Nitrogen Self-Doped Activated Carbons Derived from Bamboo Shoots as Adsorbent for Methylene Blue Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of BSNCs
2.2. Adsorption Properties of BSNCs
2.2.1. Effect of Preparation Process of BSNCs on Its Adsorption Properties
2.2.2. Effect of pH
2.2.3. Effect of Initial Concentration of MB and Contact Time
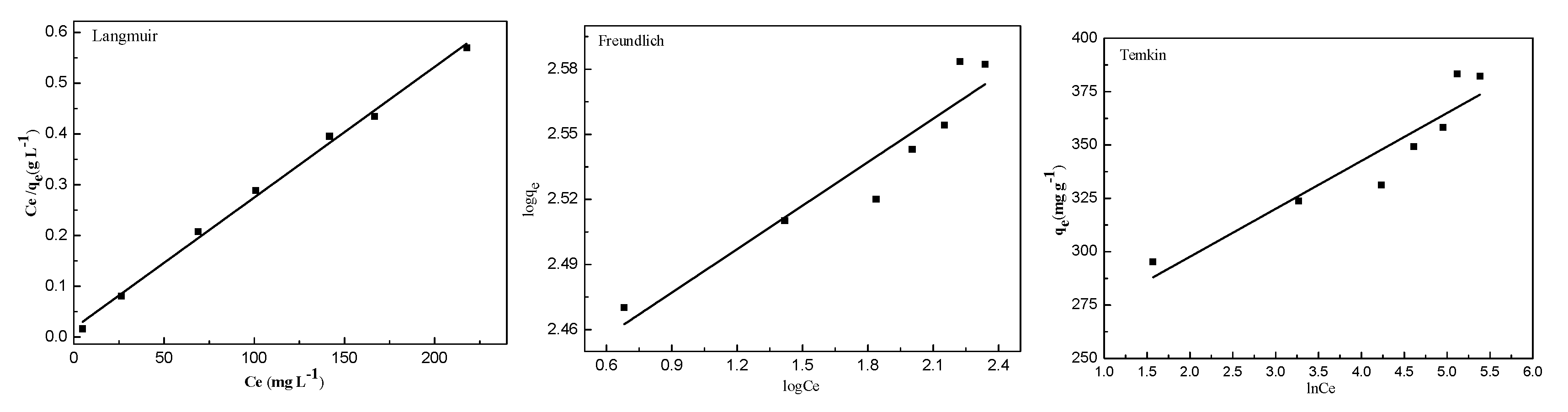
2.3. Adsorption Isotherms
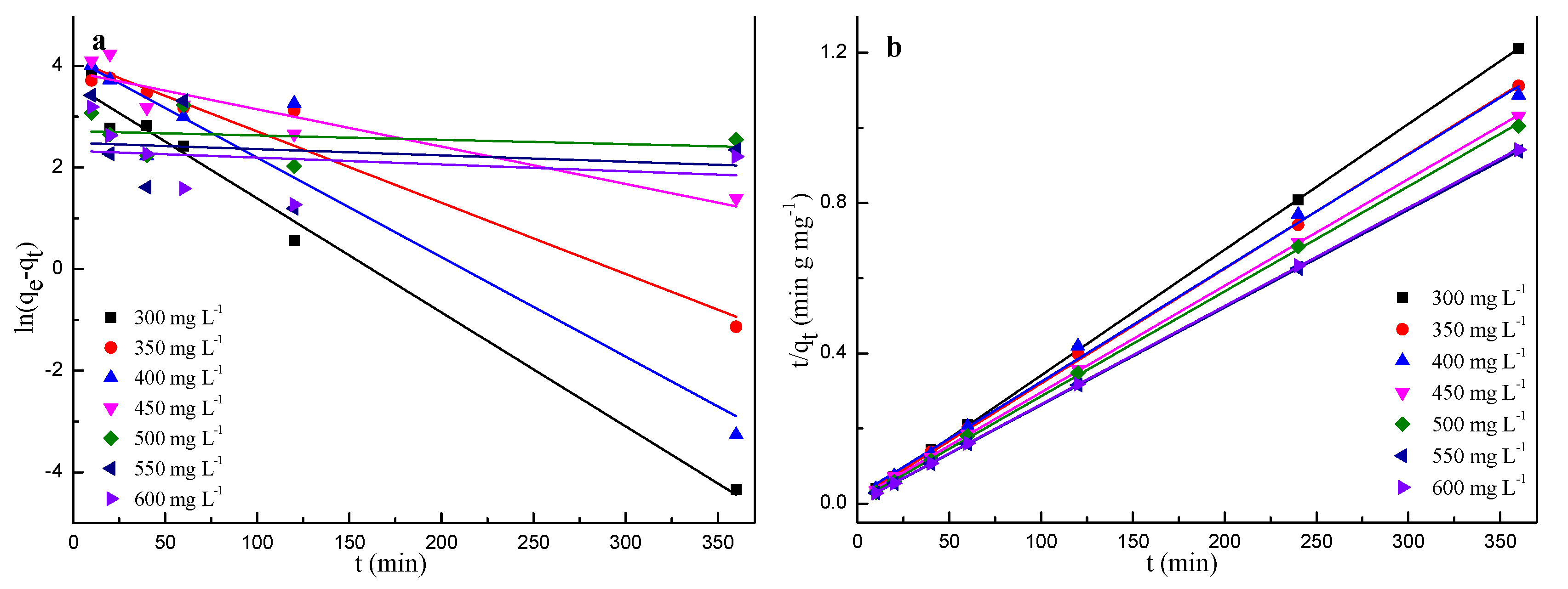
2.4. Adsorption Kinetics
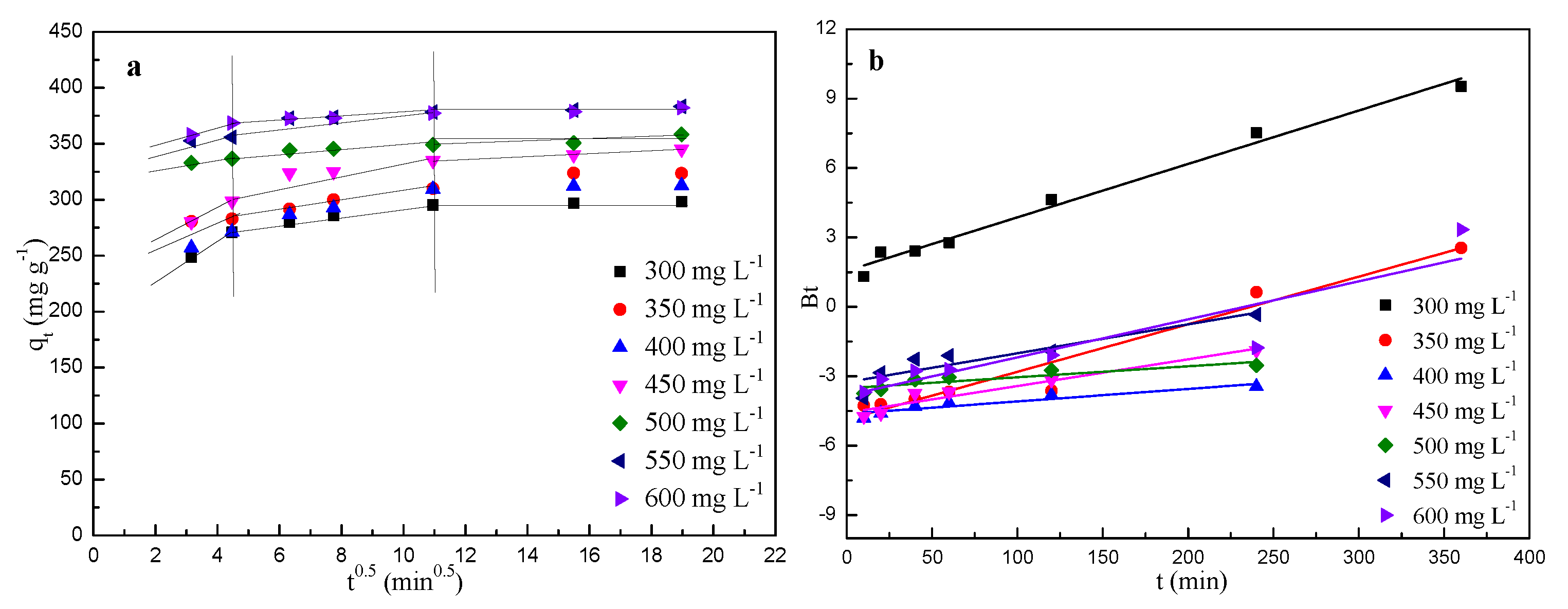
2.5. Diffusion Model
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of BSNCs
3.3. Adsorption Equilibrium
3.4. Adsorption Kinetic
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vandevivere, P.C.; Bianchi, R.; Verstraete, W. Review: Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. Cheminform 1998, 29, 289–302. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica, bast. Arabian J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Mu, N.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Worch, E. Adsorption Technology in Water Treatment; De Gruyter: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar]
- Bratby, J. Coagulation and flocculation in water and wastewater treatment-second edition. Drink. Water Treat. 2006, 5, 507–510. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Poll. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Jiao, S.; Zhao, Y.; Bi, M.; Bi, S.; Li, X.; Wang, B.; Li, C.; Dong, Y. Removal of methylene blue from water by BiFeO3/carbon fibre nanocomposite and its photocatalytic regeneration. Catalysts 2018, 8, 267. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C. Application of cross-linked stearic acid nanoparticles with dialysis membranes for methylene blue recovery. Sep. Purif. Technol. 2018, 204, 21–29. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Gouda, M.H.; Abu-Saied, M.A.; El-Shazly, Y.M.S.; Farag, H.A. Development of grafted cotton fabrics ions exchanger for dye removal applications: Methylene blue model. Desalin. Water Treat. 2015, 57, 22049–22060. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- Hameed, B.H.; EI-Khaiary, M.I. Equilibrium, kinetics and mechanism of malachite green adsorption on activated carbon prepared from bamboo by K2CO3 activation and subsequent gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef]
- Borah, L.; Goswami, M.; Phukan, P. Adsorption of methylene blue and eosin yellow using porous carbon prepared from tea waste: Adsorption equilibrium, kinetics and thermodynamics study. J. Environ. Chem. Eng. 2015, 3, 1018–1028. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, Q.Y.; Cui, Y.; Xie, F.; Li, W.J.; Li, Y.W.; Chen, M.F. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Liu, Y.; Zhou, X.Y.; Zhang, Z.Q.; Zhang, J.; Dang, F.Q. Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour. Technol. 2014, 154, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Piccin, J.S.; Gomes, C.S.; Feris, L.A.; Gutterres, M. Kinetics and isotherms of leather dye adsorption by tannery solid waste. Chem. Eng. J. 2012, 183, 30–38. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, K.; Guo, H.; Ning, X.; Hu, A.; Tang, Q.; Fan, B.; Zhu, Y.; Chen, X. Nitrogen-doped worm-like graphitized hierarchical porous carbon designed for enhancing area-normalized capacitance of electrical double layer supercapacitors. Carbon 2017, 117, 163–173. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H.; Yang, Z.; Ji, G.; Yu, B.; Liu, X.; Liu, Z. Mesoporous nitrogen-doped carbons with high nitrogen contents and ultrahigh surface areas: Synthesis and applications in catalysis. Green Chem. 2016, 18, 1976–1982. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Nursam, N.M.; Xia, F.; Sani, M.A.; Li, W.; Wang, X.D.; Caruso, R.A. High-performance coral reef-like carbon nitrides: Synthesis and application in photocatalysis and heavy metal ion adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.L.; Zhang, Y.G.; Wang, L.L.; Zhang, L.T.; Zhang, L.; Xu, L.; Liu, L. Biomass based nitrogen-doped structure-tunable versatile porous carbon materials. J. Mater. Chem. A 2017, 5, 12958–12968. [Google Scholar] [CrossRef]
- Sun, J.; Ding, Z.Q.; Gao, Q.; Xun, H.; Tang, F.; Xia, E.D. Major chemical constituents of bamboo shoots (Phyllostachys pubescens): Qualitative and quantitative research. J. Agric. Food Chem. 2016, 64, 2498–2505. [Google Scholar] [CrossRef]
- Moosa, A.A.; Ridha, A.M.; Kadhi, N.A. Use of biocomposite adsorbents for the removal of methylene blue dye from aqueous solution. Am. J. Mater. Sci. 2016, 6, 135–146. [Google Scholar]
- Chen, L.; Li, Y.; Du, Q.; Wang, Z.; Xia, Y.; Yedinak, E.; Lou, J.; Ci, L. High performance agar/graphene oxide composite aerogel for methylene blue removal. Carbohydr. Polym. 2017, 155, 345–353. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Chu, L. Chlorine-functionalized reduced graphene oxide for methylene blue removal. RSC Adv. 2015, 5, 52466–52472. [Google Scholar] [CrossRef]
- Kharismadewi, D.; Haldorai, Y.; Nguyen, V.H.; Tuma, D.; Shim, J.J. Synthesis of graphene oxide-poly (2-hydroxyethyl methacrylate) composite by dispersion polymerization in supercritical CO2: Adsorption behavior for the removal of organic dye. Compos. Interface 2016, 23, 1–21. [Google Scholar] [CrossRef]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhu, J.J.; Wang, T.; Zhao, Y.X.; Zhao, Z. Mesoporous graphitic carbon nitride: A new and efficient adsorbent for adsorption removal of methylene blue. Sci. Sin. Chim. 2016, 46, 784–790. [Google Scholar] [CrossRef]
- Hameed, B.H. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J. Hazard. Mater. 2009, 162, 939–944. [Google Scholar] [CrossRef]
- Oei, B.C.; Ibrahim, S.; Wang, S.; Ang, H.M. Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresour. Technol. 2009, 100, 4292–4295. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Chem. Phys. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the adsorption in solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physic. USSR 1960, 12, 217–222. [Google Scholar]
- Bao, C.; Ma, J.; Zhou, L.; Shao, Y.; Wu, Q.; Fei, W. Self-template synthesis of hierarchical magnetic porous carbon fibers derived from Fe(BTC)-coated bamboo fibers for fast removal of methylene blue. RSC Adv. 2015, 5, 87616–87625. [Google Scholar] [CrossRef]
- Feng, Y.F.; Zhou, H.; Liu, G.H.; Qiao, J.; Wang, J.H.; Lu, H.Y.; Yang, L.Z.; Wu, Y.H. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef]
- Ünal, G.; Kocabıyık, B.; Üner, O. Adsorptive removal of methylene blue from aqueous solution by the activated carbon obtained from the fruit of catalpa bignonioides. Water Air Soil Poll. 2015, 226, 1–14. [Google Scholar]
- Jia, Z.G.; Li, Z.Y.; Ni, T.; Li, S. Adsorption of low-cost absorption materials based on biomass (Cortaderia selloana flower spikes) for dye removal: Kinetics, isotherms and thermodynamic studies. J. Mol. Liq. 2017, 229, 285–292. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 68, 722–730. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloester stoffe. Kungliga Sven. Vetenskapsakad Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, S.; Xu, S.; Liu, C.; Chen, F.; Wang, D.; Liu, S.; Chen, Z.; Wu, Z. Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite. Chin. J. Chem. Eng. 2016, 24, 190–195. [Google Scholar] [CrossRef]
- Gao, S.; Liu, L.; Tang, Y.; Jia, D.; Zhao, Z.; Wang, Y. Coal based magnetic activated carbon as a high performance adsorbent for methylene blue. J. Porous Mat. 2016, 23, 877–884. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sani. Eng. Division 1963, 89, 31–59. [Google Scholar]
- Boyd, G.E.; Myers, L.S., Jr.; Adamson, A.W. The exchange adsorption of ions from aqueous solutions by organic zeolites. III. performance of deep adsorbent beds under non-equilibrium conditions. JACS 1947, 69, 2849–2859. [Google Scholar] [CrossRef]
- Mi, B.B.; Chen, X.F.; Jiang, C.L.; Wang, J.X.; Chen, X.J.; Zhang, B.; Liu, X.M.; Liu, Z.J.; Fei, B.H. Nitrogen-doped porous carbon derived from bamboo shoot as solid base catalyst for Knoevenagel condensation and transesterification reactions. Catalysts 2018, 8, 232. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

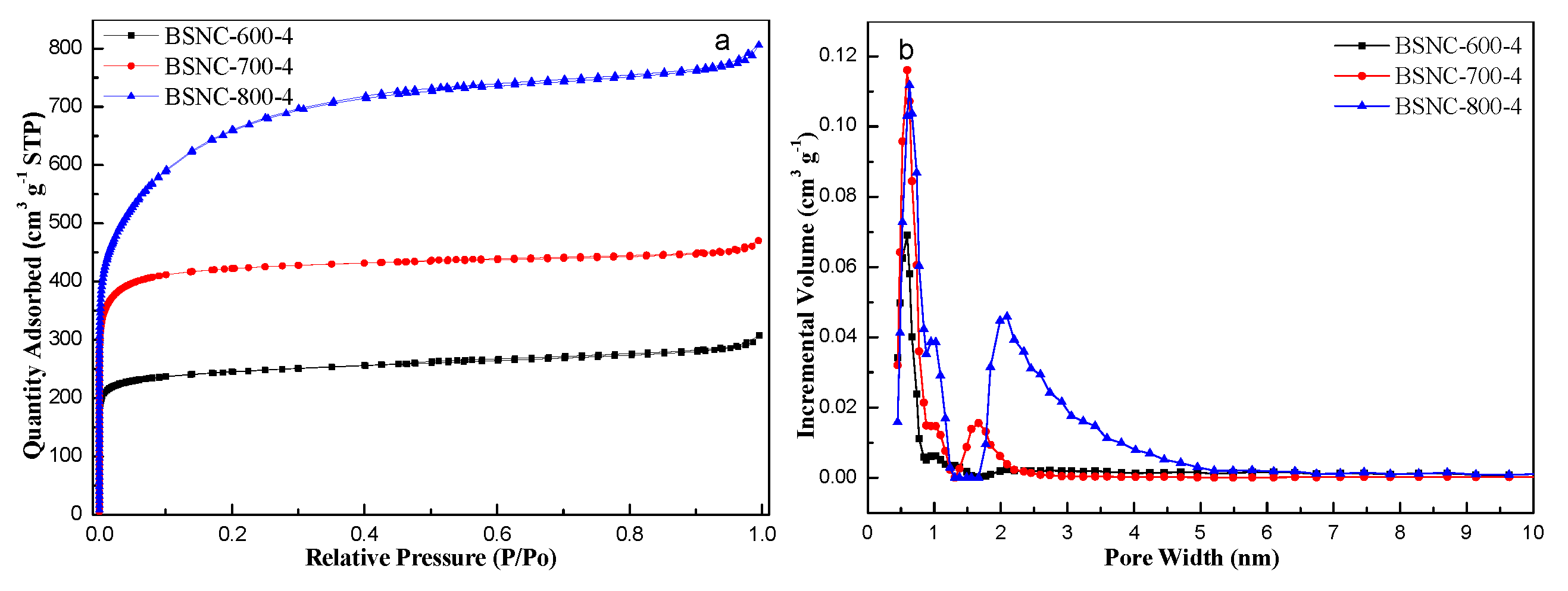
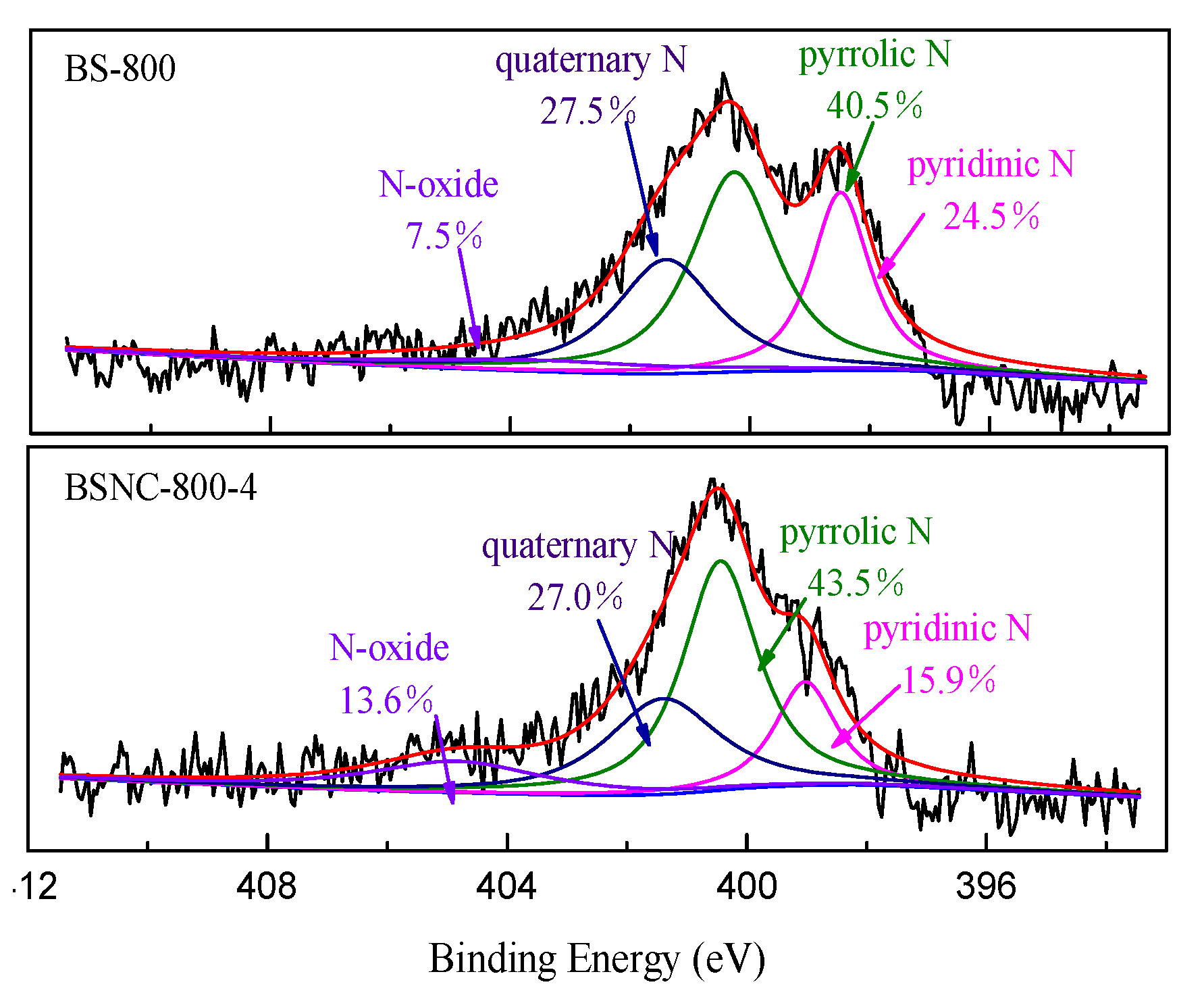
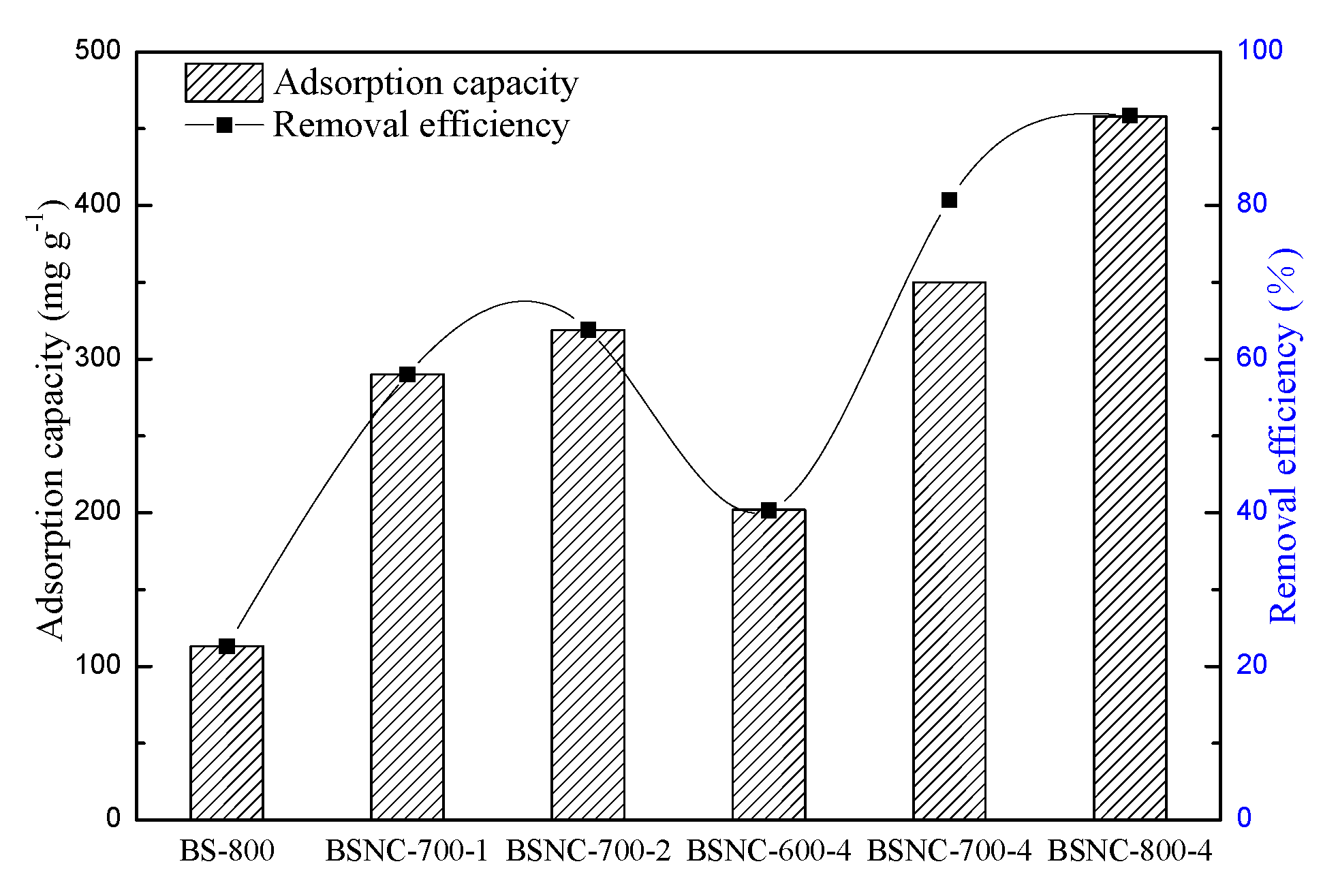


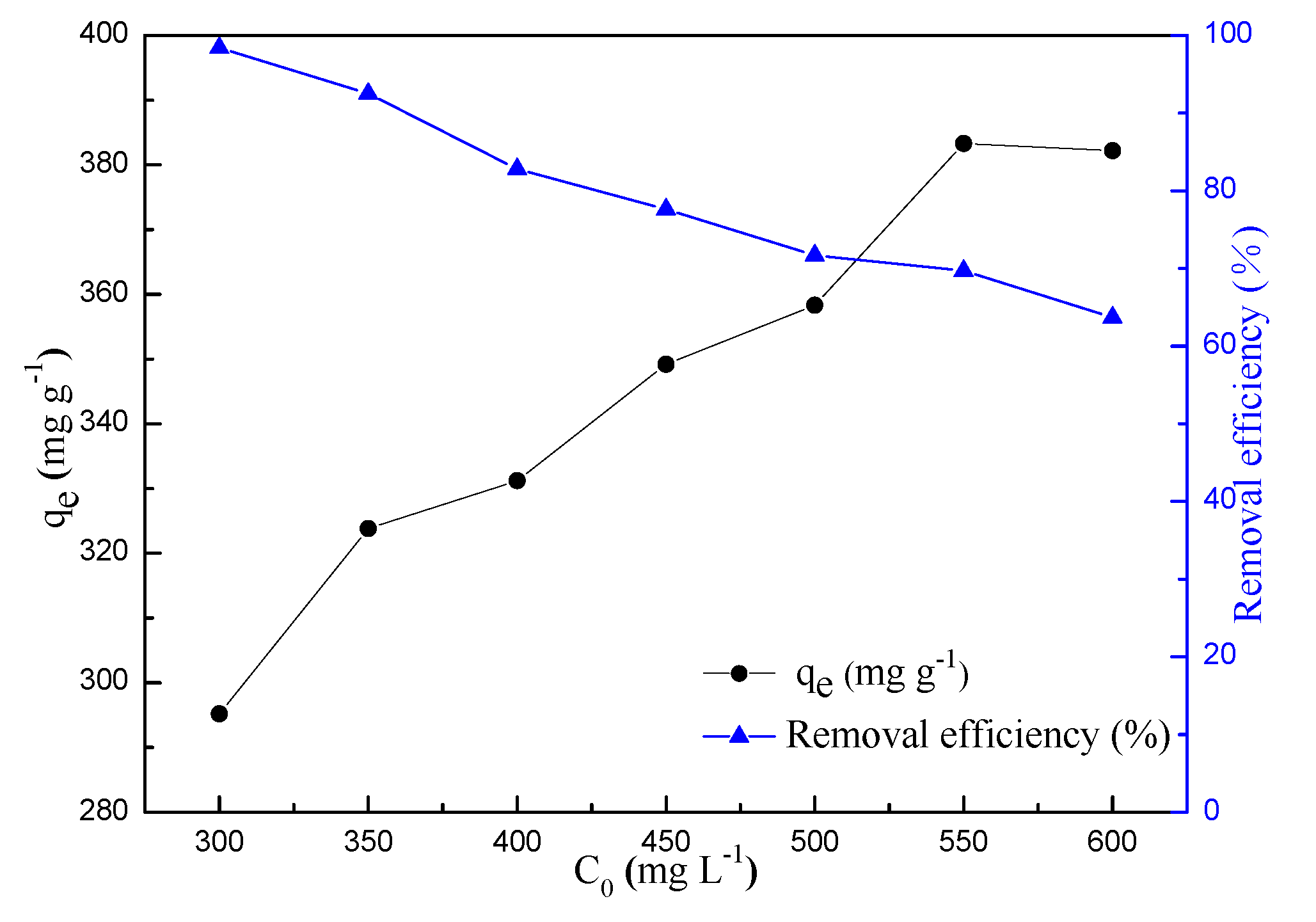
| Samples | SBET (m2 g−1) | VT (m3 g−1) | Vµ (m3 g−1) | Dp (nm) | N (wt%) | H (wt%) | C (wt%) | O (wt%) |
|---|---|---|---|---|---|---|---|---|
| BS-800 | 12.2 | - | - | - | 4.64 | 1.05 | 63.87 | 28.72 |
| BSNC-700-1 | 1208.2 | - | - | 1.97 | 2.71 | 0.91 | 67.27 | 28.83 |
| BSNC-700-2 | 1215.1 | 0.59 | 0.52 | 1.94 | 2.63 | 0.87 | 84.12 | 12.19 |
| BSNC-600-4 | 962.8 | 0.48 | 0.34 | 1.97 | 4.65 | 1.96 | 69.18 | 23.97 |
| BSNC-700-4 | 1475.5 | 0.73 | 0.63 | 1.97 | 2.79 | 0.90 | 80.37 | 15.78 |
| BSNC-800-4 | 2270.9 | 1.25 | 0.94 | 2.19 | 1.06 | 0.80 | 43.43 | 54.36 |
| Isotherms | Parameters | ||
|---|---|---|---|
| Langmuir | Q0 (mg g−1) | KL (L mg−1) | R2 |
| 384.6 | 0.148 | 0.9962 | |
| Freundlich | KF ((mg g−1) (L mg−1)1/n) | n | R2 |
| 261.2 | 1.50 | 0.9043 | |
| Temkin | KT | B | R2 |
| 11.26 | 22.4 | 0.8808 | |
| C0 (mg g−1) | qe,exp (mg g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | ||
| 300 | 297.0 | 0.0537 | 37.7 | 0.98 | 0.0016 | 303.0 | 0.99 |
| 350 | 323.8 | 0.1935 | 60.1 | 0.76 | 0.0007 | 322.6 | 0.99 |
| 400 | 331.2 | 0.0101 | 57.2 | 0.59 | 0.0004 | 333.3 | 0.99 |
| 450 | 349.2 | 0.0168 | 48.5 | 0.87 | 0.0006 | 357.1 | 0.99 |
| 500 | 358.3 | 0.0481 | 51.6 | 0.71 | 0.0012 | 357.1 | 0.99 |
| 550 | 384.1 | 0.0288 | 15.8 | 0.84 | 0.0029 | 384.6 | 0.99 |
| 600 | 382.2 | 0.0378 | 27.8 | 0.84 | 0.0022 | 384.6 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, B.; Wang, J.; Xiang, H.; Liang, F.; Yang, J.; Feng, Z.; Zhang, T.; Hu, W.; Liu, X.; Liu, Z.; et al. Nitrogen Self-Doped Activated Carbons Derived from Bamboo Shoots as Adsorbent for Methylene Blue Adsorption. Molecules 2019, 24, 3012. https://doi.org/10.3390/molecules24163012
Mi B, Wang J, Xiang H, Liang F, Yang J, Feng Z, Zhang T, Hu W, Liu X, Liu Z, et al. Nitrogen Self-Doped Activated Carbons Derived from Bamboo Shoots as Adsorbent for Methylene Blue Adsorption. Molecules. 2019; 24(16):3012. https://doi.org/10.3390/molecules24163012
Chicago/Turabian StyleMi, Bingbing, Jingxin Wang, Hongzhong Xiang, Fang Liang, Jianfei Yang, Zixing Feng, Tao Zhang, Wanhe Hu, Xianmiao Liu, Zhijia Liu, and et al. 2019. "Nitrogen Self-Doped Activated Carbons Derived from Bamboo Shoots as Adsorbent for Methylene Blue Adsorption" Molecules 24, no. 16: 3012. https://doi.org/10.3390/molecules24163012





