Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite
Abstract
:1. Introduction
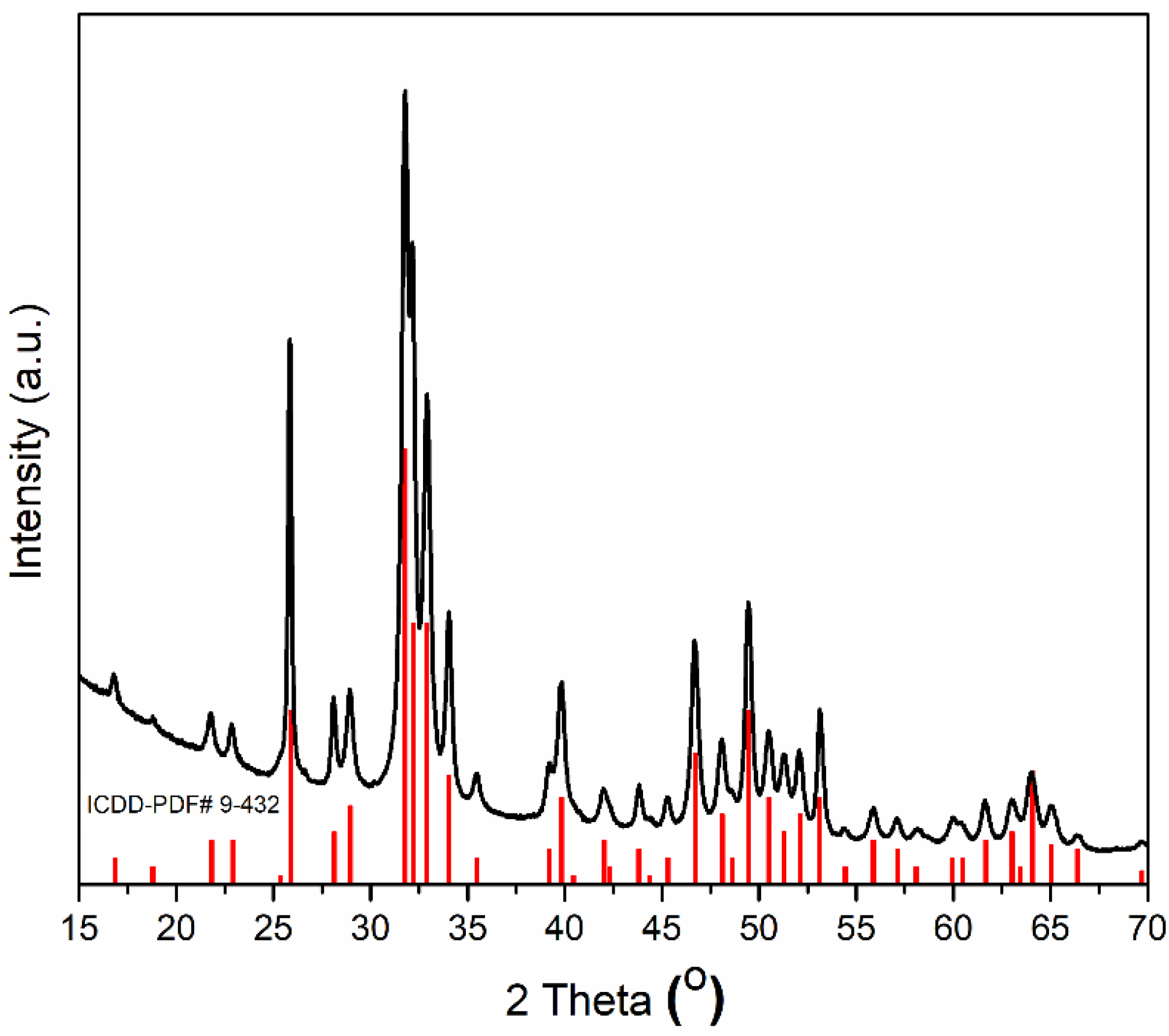
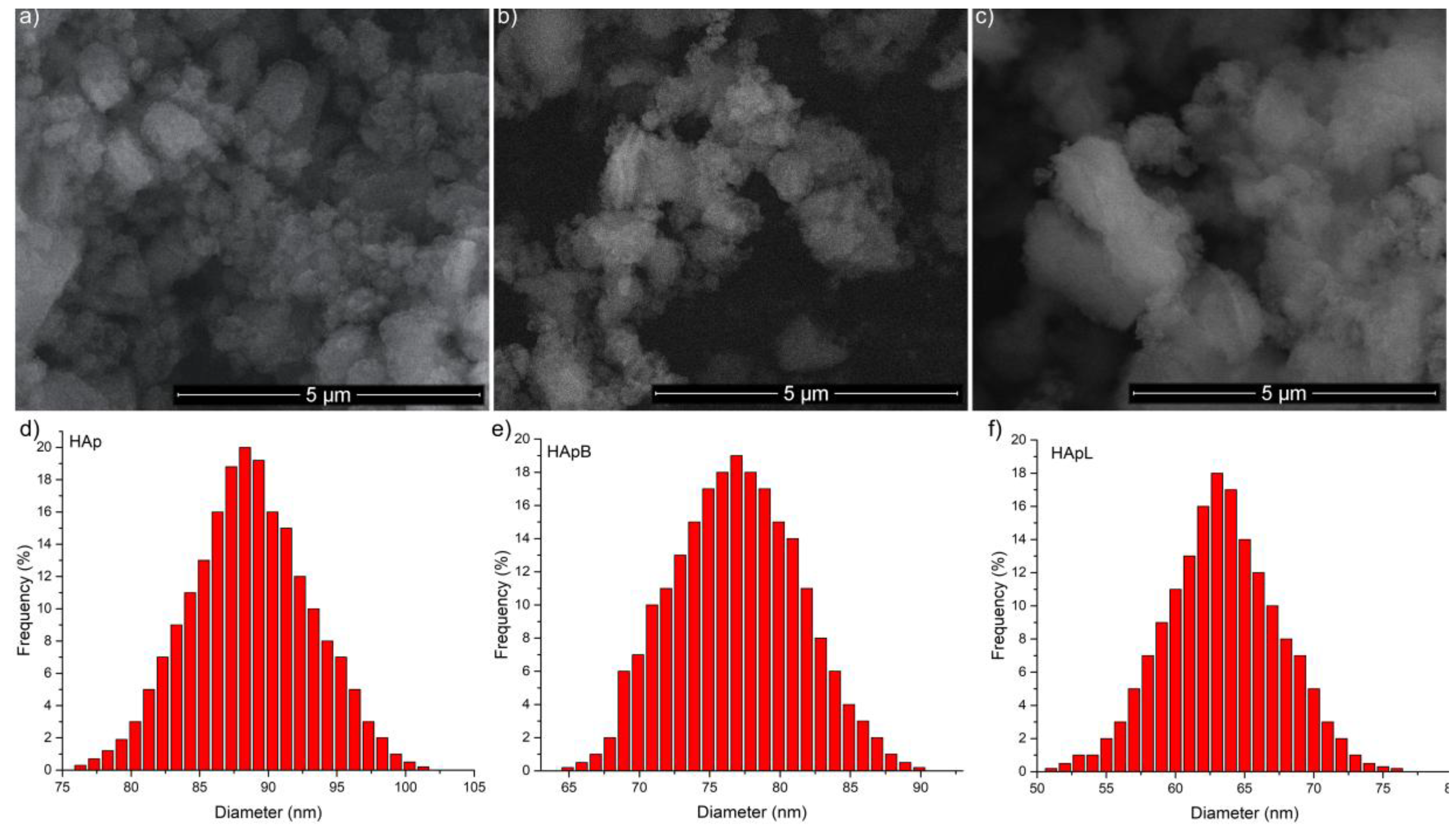
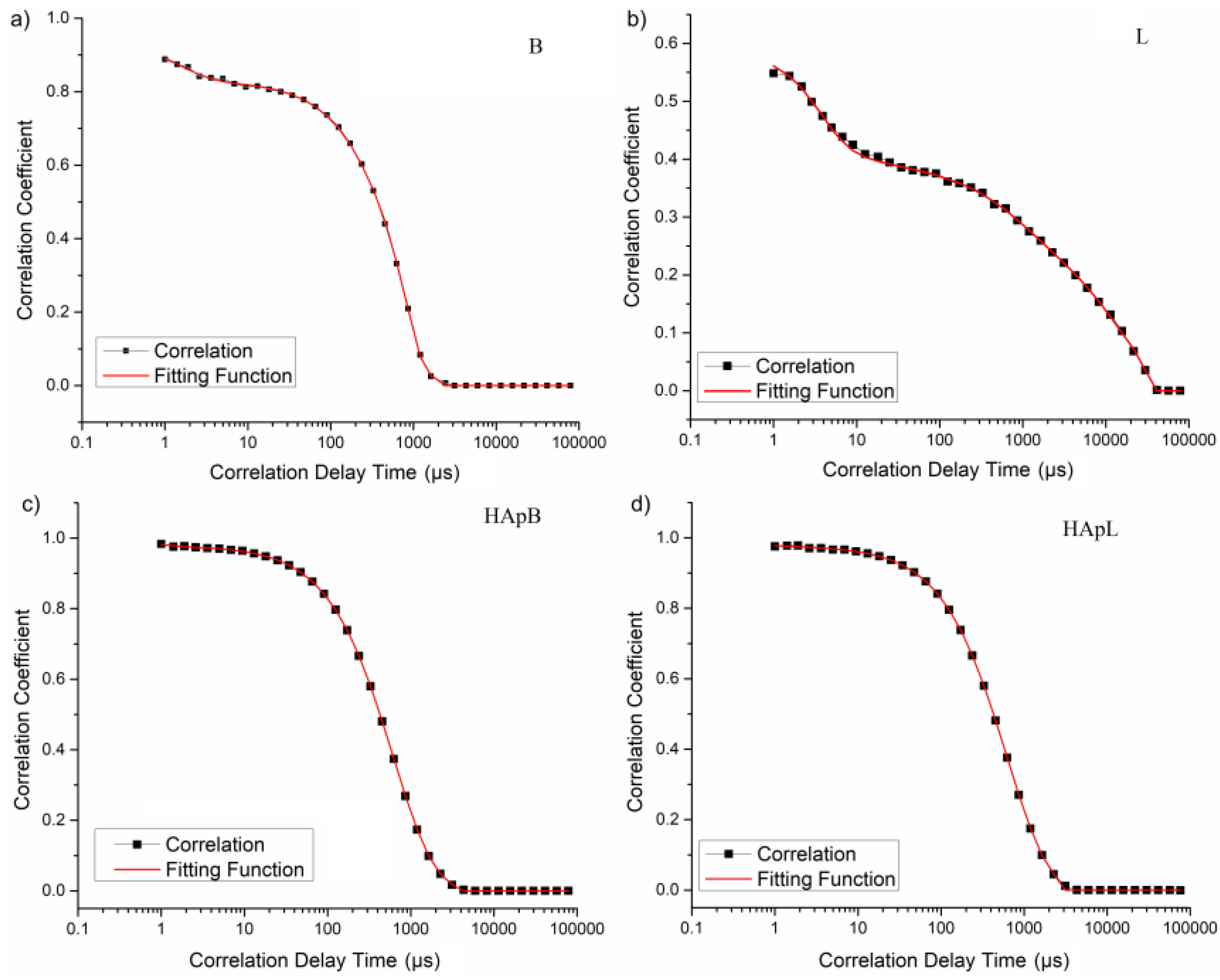
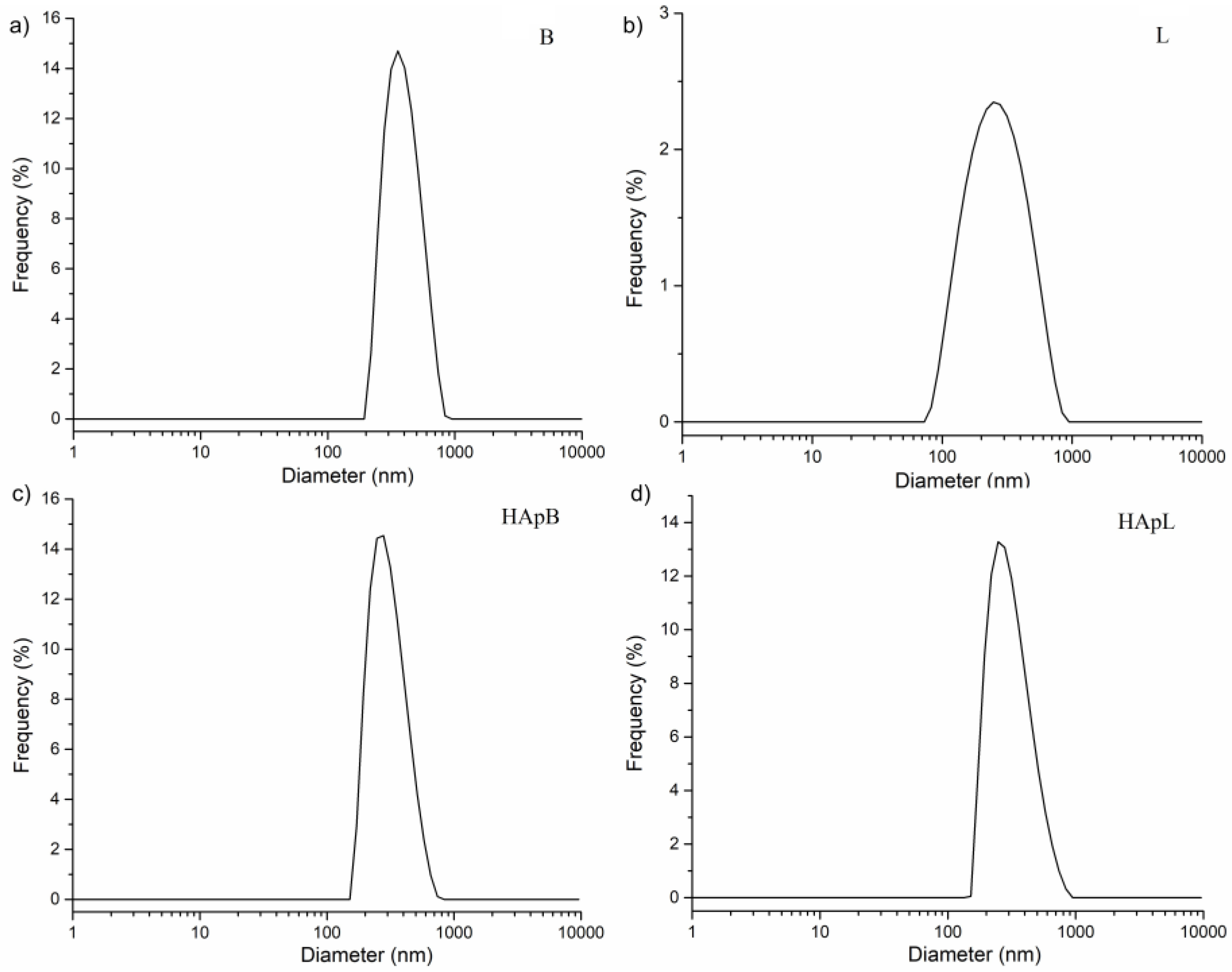
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roller, S.; Ernest, N.; Buckle, J. The Antimicrobial Activity of High-Necrodane and Other Lavender Oils on Methicillin-Sensitive and -Resistant Staphylococcus aureus (MSSA and MRSA). J Altern. Complement. Med. 2009, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, E.; Marselos, M.; Sakellaridis, N.; Constantinidis, T.; Skaltsa, H. Ethnopharmacological approach to the herbal medicines of the “Antidotes” in Nikolaos Myrepsos’ Dynameron. J. Ethnopharmacol. 2015, 163, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Ba-Hamdan, A.H.A.; Aly, M.M.; Bafeel, S.O. Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Ocimum basilicum, Collected from Jeddah Region, Saudi Arabia. J. Microbiol. Res. 2014, 4, 1–9. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food. Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E. The Essential Oils; D. Van Nostrand: New York, NY, USA, 1948. [Google Scholar]
- Boyle, W. Spices and essential oils as preservatives. The American Perfumer and Essential Oil Review; Robbins Perfumer Co.: New York, NY, USA, 1955; Volume 66, pp. 25–28. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.E. Natural antimicrobials from plants. In New Methods of Food Preservation; Gould, G.W., Ed.; Blackie Academic and Professional: London, UK, 1995; pp. 58–89. [Google Scholar]
- Tuley de Silva, K. (Ed.) A Manual on the Essential Oil Industry; United Nations Industrial Development Organization: Vienna, Austria, 1996. [Google Scholar]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food. Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Bishop, C.D. Antiviral activity of the essential oil of Melaleuca alternifolia (Maiden and Betche) Cheel (tea tree) against tobacco mosaic virus. J. Essent. Oil Res. 1995, 7, 641–644. [Google Scholar] [CrossRef]
- Azzouz, M.A.; Bullerman, L.B. Comparative antimycotic effects of selected herbs, spices, plant components and commercial antifungal agents. J. Food Protect. 1982, 45, 1298–1301. [Google Scholar] [CrossRef]
- Juglal, S.; Govinden, R.; Odhav, B. Spice oils for the control of co-occurring mycotoxin-producing fungi. J. Food Protect. 2002, 65, 683–687. [Google Scholar] [CrossRef]
- Pandey, R.; Kalra, A.; Tandon, S.; Mehrotra, N.; Singh, H.N.; Kumar, S. Essential oil compounds as potent source of nematicidal compounds. J. Phytopathol. 2000, 148, 501–502. [Google Scholar] [CrossRef]
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z.G. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 1992, 48, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Manabe, A.; Nakayama, S.; Sakamoto, K. Effects of essential oils on erythrocytes and hepatocytes from rats and dipalitoyl phophatidylcholine-liposomes. Jpn. J. Pharmacol. 1987, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczynska, A.; Sikora, M.; Kalemba, D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of essential oil of Melaleuca alternifola (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential Oils and Their Principal Constituents as Antimicrobial Agents for Synthetic Packaging Films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Santos, R.; Andrade, M.; Ramos de Melob, N.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of Chemical Composition, Antioxidant and Antimicrobial Activity of Lavender (Lavandula angustifolia L.) Essential Oils Extracted by Supercritical CO2, Hexane and Hydrodistillation. Food Bioprocess. Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Dini, I. Chapter 14—Use of Essential Oils in Food Packaging. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 139–147. [Google Scholar]
- Echegoyen, Y.; Nerín, C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; Aznar, M.; Salafranca, J.; Nerín, C. Raman spectroscopy, electronic microscopy and SPME-GC-MS to elucidate the mode of action of a new antimicrobial food packaging material. Anal. Bioanal. Chem. 2017, 409, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.; Becerril, R.; Nerin, C.; Bosetti, O. Development of a multilayer antimicrobial packaging material for tomato puree using an innovative technology. LWT-Food Sci. Technol. 2016, 72, 361–367. [Google Scholar] [CrossRef]
- Wrona, M.; Bentayeb, K.; Nerín, C. A novel active packaging for extending the shelf-life of fresh mushrooms (Agaricus bisporus). Food Control 2015, 54, 200–207. [Google Scholar] [CrossRef]
- Preedy, V. (Ed.) Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Peter, K.V. (Ed.) Handbook of Herbs and Spices; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Porto, C.D.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Kıvrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa Officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bensmira, M.; Jiang, B.; Nsabimana, C.; Jian, T. Effect of Lavender and Thyme incorporation in sunflowerseed oil on its resistance to frying temperatures. Food Res. Int. 2007, 40, 341–346. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Roller, S. Natural Antimicrobials for the Minimal Processing of Foods; Woodhead Publishing Ltd/CRC Press: Cambridge, UK, 2003. [Google Scholar]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thymeessential oils in inhibiting Botrytis cinerea and their application in strawberry packaging system. LWT-Food Sci. Technol. 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Zareen, A.; Gardezi, D.A.; Naeemullah, M.; Masood, M.S.; Tahira, R. Screening of Antibacterial Potential of Siam Queen, Holy Basil and Italian Basil Essential Oils. J. Med. Plant. Stud. 2014, 2, 63–68. [Google Scholar]
- Walsh, S.E.; Maillard, J.Y.; Russel, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.J. Activity and mechanism of action of selected biocidal agents on Gram-positive and negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The Potential of Use Basil and Rosemary Essential Oils as Effective Antibacterial Agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef] [PubMed]
- Sharafati-Chaleshtori, R.; Rokni, N.; Rafieian-Kopaei, M.; Drees, F.; Salehi, E. Antioxidant and Antibacterial Activity of Basil (Ocimum basilicum L.) Essential Oil in Beef Burger. J. Agric. Sci. Technol. 2015, 17, 817–826. [Google Scholar]
- Nakamura, C.V.; Nakamura, T.V.; Bando, E.; Melo, A.F.N.; Cortez, D.A.G.; Filho, B.P.D. Antibacterial activity of Ocimum gratissimum L. essential oil. Mem. Inst. Oswaldo Cruz 1999, 94, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Matasyoh, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Thairu, A.W.M.; Mukiama, T.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. Afr. J. Biotechnol. 2007, 6, 760–765. [Google Scholar]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Raho, G.B.; Abouni, B. Escherichia coli and Staphylococcus aureus most common source of infection. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015. [Google Scholar]
- Tiemersma, E.W.; Bronzwaers, S.L.; Lyytikäinen, O.; Degener, J.E.; Schrijnemakers, P.; Bruinsma, N.; Monen, J.; Witte, W.; Grundman, H.; European Antimicrobial Resistance Surveillance System Participants. European antimicrobial resistance surveillance system participants: Methicillin-resistant Staphylococcus aureus in Europe. Emerg. Infect. Dis. 2004, 10, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Kolmasn, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhayay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (HAp) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2015; pp. 143–159. [Google Scholar]
- Ginebra, M.P.; Canal, C.; Espagnol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Lema, J.M.; Martinez, S.S. (Eds.) Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environmen; IWA Publishing: London, UK, 2017. [Google Scholar]
- Giesen, A. Presentation: Precovery with the Crystalactor® process. Presented at the BALTIC 21 Phosphorus Recycling and Good Agricultural Management Practice, Berlin, Germany, 28–30 September 2009. [Google Scholar]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and Antimicrobial Activity of Silver-Doped Hydroxyapatite Nanoparticles. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Cerium-doped hydroxyapatite nanoparticles synthesized by the co-precipitation method. J. Serb. Chem. Soc. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in Essential Oil Composition of Basil (Ocimum basilicum L.) Italian Cultivars Related to Morphological Characteristics. J. Agric. Food Chem. 1996, 44, 3926–3929. [Google Scholar] [CrossRef]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid h ills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. A 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guegan, R.; Motelica-Heino, M.; Predoi, D. Structural and biological assesement of zinc doped hydroxyapatite nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Inhibiting effect of zinc on hydroxylapatite crystallization. J. Inorg. Biochem. 1995, 58, 49–58. [Google Scholar] [CrossRef]
- Bodycomb, J. HORIBA Scientific Home Page. Available online: http://www.horiba.com/us/particle (accessed on 20 November 2017).
- Lucio, D.; Irache, J.M.; Font, M.; Martínez-Ohárriz, M.C. Nanoaggregation of inclusion complexes of glibenclamide with cyclodextrins. Int. J. Pharm. 2017, 519, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, T.; Liu, Z.; Guo, Y.; Li, C.; Dong, C.; Shuang, S. An ultra-sensitive sensor based on β-cyclodextrin modified magnetic graphene oxide for detection of tryptophan. J. Electroanal. Chem. 2016, 781, 363–370. [Google Scholar] [CrossRef]
- Predoi, D.; Predoi, M.V.; Iconaru, S.L.; Ech Cherif El Kettani, M.; Leduc, D.; Prodan, A.M. Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution. Materials 2017, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Graham, C. Complementary therapies: In the scent of a good night’s sleep. Nurs. Stand. 1995, 9, 21. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Garcia Rehder, V.L.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnophramacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, C.; Gheorghe, I.; Coban, S.; Drumea, V.; Chifiriuc, M.C.; Otilia Banu, O.; Bezirtzoglou, E.; Lazăr, V. Rosmarinus officinalis essential Oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR strains. Rom. Biotechnol. Lett. 2016, 21, 11782–11790. [Google Scholar]
- Nuding, S.; Zabel, L.T. Detection, Identification, and Susceptibility Testing of Bacteria by Flow Cytometry. J. Bacteriol. Parasitol. 2013, S5. [Google Scholar] [CrossRef]
- Oxford University Press. Oxford English Dictionary, 2nd ed.; Note however that Upson and Andrews refer to Research on Bathing in the Roman Empire, and State that There Is No Mention of the Use of Lavender in Works on This Subject; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Fernie, W.T. Herbal Simples; ASIN: B0014W4WNE; John Wright & Co.: Bristol, UK, 1895. [Google Scholar]
- Upson, T.; Andrews, S. The Genus Lavandula; Retrieved, 2004; Royal Botanic Gardens, Kew: London, UK, 2004; ISBN 9780881926422. [Google Scholar]
- Soule, J.A. Father Kino’s Herbs: Growing & Using Them Today; Tierra del Sol Institute Press: Tucson, AZ, USA, 2011. [Google Scholar]
- Arrowsmith, N. Essential Herbal Wisdom: A Complete Exploration of 50 Remarkable Herbs; Llewellyn Worldwide: Woodbury, MN, USA, 2009; pp. 1–576. ISBN 978-0-7387-1488-2. [Google Scholar]
- Moon, T.; Wilkinson, J.M.; Cavanagh, H.M. Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflat. Parasitol. Res. 2006, 99, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M. (Ed.) Lavender: The Genus Lavandula; Taylor and Francis: Abingdon, UK, 2002; ISBN 9780203216521. [Google Scholar]
- Mith, H.; Yayi-Ladékan, E.; Kpoviessi, S.D.S.; Bokossa, I.Y.; Moudachirou, M.; Daube, G.; Clinquart, A. Chemical Composition and Antimicrobial Activity of Essential Oils of Ocimum basilicum, Ocimum canum and Ocimum gratissimum in Function of Harvesting Time. JEOP. 2016, 19, 1413–1425. [Google Scholar] [CrossRef]
- Dube, S.; Upadhyay, P.D.; Tripathi, S.C. Antifungal, physicochemical, and insect-repelling activity of the essential oil of Ocimum basilicum. Can. J. Bot. 1989, 67, 2085–2087. [Google Scholar] [CrossRef]
- Silver, L.L.; Bostian, K.A. Discovery and development of new antibiotics: The problem of antibiotic resistance. Antimicrob. Agents Chemother. 1993, 37, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Nicole, L.; Kavanaugh, N.L.; Katharina Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid.-Based Complement. Altern. Med. 2014, 651593. [Google Scholar] [CrossRef] [PubMed]
- Sinha, G.K.; Gulati, B.C. Antibacterial and antifungal study of some essential oils and some of their constituents. Indian Perfum. 1990, 34, 126–129. [Google Scholar]
- Lis-Balchin, M.; Hart, S.; Deans, S.G.; Eaglesham, E. Comparison of the pharmacological and antimicrobial action of commercial plant essential oils. J. Herbs Spices Med. Plants 1996, 4, 69–86. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour. Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Nelson, R.R.S. In vitro activities of five plant essential olis against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. J. Antimicrob. Chemother. 1997, 40, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Braux, A. GMO 101—A Practical Guide to Genetically Engineered Food; Holistic Nutrition, Exacutive Chef and Culinary Nutritionist; Alain Braux International Publishing, I.I.C.: Lexington, KY, USA, 2014; ISBN 0.9842883-5-X. [Google Scholar]
- FLUIDINOVA Home Page. Available online: http://www.fluidinova.com/company-nano-hydroxyapatite-manufacturer-and-supplier (accessed on 20 November 2017).
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Chanamai, R.; Horn, G.; McClements, D.J. Influence of Oil Polarity on Droplet Growth in Oil-in-Water Emulsions Stabilized by a Weakly Adsorbing Biopolymer or a Nonionic Surfactant. J. Colloid Interface. Sci. 2002, 247, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Yanke, T. Material review: Kashmir Lavender Oil. Perfum. Flavor. 2007, 32, 40–44. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatile by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadruple Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Limban, C.; Chifiriuc, M.C. Antibacterial Activity of New Dibenzoxepinone Oximes with Fluorine and Trifluoromethyl Group Substituents. Int. J. Mol. Sci. 2011, 12, 6432–6444. [Google Scholar] [CrossRef] [PubMed]
- Limban, C.; Marutescu, L.; Chifiriuc, M.C. Synthesis, Spectroscopic Properties and Antipathogenic Activity of New Thiourea Derivatives. Molecules 2011, 16, 7593–7607. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Palade, R.; Israil, A.M. Comparative analysis of disk diffusion and liquid medium microdillution methods fortesting the antibiotic susceptibility patterns of anaerobic bacterial strains isolated from intrabdominal infections. Biointerface Res. Appl. Chem. 2011, 1, 209–220. [Google Scholar]
- Rashid, M.A.; Ashraf, A.; Nazir, S.; Nazir, S.; Nadeem, R.; Iqbal, J.; Jabbar, S.; Ahmed, A.; Tareen, R.B. Chemical composition and biological (antioxidant, antimicrobial and haemolyitc) activities of essential oils of an endemic plant (Thymus linearis subsp. hedgei Jalas). Rom. Biotechnol. Lett. 2017, 22, 12560–12567. [Google Scholar]
- Atak, M.; Mavi, K.; Uremis, I. Bio-herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11159. [Google Scholar]
- Chifiriuc, M.C.; Stecoza, C.; Dracea, O.; Larion, C.; Israil, A.M. Antimicrobial activity of some new O-acyloximino-dibenzo[b,e]thiepins and O-acyloximino-dibenzo[b,e]thiepin-5,5-dioxides against planktonic cells. Rom. Biotechnol. Lett. 2010, 15, 5134–5139. [Google Scholar]
- Marutescu, L.; Limban, C.; Chifiriuc, M.C.; Missir, A.V.; Chirita, I.C.; Caproiu, M.T. Studies on the antimicrobial activity of new compounds containing thiourea function. Biointerface Res. Appl. Chem. 2011, 1, 236–241. [Google Scholar]
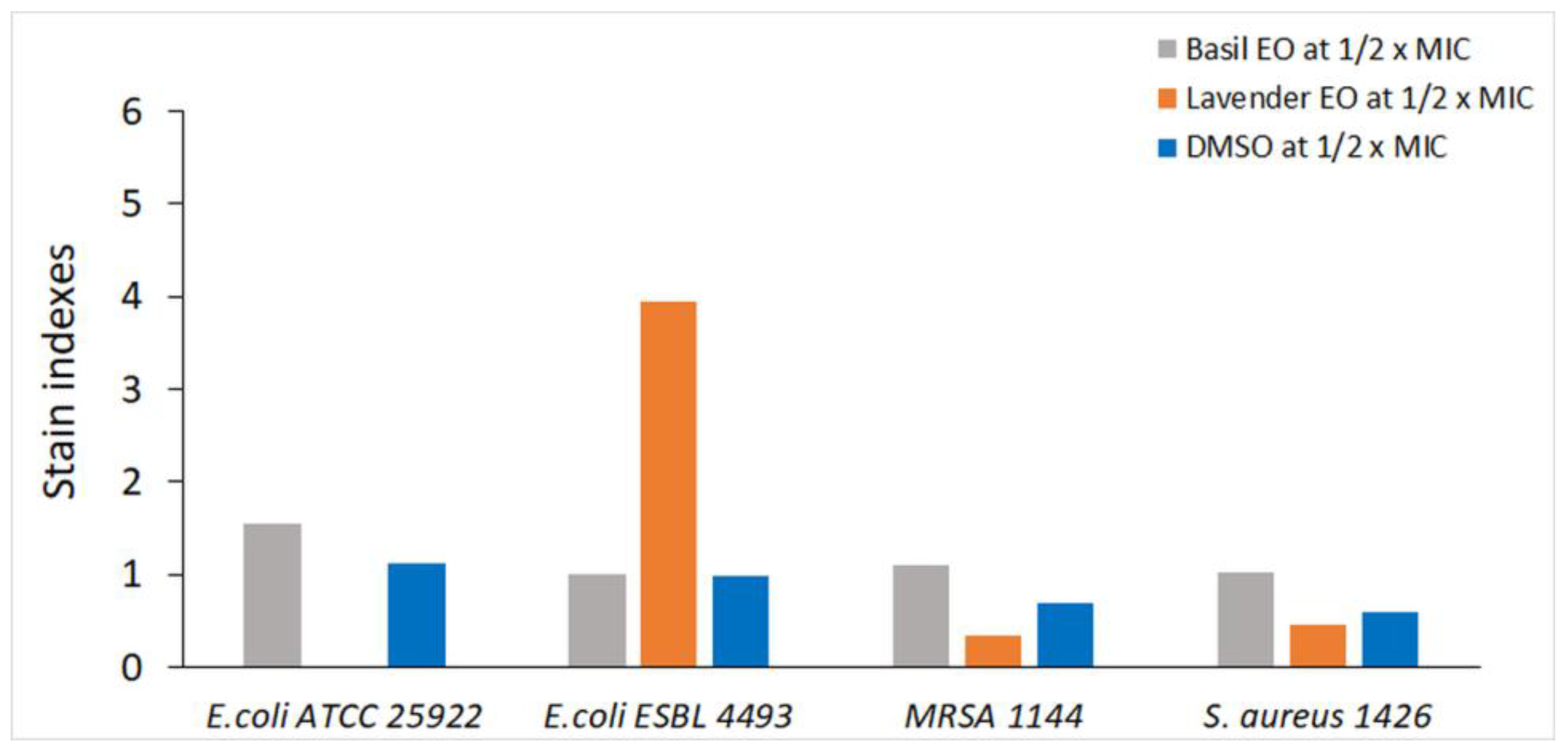
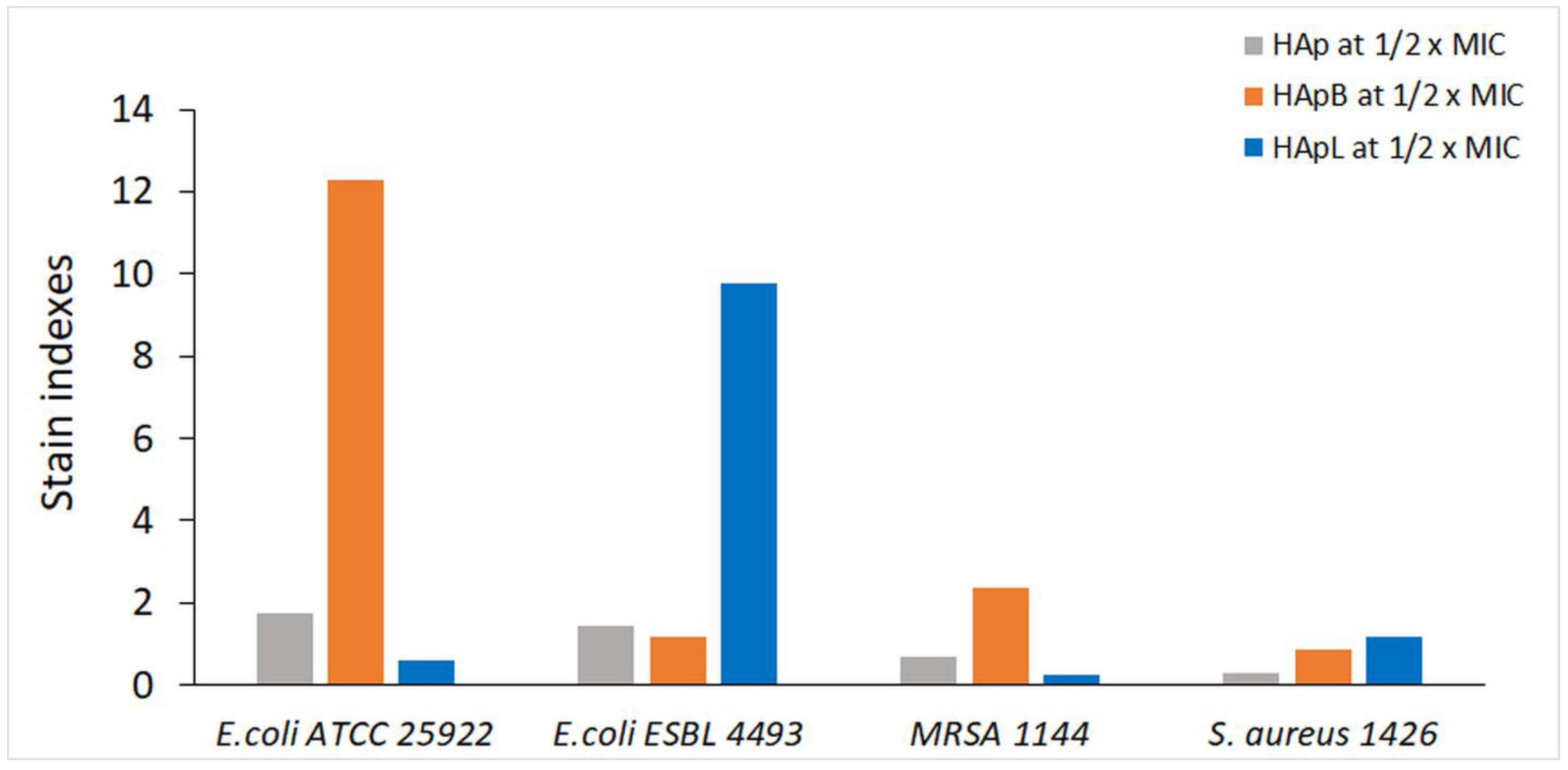
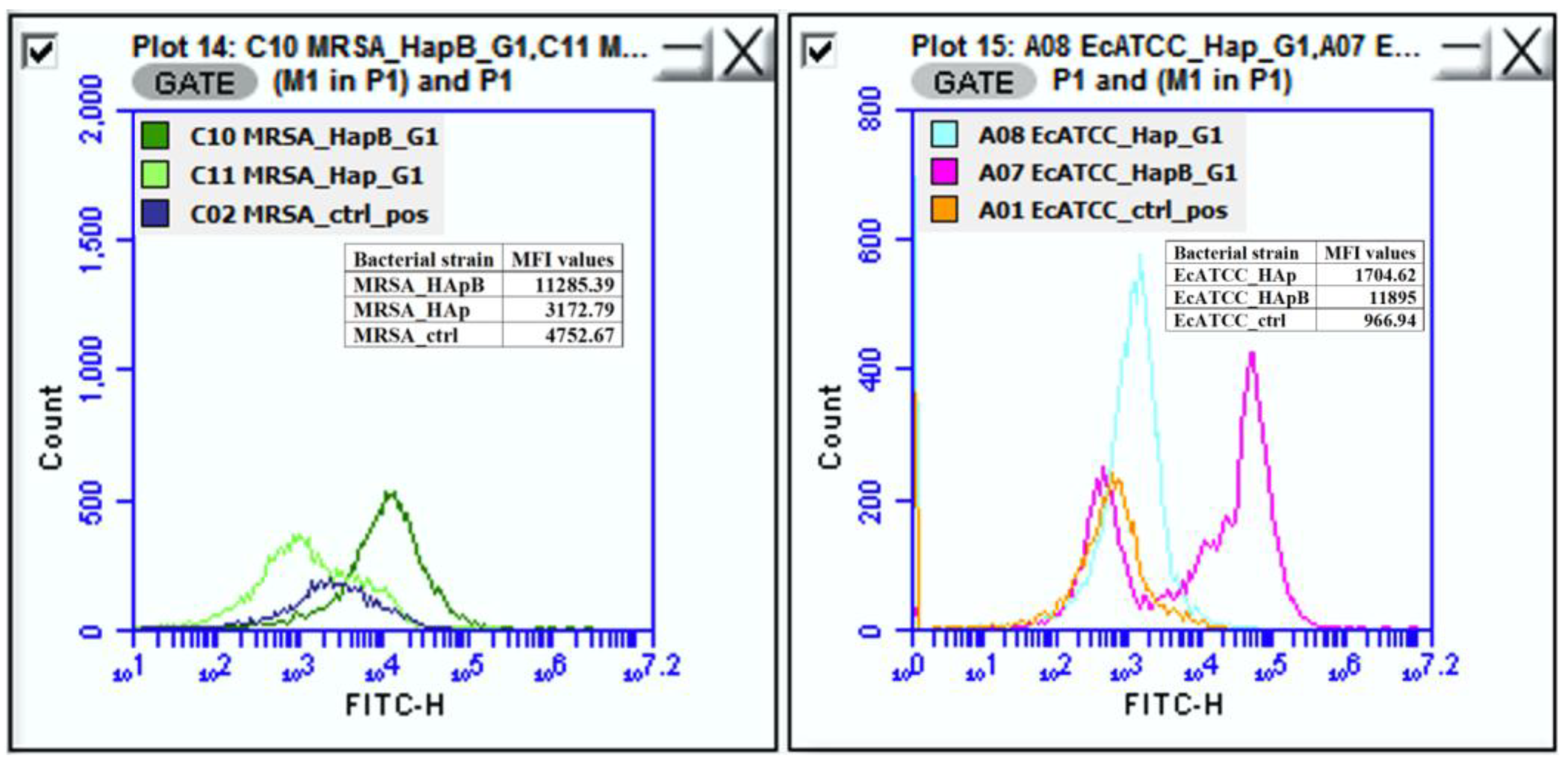
| No. | Compound | % of Total |
|---|---|---|
| Ocimum basilicum L. | ||
| 1 | Linalool | 65.95 |
| 2 | 1,8 cineole | 10.57 |
| 3 | τ-cadinol | 4.74 |
| 4 | Eugenol | 3.76 |
| 5 | α-trans-bergamotene | 2.35 |
| 6 | α-terneol | 1.04 |
| 7 | Borneol | 0.9 |
| 8 | germacrene D | 1.38 |
| 9 | α-cadinene | 1.02 |
| Identified from total area | 91.71 |
| No. | Compound | % of Total |
|---|---|---|
| Lavandula angustifolia | ||
| 1 | Linalool | 47.55 |
| 2 | Borneol | 8.52 |
| 3 | Camphor | 9.67 |
| 4 | terpinene-4-ol | 3.8 |
| 5 | Myrcene | 0.68 |
| 6 | Camphene | 0.56 |
| 7 | α-pinene | 0.54 |
| 8 | Sabinene | 0.5 |
| 9 | Limonene | 0.24 |
| 10 | β-phellandrene | 0.16 |
| 11 | α-terpinene | 0.06 |
| 12 | 1-8-cineole | 8.6 |
| 13 | linalool acetate | 3.75 |
| 14 | α-terpineol | 1.35 |
| 15 | Cryptone | 1.25 |
| 16 | geranyl acetate | 0.98 |
| Identified from total area | 79.61 |
| Sample | SEM (nm) | DLS (nm) |
|---|---|---|
| HAp | 88.5 ± 3 | 273.86 ± 2 |
| HApB | 76.8 ± 5 | 265 ± 2 |
| HApL | 63.3 ± 6 | 257.76 ± 2 |
| Plant EOs and Plant EOs-HAp Combinations (Concentration) | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| Lavander EO | HapL (10 mg/mL) | BasilEO | HApB (10 mg/mL) | Hap (10 mg/mL) | DMSO | |
| Bacterial strain | ||||||
| E. coli ATCC 25922 | 20 ± 1 | 15 ± 1 | 9 ± 2 | 7 ± 1 | *- | *- |
| E. coli ESBL 4493 | 16 ± 0.5 | 10 ± 2 | 8 ± 1 | 6 ± 1 | *- | *- |
| S. aureus 1426 | 25 ± 1 | 13 ± 2 | 10 ± 1 | 8 ± 1 | *- | *- |
| MRSA 1144 | 24 ± 0.5 | 10 ± 2 | 7 ± 2 | 6 ± 1 | *- | *- |
| Plant EOs Bacterial Strain | Lavander EO (1:1) | Basil EO (1:1) | ||
|---|---|---|---|---|
| MIC (%) | MBC (%) | MIC (%) | MBC (%) | |
| E. coli ATCC 25922 | <0.1 | <0.1 | 25 | 40 |
| E. coli ESBL 4493 | 0.19 | 0.19 | 30 | 45 |
| S. aureus 1426 | 0.78 | 1.56 | 40 | 50 |
| MRSA 1144 | 0.78 | 1.56 | 45 | 50 |
| Sample | HApL | HApB | HAp | |||
|---|---|---|---|---|---|---|
| Bacterial Strain | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) |
| E. coli ATCC 25922 | 0.15 | 0.31 | 5 | 5 | 5 | 5 |
| E. coli ESBL 4493 | 0.62 | 0.62 | >5 | >5 | >5 | >5 |
| S. aureus 1426 | 0.31 | 0.62 | 5 | >5 | 5 | >5 |
| MRSA 1144 | 0.31 | 0.62 | 5 | >5 | 5 | >5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. https://doi.org/10.3390/nano8050291
Predoi D, Iconaru SL, Buton N, Badea ML, Marutescu L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials. 2018; 8(5):291. https://doi.org/10.3390/nano8050291
Chicago/Turabian StylePredoi, Daniela, Simona Liliana Iconaru, Nicolas Buton, Monica Luminita Badea, and Luminita Marutescu. 2018. "Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite" Nanomaterials 8, no. 5: 291. https://doi.org/10.3390/nano8050291




