Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population
Abstract
:1. Introduction
2. Methods
3. Results

| N | % | Mean | Std | |
|---|---|---|---|---|
| Vitamin D supplementation (IU per day) | 13,987 | 2484.6 | 3993.8 | |
| Plasma 25(OH)D level nmol/L | 13,987 | 88.9 | 47.6 | |
| Age (Years) | ||||
| <40 | 5270 | 37.7 | ||
| 40 to 49 | 3123 | 22.3 | ||
| 50 to 59 | 3528 | 25.2 | ||
| 60+ | 2066 | 14.8 | ||
| Gender | ||||
| Female | 6760 | 48.3 | ||
| Male | 7227 | 51.7 | ||
| Weight Status | ||||
| Normal weight | 4470 | 33.1 | ||
| Underweight | 184 | 1.4 | ||
| Overweight | 4943 | 36.7 | ||
| Obesity | 3888 | 28.8 | ||
| Season | ||||
| Winter | 4685 | 33.5 | ||
| Spring | 4198 | 30.0 | ||
| Summer | 2717 | 19.4 | ||
| Fall | 2387 | 17.1 |

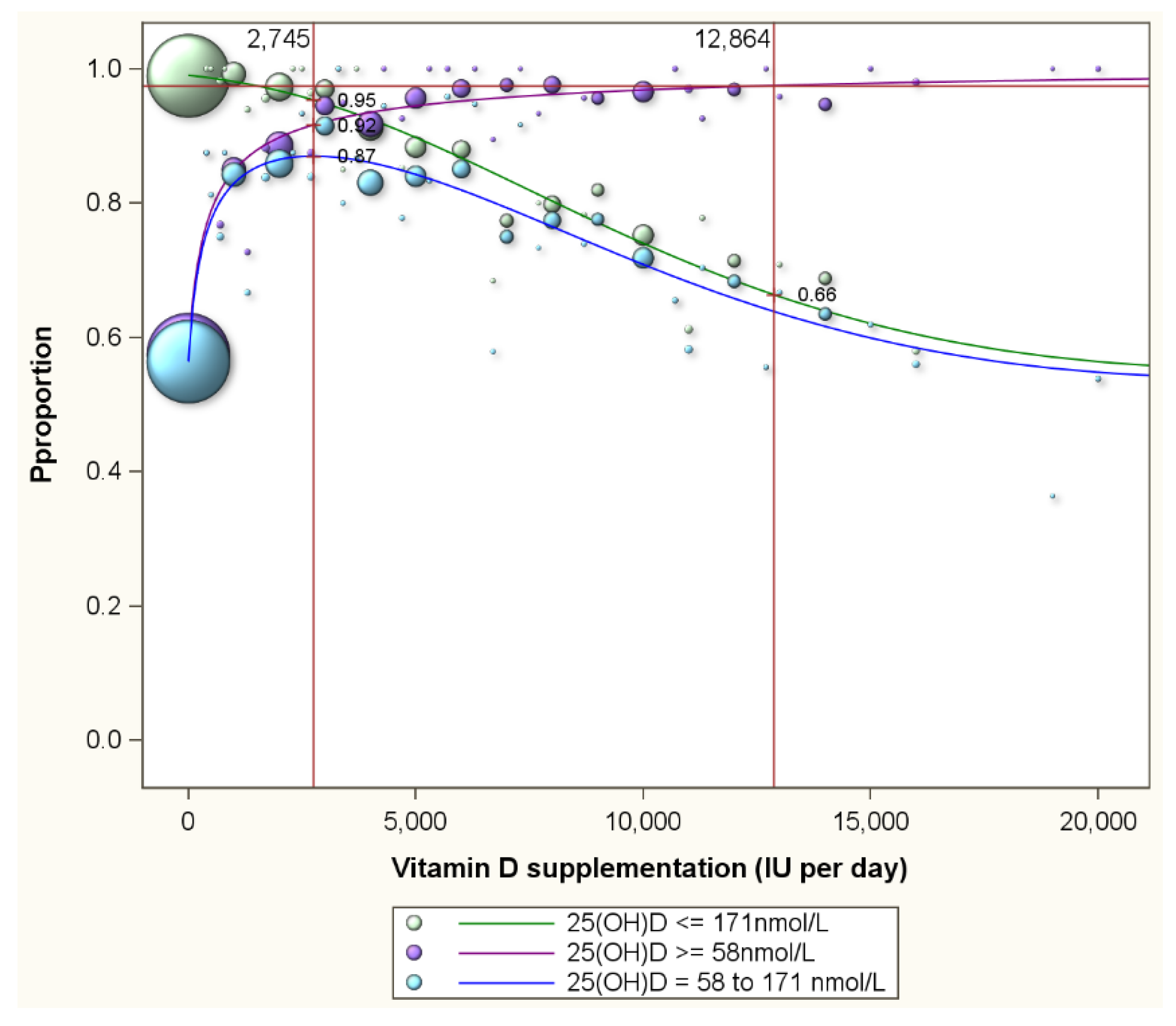
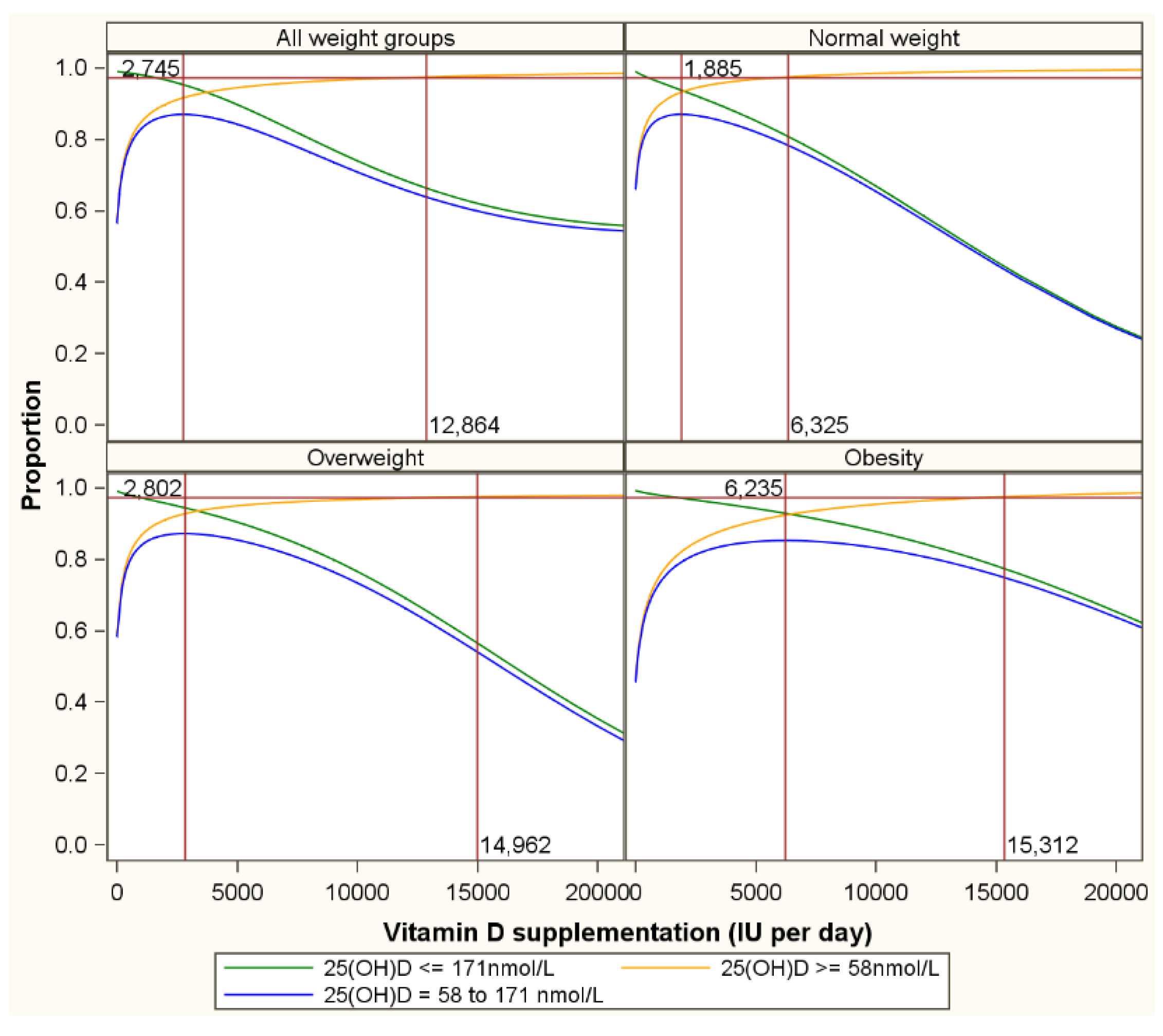
| LOWER SERUM 25(OH)D TARGETS (in nmol/L) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 50 | 58 | 75 | 100 | 150 | |||||||||||||||||||||
| Proportion | Proportion | Proportion | Proportion | Proportion | Proportion | |||||||||||||||||||||
| Weight Status | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | Optimal Intake (IU) | Between Lower and Upper | Above Lower | Below Upper | ||
| Upper Serum 25(OH)D Targets (in nmol/L) | 100 | Normal weight | 104 | 0.70 | 0.91 | 0.79 | 238 | 0.63 | 0.87 | 0.76 | 377 | 0.56 | 0.83 | 0.73 | 837 | 0.36 | 0.70 | 0.66 | ||||||||
| Overweight | 129 | 0.74 | 0.90 | 0.84 | 301 | 0.65 | 0.85 | 0.79 | 513 | 0.57 | 0.81 | 0.75 | 1108 | 0.36 | 0.69 | 0.67 | ||||||||||
| Obesity | 364 | 0.77 | 0.91 | 0.87 | 755 | 0.66 | 0.84 | 0.82 | 1177 | 0.55 | 0.77 | 0.77 | 2603 | 0.31 | 0.64 | 0.67 | ||||||||||
| All weight groups | 349 | 0.77 | 0.93 | 0.84 | 552 | 0.69 | 0.88 | 0.81 | 719 | 0.61 | 0.82 | 0.79 | 1048 | 0.41 | 0.65 | 0.75 | ||||||||||
| 125 | Normal weight | 323 | 0.85 | 0.95 | 0.90 | 606 | 0.79 | 0.92 | 0.87 | 886 | 0.74 | 0.89 | 0.85 | 1798 | 0.58 | 0.79 | 0.79 | |||||||||
| Overweight | 622 | 0.88 | 0.96 | 0.93 | 939 | 0.83 | 0.92 | 0.91 | 1209 | 0.78 | 0.88 | 0.90 | 1808 | 0.61 | 0.75 | 0.86 | ||||||||||
| Obesity | 830 | 0.87 | 0.94 | 0.93 | 1717 | 0.79 | 0.90 | 0.89 | 2683 | 0.71 | 0.85 | 0.85 | 4960 | 0.52 | 0.74 | 0.78 | ||||||||||
| All weight groups | 669 | 0.88 | 0.96 | 0.93 | 1025 | 0.82 | 0.92 | 0.91 | 1322 | 0.76 | 0.87 | 0.89 | 1941 | 0.59 | 0.73 | 0.86 | 4138 | 0.27 | 0.57 | 0.70 | ||||||
| 150 | Normal weight | 499 | 0.91 | 0.96 | 0.95 | 889 | 0.87 | 0.94 | 0.93 | 1280 | 0.82 | 0.91 | 0.91 | 2650 | 0.68 | 0.83 | 0.85 | 6208 | 0.44 | 0.74 | 0.71 | |||||
| Overweight | 731 | 0.91 | 0.96 | 0.95 | 1274 | 0.86 | 0.93 | 0.93 | 1779 | 0.82 | 0.90 | 0.92 | 3156 | 0.68 | 0.81 | 0.87 | 6479 | 0.42 | 0.67 | 0.76 | ||||||
| Obesity | 1620 | 0.92 | 0.97 | 0.96 | 3181 | 0.87 | 0.94 | 0.93 | 4640 | 0.81 | 0.90 | 0.91 | 7250 | 0.66 | 0.80 | 0.86 | 9862 | 0.40 | 0.61 | 0.80 | ||||||
| All weight groups | 1012 | 0.93 | 0.97 | 0.96 | 1535 | 0.88 | 0.94 | 0.95 | 1978 | 0.83 | 0.90 | 0.94 | 2949 | 0.68 | 0.78 | 0.90 | 5643 | 0.44 | 0.65 | 0.78 | ||||||
| 171 | Normal weight | 774 | 0.94 | 0.97 | 0.97 | 1340 | 0.90 | 0.95 | 0.95 | 1885 | 0.87 | 0.93 | 0.94 | 3656 | 0.75 | 0.86 | 0.89 | 7209 | 0.55 | 0.78 | 0.78 | |||||
| Overweight | 1276 | 0.94 | 0.97 | 0.97 | 2114 | 0.91 | 0.95 | 0.96 | 2802 | 0.87 | 0.93 | 0.94 | 4611 | 0.76 | 0.85 | 0.91 | 8270 | 0.55 | 0.73 | 0.82 | ||||||
| Obesity | 2243 | 0.94 | 0.97 | 0.97 | 4344 | 0.90 | 0.95 | 0.95 | 6235 | 0.85 | 0.92 | 0.93 | 9451 | 0.73 | 0.84 | 0.89 | 12,843 | 0.50 | 0.67 | 0.83 | ||||||
| All weight groups | 1414 | 0.95 | 0.97 | 0.98 | 2133 | 0.91 | 0.95 | 0.96 | 2745 | 0.87 | 0.92 | 0.95 | 4108 | 0.74 | 0.82 | 0.92 | 6829 | 0.54 | 0.70 | 0.84 | 10,373 | 0.14 | 0.41 | 0.73 | ||
| 200 | Normal weight | 1199 | 0.96 | 0.98 | 0.98 | 2038 | 0.94 | 0.97 | 0.97 | 2849 | 0.91 | 0.95 | 0.96 | 5479 | 0.82 | 0.90 | 0.92 | 10,008 | 0.69 | 0.86 | 0.83 | |||||
| Upper Serum 25(OH)D Targets (in nmol/L) | Overweight | 2082 | 0.96 | 0.98 | 0.98 | 3321 | 0.94 | 0.96 | 0.97 | 4250 | 0.91 | 0.94 | 0.96 | 6702 | 0.82 | 0.88 | 0.94 | 11,120 | 0.67 | 0.80 | 0.87 | |||||
| Obesity | 3527 | 0.96 | 0.98 | 0.98 | 6386 | 0.94 | 0.97 | 0.97 | 8714 | 0.90 | 0.95 | 0.95 | 12,365 | 0.80 | 0.88 | 0.92 | 16,112 | 0.60 | 0.73 | 0.87 | ||||||
| All weight groups | 1883 | 0.96 | 0.98 | 0.98 | 3243 | 0.93 | 0.96 | 0.97 | 4491 | 0.90 | 0.94 | 0.96 | 7297 | 0.80 | 0.87 | 0.93 | 9423 | 0.66 | 0.76 | 0.90 | 12,627 | 0.30 | 0.46 | 0.84 | ||
| 250 | Normal weight | 2319 | 0.98 | 0.99 | 0.99 | 3971 | 0.96 | 0.98 | 0.98 | 5433 | 0.95 | 0.97 | 0.98 | |||||||||||||
| Overweight | 3980 | 0.98 | 0.99 | 0.99 | 5981 | 0.96 | 0.98 | 0.99 | 7281 | 0.94 | 0.96 | 0.98 | ||||||||||||||
| Obesity | 5433 | 0.98 | 0.99 | 0.99 | 11,402 | 0.96 | 0.99 | 0.98 | 16,917 | 0.95 | 0.98 | 0.97 | ||||||||||||||
| All weight groups | 3543 | 0.98 | 0.99 | 0.99 | 5918 | 0.96 | 0.98 | 0.98 | 7914 | 0.94 | 0.96 | 0.98 | 11,972 | 0.87 | 0.92 | 0.96 | 11,922 | 0.74 | 0.78 | 0.96 | 15,623 | 0.43 | 0.50 | 0.93 | ||
| 300 | Normal weight | 3307 | 0.99 | 0.99 | 1.00 | 5228 | 0.98 | 0.98 | 0.99 | |||||||||||||||||
| Overweight | 5,042 | 0.98 | 0.99 | 0.99 | 7433 | 0.97 | 0.98 | 0.99 | ||||||||||||||||||
| Obesity | 8767 | 0.99 | 0.99 | 1.00 | 13,065 | 0.98 | 0.99 | 0.99 | ||||||||||||||||||
| All weight groups | 5163 | 0.98 | 0.99 | 0.99 | 8225 | 0.97 | 0.98 | 0.99 | 10,560 | 0.96 | 0.97 | 0.99 | 14,955 | 0.90 | 0.93 | 0.97 | 13,049 | 0.77 | 0.79 | 0.98 | 16,859 | 0.47 | 0.51 | 0.96 | ||
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Authors Contributions
Conflict of interest
Appendix
| Study | Gender of Participants | Vitamin D Supplement | Sub Group | Dose (IU) | Follow Up in Weeks | Number of Participants | Mean 25(OH)D in nmol/L | SD in nmol/L |
|---|---|---|---|---|---|---|---|---|
| Ala-Houhala, M., et al., 1988 [17] | Both | None | 0 | 52 | 27 | 43.3 | 19.5 | |
| Not specified | 400 | 52 | 24 | 71.3 | 23.8 | |||
| Aloia, J., et al., 2010 [18] | Both | D3 | 4000 | 13 | 35 | 111.7 | 30.0 | |
| Aloia, J.F., et al., 2005 [19] | Female | D3 | 800 | 13 | 104 | 70.8 | 22.9 | |
| Biancuzzo, R.M., et al., 2010 [20] | Both | None | 0 | 11 | 17 | 45.3 | 16.0 | |
| D3 | In orange juice | 1000 | 11 | 16 | 76.8 | 21.3 | ||
| In capsules | 1000 | 11 | 18 | 70.0 | 27.5 | |||
| D2 | In orange juice | 1000 | 11 | 20 | 66.0 | 18.5 | ||
| In capsules | 1000 | 11 | 15 | 68.5 | 26.3 | |||
| Bolton-Smith, C., et al., 2007 [21] | Female | None | 0 | 52 | 55 | 47.8 | 17.3 | |
| 104 | 55 | 48.8 | 13.3 | |||||
| D3 | Vit D + Calcium | 400 | 52 | 55 | 74.3 | 15.3 | ||
| 104 | 55 | 74.5 | 15.0 | |||||
| Vit D + Vit K + Calcium | 400 | 52 | 58 | 73.0 | 16.3 | |||
| 104 | 58 | 70.8 | 16.5 | |||||
| Cashman, K.D., et al., 2012 [22] | Both | None | 0 | 5 | 13 | 39.7 | 11.1 | |
| 10 | 13 | 41.2 | 11.1 | |||||
| D3 | 800 | 5 | 16 | 64.1 | 9.5 | |||
| 10 | 16 | 69.0 | 8.7 | |||||
| Dawson-Hughes, B., et al., 1991 [23] | Female | None | 0 | 48 | 121 | 60.6 | 28.5 | |
| D3 | 400 | 48 | 125 | 92.1 | 23.6 | |||
| Dawson-Hughes, B., et al., 1995 [24] | Female | Not specified | 100 | 39 | 124 | 66.3 | 25.5 | |
| 700 | 39 | 123 | 100.1 | 24.5 | ||||
| Diamond, T., et al., 2013 [25] | Both | D3 | 2000 | 12 | 11 | 75.3 | 15.9 | |
| 5000 | 12 | 15 | 114.4 | 22.2 | ||||
| Gallagher, J.C., et al., 2014 [26] | Both | D3 | African American women | 2400 | 52 | 20 | 97.8 | 16.5 |
| White women | 2400 | 52 | 5 | 108.0 | 14.3 | |||
| Harris, S.S., et al., 2012 [27] | Both | None | 0 | 12 | 43 | 37.4 | 16.1 | |
| D3 | 4000 | 12 | 46 | 81.1 | 27.9 | |||
| Holick, M.F., et al., 2008 [28] | Both | None | 0 | 11 | 16 | 47.0 | 19.8 | |
| D3 | 1000 | 11 | 20 | 72.3 | 27.5 | |||
| D2 | 1000 | 11 | 18 | 67.0 | 24.0 | |||
| D3+D2 | 1000 | 11 | 14 | 71.0 | 19.3 | |||
| Jensen, C., et al., 2002 [29] | Female | Not specified | 400 | 52 | 22 | 50.3 | 19.1 | |
| 104 | 22 | 80.4 | 21.6 | |||||
| 156 | 22 | 76.6 | 22.1 | |||||
| Karkkainen, M.K., et al., 2010 [30] | Female | D3 | 0 | 156 | 287 | 55.9 | 21.8 | |
| 800 | 156 | 306 | 74.6 | 21.9 | ||||
| Kilpinen-Loisa, P., et al., 2009 [31] | Both | D3 | 800 | 26 | 72 | 82.0 | 21.0 | |
| Kyriakidou-Himonas, M., et al., 1999 [32] | Female | D3 | 800 | 12 | 10 | 63.3 | 11.1 | |
| Lagunova, Z., et al., 2013 [33] | Both | D3 | 2000 | 4 | 11 | 78.9 | 15.3 | |
| Lappe, J.M., et al., 2007 [34] | Female | None | 0 | 52 | 446 | 71.1 | 19.8 | |
| D3 | 1100 | 52 | 288 | 96.0 | 21.4 | |||
| Li-Ng, M., et al., 2009 [35] | Male | D3 | 2000 | 12 | 78 | 88.5 | 23.2 | |
| Logan, V.F., et al., 2013 [36] | Both | None | 0 | 4 | 13 | 71.0 | 15.3 | |
| 8 | 13 | 61.0 | 15.3 | |||||
| 13 | 13 | 44.0 | 15.3 | |||||
| 25 | 13 | 37.0 | 17.9 | |||||
| D3 | 1000 | 4 | 23 | 83.0 | 12.2 | |||
| 8 | 23 | 83.0 | 14.7 | |||||
| 13 | 23 | 80.0 | 17.1 | |||||
| 25 | 23 | 80.0 | 19.6 | |||||
| D2 | 1000 | 4 | 25 | 70.0 | 12.9 | |||
| 8 | 25 | 66.0 | 12.9 | |||||
| 13 | 25 | 62.0 | 14.7 | |||||
| 25 | 25 | 56.0 | 11.0 | |||||
| Molgaard, C., et al., 2010 [37] | Female | None | 0 | 26 | 73 | 47.0 | 20.0 | |
| 52 | 73 | 39.7 | 17.7 | |||||
| D3 | 200 | 26 | 74 | 54.5 | 12.0 | |||
| 52 | 74 | 52.9 | 16.3 | |||||
| 400 | 26 | 73 | 59.4 | 13.2 | ||||
| 52 | 74 | 57.9 | 14.3 | |||||
| Nelson, M.L., et al., 2009 [38] | Female | None | 0 | 52 | 31 | 72.7 | 27.8 | |
| D3 | 800 | 52 | 31 | 97.4 | 31.3 | |||
| Oosterwerff, M.M., et al., 2014 [39] | Both | None | 0 | 8 | 53 | 24.0 | 16.0 | |
| 16 | 53 | 23.0 | 15.0 | |||||
| D3 | 1200 | 8 | 57 | 58.0 | 12.0 | |||
| 16 | 57 | 60.0 | 16.0 | |||||
| Orwoll, E.S., et al., 1988 [41] | Male | None | 0 | 26 | 46 | 55.0 | 15.0 | |
| 52 | 46 | 60.0 | 18.0 | |||||
| D3 | 1000 | 26 | 46 | 75.0 | 18.0 | |||
| 52 | 46 | 85.0 | 20.0 | |||||
| Orwoll, E.S., et al., 1989 [40] | Both | None | 0 | 104 | 14 | 45.0 | 22.5 | |
| D3 | 1600 | 52 | 14 | 240.0 | 97.5 | |||
| 104 | 17 | 225.0 | 87.5 | |||||
| Pignotti, G.A., et al., 2010 [42] | Female | None | 0 | 13 | 29 | 58.8 | 24.7 | |
| D3 | 400 | 13 | 29 | 59.5 | 17.5 | |||
| Putman, M.S., et al., 2013 [43] | Both | D3 | 200 | 11 | 25 | 28.9 | 7.0 | |
| 1000 | 11 | 29 | 30.1 | 6.6 | ||||
| Rajakumar, K., et al., 2008 [44] | Both | D3 | Non-obese | 400 | 4 | 18 | 72.8 | 16.8 |
| Obese | 400 | 4 | 21 | 65.5 | 20.3 | |||
| Schaafsma, A., et al., 2000 [45] | Female | D3 | 400 | 13 | 46 | 108.0 | 25.0 | |
| 26 | 46 | 111.0 | 23.0 | |||||
| 52 | 46 | 121.0 | 27.0 | |||||
| Schnatz, P.F., et al., 2014 [46] | Female | None | 0 | 104 | 291 | 45.5 | 23.7 | |
| D3 | 400 | 104 | 285 | 60.8 | 30.5 | |||
| Schou, A.J., et al., 2003 [47] | Both | None | Placebo then supplementation | 0 | 4 | 10 | 33.7 | 10.4 |
| Supplementation then placebo | 0 | 4 | 10 | 32.3 | 13.0 | |||
| D3 | Placebo then supplementation | 600 | 4 | 10 | 50.2 | 14.2 | ||
| Supplementation then placebo | 600 | 4 | 10 | 43.4 | 9.2 | |||
| Smith, S.M., et al., 2009 [48] | Both | None | 0 | 18 | 18 | 38.0 | 17.0 | |
| 25 | 18 | 34.0 | 12.0 | |||||
| D3 | 400 | 18 | 19 | 55.0 | 19.0 | |||
| 25 | 19 | 57.0 | 15.0 | |||||
| 1000 | 18 | 18 | 63.0 | 20.0 | ||||
| 25 | 18 | 63.0 | 25.0 | |||||
| 2000 | 18 | 7 | 71.0 | 20.0 | ||||
| 25 | 7 | 71.0 | 23.0 | |||||
| Van Der Klis, F.R., et al., 1996 [49] | Female | None | 0 | 5 | 41 | 58.5 | 10.7 | |
| D3 | 400 | 5 | 19 | 87.9 | 28.1 | |||
| 800 | 5 | 19 | 87.9 | 28.1 | ||||
| 19 | 102.6 | 28.8 | ||||||
| Vieth, R., et al., 2001 [50] | Both | D3 | 1000 | 12 | 33 | 68.7 | 16.9 | |
| 4000 | 12 | 28 | 96.4 | 14.6 | ||||
| Wamberg, L., et al., 2013 [51] | Both | None | 0 | 26 | 26 | 46.8 | 21.2 | |
| D3 | 7000 | 26 | 26 | 110.0 | 17.3 | |||
| Zwart, S.R., et al., 2011 [52] | Both | Not specified | 2000 | 13 | 15 | 73.0 | 17.0 | |
| 26 | 15 | 79.0 | 16.0 |
References
- Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Maxmen, A. Nutrition advice: The vitamin D-lemma. Nature 2011, 475, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Gummert, J.F.; Borgermann, J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: A systematic review. Eur. J. Nutr. 2014, 53, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Van Groningen, L.; Opdenoordt, S.; van Sorge, A.; Telting, D.; Giesen, A.; de Boer, H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2010, 162, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar] [PubMed]
- Janz, T.; Pearson, C. Vitamin D Blood Levels of Canadians. In Health at a Glance, Statistics Canada (Catalogue No. 82–624-X); Statistics Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- Greene-Finestone, L.; Berger, C.; de Groh, M.; Hanley, D.; Hidiroglou, N.; Sarafin, K.; Poliquin, S.; Krieger, J.; Richards, J.; Goltzman, D. 25-Hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. Osteoporos. Int. 2011, 22, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, P.J.; Ekwaru, J.P. A Statistical Error in the Estimation of the Recommended Dietary Allowance for Vitamin D. Nutrients 2014, 6, 4472–4475. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.; Garland, C.; Baggerly, C.; French, C.; Gorham, E.; Letter to Veugelers, P.J.; Ekwaru, J.P. A Statistical Error in the Estimation of the Recommended Dietary Allowance for Vitamin D. Nutrients 2015, 7, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; French, C.B.; Baggerly, L.L.; Heaney, R.P. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011, 31, 607–611. [Google Scholar] [PubMed]
- Pham, T.M.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J. The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program. Nutrients 2015, 7, 7271–7284. [Google Scholar] [CrossRef] [PubMed]
- Ekwaru, J.P.; Ohinmaa, A.; Veugelers, P.J. The effectiveness of a preventive health program and vitamin D status in improving health-related quality of life of older Canadians. Qual. Life Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R.; Bassett, G., Jr. Regression quantiles. Econom. J. Econom. Soc. 1978, 46, 33–50. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.A.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Ala-Houhala, M.; Koskinen, T.; Koskinen, M.; Visakorpi, J.K. Double blind study on the need for vitamin D supplementation in prepubertal children. Acta Paediatr. Scand. 1988, 77, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.; Bojadzievski, T.; Yusupov, E.; Shahzad, G.; Pollack, S.; Mikhail, M.; Yeh, J. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J. Clin. Endocrinol. Metab. 2010, 95, 3216–3224. [Google Scholar] [PubMed]
- Aloia, J.F.; Talwar, S.A.; Pollack, S.; Yeh, J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch. Intern. Med. 2005, 165, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Biancuzzo, R.M.; Young, A.; Bibuld, D.; Cai, M.H.; Winter, M.R.; Klein, E.K.; Ameri, A.; Reitz, R.; Salameh, W.; Chen, T.C.; et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 2010, 91, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Smith, C.; McMurdo, M.E.; Paterson, C.R.; Mole, P.A.; Harvey, J.M.; Fenton, S.T.; Prynne, C.J.; Mishra, G.D.; Shearer, M.J. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J. Bone Miner. Res. 2007, 22, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Seamans, K.M.; Lucey, A.J.; Stocklin, E.; Weber, P.; Kiely, M.; Hill, T.R. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am. J. Clin. Nutr. 2012, 95, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Dallal, G.E.; Krall, E.A.; Harris, S.; Sokoll, L.J.; Falconer, G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann. Intern. Med. 1991, 115, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E.; Falconer, G.; Green, C.L. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am. J. Clin. Nutr. 1995, 61, 1140–1145. [Google Scholar] [CrossRef]
- Diamond, T.; Wong, Y.K.; Golombick, T. Effect of oral cholecalciferol 2000 versus 5000 IU on serum vitamin D, PTH, bone and muscle strength in patients with vitamin D deficiency. Osteoporos. Int. 2013, 24, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Jindal, P.S.; Smith, L.M. Vitamin D supplementation in young White and African American women. J. Bone Miner. Res. 2014, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Pittas, A.G.; Palermo, N.J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes. Metab. 2012, 14, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Holloway, L.; Block, G.; Spiller, G.; Gildengorin, G.; Gunderson, E.; Butterfield, G.; Marcus, R. Long-term effects of nutrient intervention on markers of bone remodeling and calciotropic hormones in late-postmenopausal women. Am. J. Clin. Nutr. 2002, 75, 1114–1120. [Google Scholar] [PubMed]
- Karkkainen, M.K.; Tuppurainen, M.; Salovaara, K.; Sandini, L.; Rikkonen, T.; Sirola, J.; Honkanen, R.; Arokoski, J.; Alhava, E.; Kroger, H. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65–71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas 2010, 65, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen-Loisa, P.; Arvio, M.; Ilvesmaki, V.; Makitie, O. Vitamin D status and optimal supplementation in institutionalized adults with intellectual disability. J. Intellect. Disabil. Res. JIDR 2009, 53, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou-Himonas, M.; Aloia, J.F.; Yeh, J.K. Vitamin D supplementation in postmenopausal black women. J. Clin. Endocrinol. Metab. 1999, 84, 3988–3990. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.C.; Aksnes, L.; Holick, M.F.; Iani, V.; Bruland, O.S.; Moan, J. Effect of vitamin D supplementation and ultraviolet B exposure on serum 25-hydroxyvitamin D concentrations in healthy volunteers: A randomized, crossover clinical trial. Br. J. Dermatol. 2013, 169, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [PubMed]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Logan, V.F.; Gray, A.R.; Peddie, M.C.; Harper, M.J.; Houghton, L.A. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br. J. Nutr. 2013, 109, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Molgaard, C.; Larnkjaer, A.; Cashman, K.D.; Lamberg-Allardt, C.; Jakobsen, J.; Michaelsen, K.F. Does vitamin D supplementation of healthy Danish Caucasian girls affect bone turnover and bone mineralization? Bone 2010, 46, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Blum, J.M.; Hollis, B.W.; Rosen, C.; Sullivan, S.S. Supplements of 20 microg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J. Nutr. 2009, 139, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Oosterwerff, M.M.; Eekhoff, E.M.; van Schoor, N.M.; Boeke, A.J.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; McClung, M.R.; Oviatt, S.K.; Recker, R.R.; Weigel, R.M. Histomorphometric effects of calcium or calcium plus 25-hydroxyvitamin D3 therapy in senile osteoporosis. J. Bone Miner. Res. 1989, 4, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Weigel, R.M.; Oviatt, S.K.; McClung, M.R.; Deftos, L.J. Calcium and cholecalciferol: Effects of small supplements in normal men. Am. J. Clin. Nutr. 1988, 48, 127–130. [Google Scholar] [PubMed]
- Pignotti, G.A.; Genaro, P.S.; Pinheiro, M.M.; Szejnfeld, V.L.; Martini, L.A. Is a lower dose of vitamin D supplementation enough to increase 25(OH)D status in a sunny country? Eur. J. Nutr. 2010, 49, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Putman, M.S.; Pitts, S.A.; Milliren, C.E.; Feldman, H.A.; Reinold, K.; Gordon, C.M. A randomized clinical trial of vitamin D supplementation in healthy adolescents. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2013, 52, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; Fernstrom, J.D.; Holick, M.F.; Janosky, J.E.; Greenspan, S.L. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008, 16, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.; Muskiet, F.A.; Storm, H.; Hofstede, G.J.; Pakan, I.; van der Veer, E. Vitamin D(3) and vitamin K(1) supplementation of Dutch postmenopausal women with normal and low bone mineral densities: Effects on serum 25-hydroxyvitamin D and carboxylated osteocalcin. Eur. J. Clin. Nutr. 2000, 54, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Schnatz, P.F.; Jiang, X.; Vila-Wright, S.; Aragaki, A.K.; Nudy, M.; O’Sullivan, D.M.; Jackson, R.; LeBlanc, E.; Robinson, J.G.; Shikany, J.M.; et al. Calcium/vitamin D supplementation, serum 25-hydroxyvitamin D concentrations, and cholesterol profiles in the Women’s Health Initiative calcium/vitamin D randomized trial. Menopause 2014, 21, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Schou, A.J.; Heuck, C.; Wolthers, O.D. A randomized, controlled lower leg growth study of vitamin D supplementation to healthy children during the winter season. Ann. Hum. Biol. 2003, 30, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Gardner, K.K.; Locke, J.; Zwart, S.R. Vitamin D supplementation during Antarctic winter. Am. J. Clin. Nutr. 2009, 89, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Van Der Klis, F.R.; Jonxis, J.H.; van Doormaal, J.J.; Sikkens, P.; Saleh, A.E.; Muskiet, F.A. Changes in vitamin-D metabolites and parathyroid hormone in plasma following cholecalciferol administration to pre- and postmenopausal women in the Netherlands in early spring and to postmenopausal women in Curacao. Br. J. Nutr. 1996, 75, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Chan, P.C.; MacFarlane, G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001, 73, 288–294. [Google Scholar] [PubMed]
- Wamberg, L.; Pedersen, S.B.; Richelsen, B.; Rejnmark, L. The effect of high-dose vitamin D supplementation on calciotropic hormones and bone mineral density in obese subjects with low levels of circulating 25-hydroxyvitamin d: Results from a randomized controlled study. Calcif. Tissue Int. 2013, 93, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Mehta, S.K.; Ploutz-Snyder, R.; Bourbeau, Y.; Locke, J.P.; Pierson, D.L.; Smith, S.M. Response to vitamin D supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr Virus Reactivation. J. Nutr. 2011, 141, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Vitamin D Levels of Canadians. 2012 to 2013. Available online: http://www.statcan.gc.ca/pub/82–625-x/2014001/article/14125-eng.htm (accessed on 11 August 2015).
- Holick, M. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hagenau, T.; Vest, R.; Gissel, T.N.; Poulsen, C.S.; Erlandsen, M.; Mosekilde, L.; Vestergaard, P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos. Int. 2009, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, H.; Calvo, M.S.; Green, T.J.; Whiting, S.J. Despite mandatory fortification of staple foods, vitamin D intakes of Canadian children and adults are inadequate. J. Steroid Biochem. Mol. Biol. 2010, 121, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, L.L.; Willows, N.; Yuan, Y.; Veugelers, P.J. Dietary reference intakes for vitamin D based on the revised 2010 dietary guidelines are not being met by children in Alberta, Canada. Nutr. Res. 2015, 35, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Lichtenstein, A.H.; Dolnikowski, G.; Palermo, N.J.; Rasmussen, H. Dietary fat increases vitamin D-3 absorption. J. Acad. Nutr. Diet. 2015, 115, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, G.B.; Licata, A. Taking vitamin D with the largest meal improves absorption and results in higher serum levels of 25-hydroxyvitamin D. J. Bone Miner. Res. 2010, 25, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, F.V.; Faulhaber, G.A.; Menegatti, P.K.; Marques Lda, S.; Furlanetto, T.W. Effect of High- versus Low-Fat Meal on Serum 25-Hydroxyvitamin D Levels after a Single Oral Dose of Vitamin D: A Single-Blind, Parallel, Randomized Trial. Int. J. Endocrinol. 2011, 2011, 809069. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Yu, K.; Stolzenberg-Solomon, R.; Simon, K.C.; McCullough, M.L.; Gallicchio, L.; Jacobs, E.J.; Ascherio, A.; Helzlsouer, K.; Jacobs, K.B.; et al. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010, 19, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, E2133–E2137. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Boeing, H.; Hirche, F.; Weikert, C.; Schulze, M.B.; Gottschald, M.; Kuhn, T.; Katzke, V.A.; Teucher, B.; Dierkes, J.; et al. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: A prospective case-cohort study. Eur. J. Epidemiol. 2013, 28, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Didriksen, A.; Grimnes, G.; Hutchinson, M.S.; Kjaergaard, M.; Svartberg, J.; Joakimsen, R.M.; Jorde, R. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur. J. Endocrinol. Eur. Fed. Endocr.Soc. 2013, 169, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Engelman, C.D.; Meyers, K.J.; Iyengar, S.K.; Liu, Z.; Karki, C.K.; Igo, R.P., Jr.; Truitt, B.; Robinson, J.; Sarto, G.E.; Wallace, R.; et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J. Nutr. 2013, 143, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.G.; Tang, W.; Hootman, K.C.; Brannon, P.M.; Houston, D.K.; Kritchevsky, S.B.; Harris, T.B.; Garcia, M.; Lohman, K.; Liu, Y.; et al. Genetic and environmental factors are associated with serum 25-hydroxyvitamin D concentrations in older African Americans. J. Nutr. 2015, 145, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.P.; Robinson-Cohen, C.; de Boer, I.H.; Houston, D.K.; Lohman, K.; Liu, Y.; Kritchevsky, S.B.; Cauley, J.A.; Tanaka, T.; Ferrucci, L.; et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012, 308, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Sollid, S.T.; Hutchinson, M.Y.; Fuskevag, O.M.; Joakimsen, R.M.; Jorde, R. Large Individual Differences in Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation: Effects of Genetic Factors, Body Mass Index, and Baseline Concentration. Results from a Randomized Controlled Trial. Horm. Metab. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Institute of Medicine. How the RDA for Vitamin D Was Determined. Available online: http://iom.nationalacademies.org/Global/News%20Announcements/How-the-RDA-for-Vitamin-D-Was-Determined (accessed on 8 August 2014).
- American Geriatrics Society Workgroup on Vitamin, D.S.f.O.A. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J. Am. Geriatr. Soc. 2014, 62, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. The Vitamin D requirement in health and disease. J. Steroid Biochem. Mol. Biol. 2005, 97, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.P.; Doll, R.; Khaw, K.T. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ 2003, 326, 469. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Giovannucci, E.; Dietrich, T.; Dawson-Hughes, B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA 2005, 293, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Manson, J.E.; Pilz, S.; Marz, W.; Michaelsson, K.; Lundqvist, A.; Jassal, S.K.; Barrett-Connor, E.; Zhang, C.; et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.B.; Giovannucci, E.L.; Lipkin, M.; Newmark, H.; Holick, M.F.; Garland, F.C. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am. J. Clin.Nutr. 2011, 94, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.C.; Guy, M.; Mansi, J.L.; Peckitt, C.; Bliss, J.; Wilson, R.G.; Colston, K.W. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur. J. Cancer 2005, 41, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Comstock, G.W.; Garland, F.C.; Helsing, K.J.; Shaw, E.K.; Gorham, E.D. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 1989, 2, 1176–1178. [Google Scholar] [CrossRef]
- Grant, W.B. Solar ultraviolet irradiance and cancer incidence and mortality. Adv. Exp. Med. Biol. 2014, 810, 52–68. [Google Scholar] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Laaksi, I.; Ruohola, J.P.; Tuohimaa, P.; Auvinen, A.; Haataja, R.; Pihlajam{"a}ki, H.; Ylikomi, T. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007, 86, 714–717. [Google Scholar] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S. Vitamin D research and clinical practice: At a crossroads. JAMA 2015, 313, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
- Dror, Y.; Giveon, S.M.; Hoshen, M.; Feldhamer, I.; Balicer, R.D.; Feldman, B.S. Vitamin D levels for preventing acute coronary syndrome and mortality: Evidence of a nonlinear association. J. Clin. Endocrinol. Metab. 2013, 98, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Durup, D.; Jorgensen, H.L.; Christensen, J.; Tjonneland, A.; Olsen, A.; Halkjaer, J.; Lind, B.; Heegaard, A.M.; Schwarz, P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J. Clin. Endocrinol. Metab. 2015, 100, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shao, X.; Yao, Y.; Xu, L.; Chang, L.; Jiang, Z.; Lin, Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: New findings from an updated meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Kuhn, J.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Borgermann, J. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur. Heart J. 2013, 34, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Kuhn, J.; Ernst, J.B.; Becker, T.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Borgermann, J. 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and postoperative outcome in cardiac surgery. J. Clin. Endocrinol. Metab. 2015, 100, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dudenkov, D.V.; Yawn, B.P.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing Incidence of Serum 25-Hydroxyvitamin D Values Above 50 ng/mL: A 10-Year Population-Based Study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Shao, A.; Dawson-Hughes, B.; Hathcock, J.; Giovannucci, E.; Willett, W.C. Benefit-risk assessment of vitamin D supplementation. Osteoporos. Int. 2010, 21, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D Is Not as Toxic as Was Once Thought: A Historical and an Up-to-Date Perspective. Mayo Clin. Proc. 2015, 90, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Spedding, S.; Vanlint, S.; Morris, H.; Scragg, R. Does vitamin D sufficiency equate to a single serum 25-hydroxyvitamin D level or are different levels required for non-skeletal diseases? Nutrients 2013, 5, 5127–5139. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veugelers, P.J.; Pham, T.-M.; Ekwaru, J.P. Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients 2015, 7, 10189-10208. https://doi.org/10.3390/nu7125527
Veugelers PJ, Pham T-M, Ekwaru JP. Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients. 2015; 7(12):10189-10208. https://doi.org/10.3390/nu7125527
Chicago/Turabian StyleVeugelers, Paul J., Truong-Minh Pham, and John Paul Ekwaru. 2015. "Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population" Nutrients 7, no. 12: 10189-10208. https://doi.org/10.3390/nu7125527




