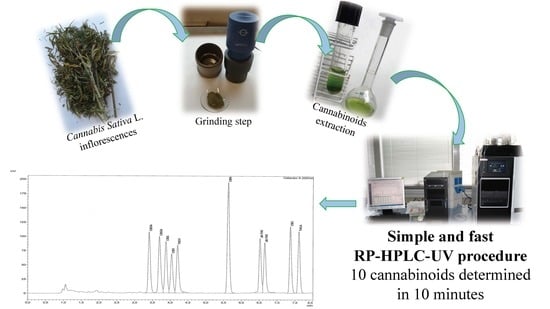
Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Validation
2.2.1. Precision
2.2.2. Repeatability, R
2.2.3. Reproducibility, R
2.2.4. Recovery
2.2.5. Detection Limit, LOD
2.2.6. Quantification Limit, LOQ
2.2.7. Linearity
2.3. Cannabinoids in Hemp Samples
3. Materials and Methods
3.1. Chemicals, Standards and Apparatus
3.2. Sampling
3.3. Cannabinoid Extraction
3.4. Preparation of Standard Solution
3.5. HPLC Conditions
3.6. Validation Parameters
3.6.1. Precision
3.6.2. Repeatability, R
3.6.3. Reproducibility, R
3.6.4. Recovery
3.6.5. Detection Limit, LOD
3.6.6. Quantification limit, LOQ
3.6.7. Linearity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Gimenez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Legge 2 Dicembre 2016, n.242. Disposizioni per la Promozione della Coltivazione e della Filiera Agroindustriale della Canapa (16G00258), GU Serie Generale n. 304 del 30-12-2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 1 June 2019).
- European Commission (EC). European Union Common Catalogue of Varieties of Agricultural Plant Species, Plant Variety Database. Available online: http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search//public/index.cfm (accessed on 1 June 2019).
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Laezza, C. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. In The Chemistry of Organic Natural Products/Progrès dans la Chimie des Substances Organiques Naturelles; Springer International Publishing: Vienna, Austria, 1967; pp. 175–213. [Google Scholar]
- Radwan, M.M.; Wanas, A.S.; Chandra, S.; ElSohly, M.A. Natural Cannabinoids of Cannabis and Methods of Analysis. In Cannabis sativa L.-Botany and Biotechnology, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 161–182. [Google Scholar]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci 2018, 41, 398–415. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Yegles, M.; Van Elsué, N.; Schneider, S. Determination of cannabinoids in hair of CBD rich extracts consumers using gas chromatography with tandem mass spectrometry (GC/MS–MS). Forensic. Sci. Int. 2018, 292, 163–166. [Google Scholar] [CrossRef]
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Leghissa, A.; Hildenbrand, Z.L.; Foss, F.W.; Schug, K.A. Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry. J. Sep. Sci 2018, 41, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wene, D.; Fan, Z.T. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Burnier, C.; Esseiva, P.; Roussel, C. Quantification of THC in Cannabis plants by fast-HPLC-DAD: A promising method for routine analyses. Talanta 2019, 192, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L.(hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic. Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef]
- Fekete, S.; Sadat-Noorbakhsh, V.; Schelling, C.; Molnár, I.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Implementation of a generic liquid chromatographic method development workflow: Application to the analysis of phytocannabinoids and Cannabis sativa extracts. J. Pharm. Biomed. Anal. 2018, 155, 116–124. [Google Scholar] [CrossRef]
- Purschke, K.; Heinl, S.; Lerch, O.; Erdmann, F.; Veit, F. Development and validation of an automated liquid-liquid extraction GC/MS method for the determination of THC, 11-OH-THC, and free THC-carboxylic acid (THC-COOH) from blood serum. Anal. Bioanal. Chem. 2016, 408, 4379–4388. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Gambaro, V. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lee, H.H.; Lee, J.F.; Chen, B.H. Urine specimen validity test for drug abuse testing in workplace and court settings. J. Food Drug Anal. 2018, 26, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Leaner and greener analysis of cannabinoids. Anal. Bioanal. Chem. 2017, 409, 3153–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; ElSohly, M.A. Determination of 11 cannabinoids in biomass and extracts of different varieties of Cannabis using high-performance liquid chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- ASTM. ASTM E685-93(2013), Standard Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid Chromatography; ASTM International: West Conshohocken, PA, USA, 2013; Available online: www.astm.org (accessed on 1 June 2019).
- Regulation (EU) No 1307/2013 of the European Parliament and of the Council of 17 December 2013 Establishing Rules for Direct Payments to Farmers under the Support Schemes within the Framework of the Common Agricultural Policy and Repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32013R1307 (accessed on 1 June 2019).
- del M. Contreras, M.; Jurado-Campos, N.; Sánchez-Carnerero Callado, C.; Arroyo-Manzanares, N.; Fernández, L.; Casano, S.; Ferreiro-Vera, C. Thermal desorption-ion mobility spectrometry: A rapid sensor for the detection of cannabinoids and discrimination of Cannabis sativa L. chemotypes. Sens. Actuators B Chem. 2018, 273, 1413–1424. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2017/1155 of 15 February 2017 Amending Delegated Regulation (EU) No 639/2014 as Regards the Control Measures Relating to the Cultivation of Hemp, Certain Provisions on the Greening Payment, the Payment for Young Farmers in Control of a Legal Person, the Calculation of the per Unit Amount in the Framework of Voluntary Coupled Support, the Fractions of Payment Entitlements and Certain Notification Requirements Relating to the Single Area Payment Scheme and the Voluntary Coupled Support, and Amending Annex X to Regulation (EU) No 1307/2013 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/reg_del/2017/1155/oj (accessed on 1 June 2019).
- De Backer, D.B.; Debrus, B.; Lebrun, P. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Jin, S.; Yu, Y.; Lee, C.; Chen, J. Analytical & Bioanalytical Techniques Classification of Cannabis Cultivars Marketed in Canada for Medical Purposes by Quantification of Cannabinoids and Terpenes Using HPLC-DAD and GC-MS. J. Anal. Bioanal. Tech. 2017, 8, 1–9. [Google Scholar]
- Thompson, M.; Ellison, S.L.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
Sample Availability: Samples of fiber-type hemp extracts are available from the authors. |


| Compound | R2 | 1 LOD (µg/mL) | 2 LOQ (µg/mL) | 3 LOD (µg/mL) | 4 LOQ (µg/mL) | Intraday (Repeatability) RSD | Interday (Repeatability) RSD | Reproducibility RSD | Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| CBDA | 0.9999 | 0.34 | 1.05 | 0.11 | 0.37 | 5.65 | 5.05 | 0.09 | 96.06 |
| CBGA | 0.9999 | 0.32 | 0.98 | 0.12 | 0.40 | 4.71 | 4.34 | 2.13 | 93.90 |
| CBG | 0.9995 | 0.62 | 1.87 | 0.13 | 0.45 | 3.34 | 2.83 | 0.91 | 94.60 |
| CBD | 0.9995 | 0.63 | 1.91 | 0.17 | 0.58 | 4.89 | 4.44 | 0.70 | 84.92 |
| THCV | 0.9989 | 0.95 | 2.87 | 0.15 | 0.49 | - | - | N.d. * | N.d. * |
| CBN | 0.9999 | 0.28 | 0.84 | 0.06 | 0.21 | 2.59 | 2.95 | 0.81 | 97.08 |
| Δ9-THC | 0.9981 | 1.25 | 3.79 | 0.15 | 0.50 | 3.05 | 3.22 | 0.13 | 99.69 |
| Δ8-THC | 0.9987 | 1.02 | 3.10 | 0.17 | 0.56 | 3.81 | 3.64 | 0.74 | 100 |
| CBC | 0.9999 | 0.29 | 0.88 | 0.11 | 0.36 | 5.3 | 4.78 | 0.89 | 98.68 |
| THCA | 0.9998 | 0.43 | 1.29 | 0.11 | 0.37 | 5.55 | 5.01 | 1.91 | 95.27 |
| Cannabinoids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | CBDA (%) | CBGA (%) | CBG (%) | CBD (%) | THCV (%) | CBN (%) | Δ9-THC (%) | Δ8-THC (%) | CBC (%) | THCA (%) |
| L11 CV% | 0.88 ± 0.04 5.05 | 0.02 ± 0.00 4.34 | 0.02 ± 0.00 2.83 | 0.48 ± 0.02 4.44 | N.d. * | 0.01 ± 0.00 2.95 | 0.06 ± 0.00 3.22 | 0.03 ± 0.00 3.64 | 0.03 ± 0.00 4.78 | 0.03 ± 0.00 5.10 |
| L5 CV% | 0.93 ± 0.06 6.48 | 0.02 ± 0.00 1.28 | 0.02 ± 0.00 1.73 | 0.45 ± 0.03 6.28 | N.d. * | 0.01 ± 0.00 1.49 | 0.06 ± 0.00 0.21 | 0.03 ± 0.00 2.20 | 0.02 ± 0.00 2.98 | 0.04 ± 0.00 7.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrioli, M.; Tura, M.; Scotti, S.; Gallina Toschi, T. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Molecules 2019, 24, 2113. https://doi.org/10.3390/molecules24112113
Mandrioli M, Tura M, Scotti S, Gallina Toschi T. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Molecules. 2019; 24(11):2113. https://doi.org/10.3390/molecules24112113
Chicago/Turabian StyleMandrioli, Mara, Matilde Tura, Stefano Scotti, and Tullia Gallina Toschi. 2019. "Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L." Molecules 24, no. 11: 2113. https://doi.org/10.3390/molecules24112113