoe, V.W.V.; Franco, O.H.; Voortman, T. Associations between Dietary Fiber Intake in Infancy and Cardiometabolic Health at School Age: The Generation R Study. Nutrients 2016, 8, 531. https://doi.org/10.3390/nu8090531
Van Gijssel RMA, Braun KVE, Kiefte-de Jong JC, Jaddoe VWV, Franco OH, Voortman T. Associations between Dietary Fiber Intake in Infancy and Cardiometabolic Health at School Age: The Generation R Study. Nutrients. 2016; 8(9):531. https://doi.org/10.3390/nu8090531
Chicago/Turabian StyleVan Gijssel, Rafaëlle M. A., Kim V. E. Braun, Jessica C. Kiefte-de Jong, Vincent W. V. Jaddoe, Oscar H. Franco, and Trudy Voortman. 2016. "Associations between Dietary Fiber Intake in Infancy and Cardiometabolic Health at School Age: The Generation R Study" Nutrients 8, no. 9: 531. https://doi.org/10.3390/nu8090531





