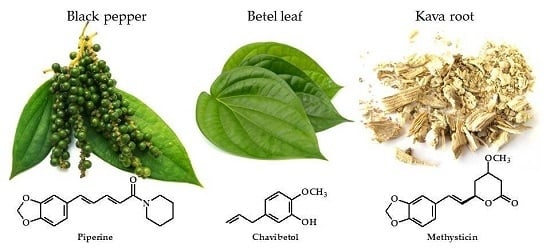
Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications
Abstract
:Table of Contents
|
1. Introduction
2. Habitat and Cultivation of Piper Plants
2.1. Habitat of Piper nigrum L.
2.2. Cultivation of Piper nigrum L.
3. Chemical Constituents of the Essential Oils of Piper Species
- (A)
- EOs dominated by monoterpene compounds
- Piper demeraranum: limonene, sabinene, β-pinene and α-pinene.
- Piper chimonanthifolium: piperitone
- Piper cubeba: sabinene and 1,8-cineole
- (B)
- EOs dominated by sesquiterpene compounds
- Piper majusculum leaf: β-caryophyllene, germacrene D and β-elemene
- Piper cernuum: β-elemene and epi-cubebol
- Piper madeiranum: β-caryophyllene and germacrene D-4-ol
- Piper duckei germacrene D and β-caryophyllene
- Piper nigrum: β-caryophyllene
- Piper lepturum var. lepturum: β-guaiene
- Piper lepturum var. angustifolium: β-bisabolene
- (C)
- EOs dominated by both monoterpene and sesquiterpene compounds.
- Piper hispidum: α-copaene and α-pinene
- Piper demeraranum: limonene and β-elemene
- Piper aduncum: camphor, viridiflorol and piperitone
- (D)
- EOs dominated by phenylpropanoid compounds
- Piper caninum, Piper auritum, Piper hispidinervum: safrole
- Piper aduncum: dillapiole
- Piper divaricatum: methyleugenol and eugenol
- Piper betle: chavibetol
- Piper patulum: 1,3,5-trimethoxy-2-propenylbenzene
- Piper klotzsdhianum: 2,4,5-trimethoxy-1-propenylbenzene
- Piper marginatum: (Z)-asarone
- (E)
- EOs dominated by benzenoid compounds
- Piper klotzsdhianum: 1-butyl-3,4-methylenedioxybenzene, 1-butyl-3,4-methylenedioxybenzene and 1-butyl-3,4-methyl- enedioxybenzene
- Piper sarmentosum: benzyl benzoate, benzyl alcohol, 2-hydroxy-benzoic acid phenylmethyl ester and 2-butenyl-benzene
- Piper harmandii: benzyl benzoate and benzyl salicylate
- (F)
- EOs dominated by non-terpenoid compounds
- Piper maclurei: methyl oleate
- Piper caldense: pentadecane
3.1. Piper aduncum L.
3.2. Piper amalago L.
3.3. Piper betle L.
3.4. Piper cubeba Bojer
3.5. Piper nigrum L.
3.6. Piper longum L.
3.7. Piper arboreum Aubl.
3.8. Piper auritum Kunth
3.9. Piper cernuum Vell.
3.10. Piper dilatatum Rich.
3.11. Piper gaudichaudianum Kunth
3.12. Piper hispidum Sw. (Including References to the Synonym Piper hispidinervum C.DC.)
3.13. Piper guineense Schumach. & Thonn
3.14. Piper marginatum Jacq.
- Chemotype I: safrole and propiopiperone
- Chemotype II: propiopiperone and p-menthα-1(7),8-diene
- Chemotype III: propiopiperone, myristicin, (E)-β-ocimene, γ-terpinene
- Chemotype IV: β-caryophyllene, α-copaene, and propiopiperone
- Chemotype V: (E)-isoosmorhizole, (E)-anethole, and isoosmorhizole
- Chemotype VI: 2-methoxy-4,5-(methylenedioxy)propiophenone, methoxy-4,5-(methylene- dioxy)propiophenone isomer 5, (E)-isoosmorhizole.
- Chemotype VII: β-caryophyllene, bicyclogermacrene, (E)-asarone.
3.15. Piper umbellatum L.
3.16. Piper tuberculatum Jacq.
- (1)
- EOs dominated by monoterpene compounds: P. aduncum, P. cubeba, P. dilatatum, P. nigrum, P. hispidum, P. guineense.
- (2)
- EOs dominated by sesquiterpene compounds: P. aduncum, P. amalgamo, P. cubeba, P. nigrum, P. arborescens, P. tuberculatum, P. umbellatum, P. cernuum, P. dilatatum, P. gaudichianum, P. hispidum, P. guineense.
- (3)
- EOs dominated by both monoterpene and sesquiterpene compounds: P. aduncum, P. cubeba, P. dilatatum, P. nigrum, P. hispidum, P. guineense.
- (4)
- EOs dominated by phenylpropanoid compounds: P. aduncum, P. betle, P. auroitum, P. gaudichiuanum, P. guineense, P. marginam.
3.17. Other Piper Species
4. Traditional Uses of Piper Species
4.1. Piper abbreviatum Opiz
4.2. Piper aduncum L.
4.3. Piper boehmeriifolium (Wall. ex Miq.) C.DC.
4.4. Piper sylvaticum Roxb.
4.5. Piper capense L.f.
4.6. Piper cubeba L.
4.7. Piper gibbilimbum C.DC.
4.8. Piper guineense Schum and Thonn
4.9. Piper longum L. (syn. P. latifolium Forst.; P. chaba Hunter)
4.10. Piper nigrum L.
4.11. Piper cavalcantei Yunck.
4.12. Piper marginatum Jacq.
4.13. Piper umbellatum L.
4.14. Piper aborescens Roxb.
4.15. Piper acutifolium Ruiz and Pav.
4.16. Piper alatabaccum Trel. & Yunck
4.17. Piper angustifolium Lam.
4.18. Piper auritum Kunth
4.19. Piper barbatum Kunth
4.20. Piper betle L.
4.21. Piper claussenianum (Miq.) C. DC.
4.22. Piper cumanense Kunth
4.23. Piper dennisii Trel.
4.24. Piper fimbriulatum C. DC.
4.25. Piper glabratum Kunth
4.26. Piper grande Vahl
4.27. Piper hayneanum C.DC.
4.28. Piper hispidum L.
4.29. Piper holtonii C.DC.
4.30. Piper jacquemontianum Kunth
4.31. Piper jericoense Trel. & Yunck
4.32. Piper lanceaefolium HBK.
4.33. Piper methysticum G.Forst
4.34. Piper multiplinervium C.DC.
4.35. Piper obrutum Trel. & Yunck.
4.36. Piper ovatum Vahl
4.37. Piper pulchrum C.DC.
4.38. Piper pyrifolium Vahl.
4.39. Piper regnellii (Miq.) C. DC.
4.40. Piper retrofractum Vahl
4.41. Piper sanvicentense Trel. & Yunck.
4.42. Piper sarmentosum Roxb.
4.43. Piper sintenense Hatus.
4.44. Piper strigosum Trel. & Yunck.
4.45. Piper stylosum Miq.
4.46. Piper tuberculatum Jacq.
4.47. Piper xanthostachyum C. DC
4.48. Piper carpunya Ruiz & Pav (syn: P. lenticellosum C.D.C.)
4.49. Piper obliquum Ruiz & Pavon
4.50. Piper laetispicum C. DC
4.51. Piper arboreum Aubl.
4.52. Piper amalago L.
4.53. Piper ribesioides Wall. (syn: P. sumatranum (Miq.) C. DC.)
4.54. Piper corcovadensis (Miq.) C. DC.
4.55. Piper futokadsura Siebold
4.56. Piper elongatum Vahl.
4.57. Piper mikanianum (Kunth) Steud
4.58. Piper medium Jacq.
4.59. Piper wallichii (Miq.) Hand.-Mazz.
4.60. Piper truncatum Vell.
4.61. Piper aequale Vahl
4.62. Piper alyreanum C.DC
4.63. Piper attenuatum Buch.-Ham. ex Miq.
4.64. Piper augustum Rudge
4.65. Piper darienense C.DC.
4.66. Piper reticulatum L.
4.67. Piper hongkongense C. DC
4.68. Piper kadsura (Choisy) Ohwi
4.69. Piper macropodum C. DC
4.70. Piper mutabile C. DC
4.71. Piper puberulilimbum C. DC
4.72. Piper yunnanense Tseng
4.73. Piper callosum Ruiz & Pav.
4.74. Piper conejoense Trel. & Yunck.
4.75. Piper novae-hollandiae Miq.
4.76. Piper mullesua Buch.-Ham. ex D. Don
4.77. Piper peltatum L.
4.78. Piper interruptum Opiz (syn: P. ribesoides Wall.)
4.79. Piper guianense (Klotzsch) C.DC.
4.80. Piper fragile Benth.
4.81. Piper coruscans Kunth.
4.82. Piper caninum Blume
4.83. Piper bantamese Blume
4.84. Piper sanctum (Miq.) Schltdl.
4.85. Piper sylvestre Lam.
4.86. Piper lanatum Roxb.
4.87. Piper porphyrophyllum N.E.Br.
4.88. Piper cernuum Vell.
4.89. Piper cordulatum C. DC.
4.90. Piper divaricatum Meyer
4.91. Piper flaviflorum C. DC.
4.92. Piper gaudichaudianum (Kunth) Kunth ex Steud
4.93. Piper hainanense Hemsl.
4.94. Piper klotzschianum Kunth.
4.95. Piper miniatum Blume
4.96. Piper aff. pedicellatum C. DC.
4.97. Piper philippinum Miq. (syn. P. kwashoense Hayata)
4.98. Piper piscatorum Trel. & Yunck.
4.99. Piper ossanum Trel.
4.100. Piper semiimmersum C. DC.
4.101. Piper submultinerve C. DC.
4.102. Piper loretoanum Trel.
4.103. Piper mediocre C.DC.
4.104. Piper sanguineispicum Trel.
4.105. Piper taiwanense Lin & Lu
4.106. Piper trichostachyon (Miq.) C. DC.
5. Food Preservative Effects of Piper Plants
5.1. Antioxidative Activity
5.2. Antimicrobial Activity
6. Antiparasitic Activities of Piper Species
7. Biological Activities Piper Plants
7.1. Antiproliferative/Anti-Cancer Properties
7.2. Anti-Inflammatory Properties
7.3. Neuropharmacological Activities
7.4. Clinical Studies
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Hernández-Álvarez, A.J.; Contreras, M.D.M.; Martorell, M.; Ramírez-Alarcón, K.; Melgar-Lalanne, G.; Matthews, K.R.; Sharifi-Rad, M.; Setzer, W.N.; Nadeem, M.; et al. Potential phytopharmacy and food applications of Capsicum spp.: A comprehensive review. Nat. Prod. Commun. 2018, 13, 1543–1556. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. (Noisy-le-grand) 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar]
- Abdolshahi, A.; Naybandi-Atashi, S.; Heydari-Majd, M.; Salehi, B.; Kobarfard, F.; Ayatollahi, S.A.; Ata, A.; Tabanelli, G.; Sharifi-Rad, M.; Montanari, C. Antibacterial activity of some Lamiaceae species against Staphylococcus aureus in yoghurt-based drink (Doogh). Cell. Mol. Biol. (Noisy-le-grand) 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef]
- Dyer, L.A.; Palmer, A. Piper A Model Genus for Studies of Phytochemistry, Ecology, and Evolution; Springer: Heidelberg, Germany, 2012; ISBN 978-0306484988. [Google Scholar]
- Raja Mazlan, R.N.A.; Rukayadi, Y.; Maulidiani, M.; Ismail, I.S. Solvent extraction and identification of active anticariogenic metabolites in Piper cubeba L. through 1H-NMR-based metabolomics approach. Molecules 2018, 23, 1730. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Ferreira, P.M.P.; Machado, C.M.L.; de Aquino, N.C.; Silveira, E.R.; Chammas, R.; Pessoa, C. Antitumour efficacy of Piper tuberculatum and piplartine based on the hollow fiber assay. Planta Med. 2015, 81, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Campelo, Y.; Ombredane, A.; Vasconcelos, A.G.; Albuquerque, L.; Moreira, D.C.; Plácido, A.; Rocha, J.; Fokoue, H.H.; Yamaguchi, L.; Mafud, A.; et al. Structure–Activity relationship of piplartine and synthetic analogues against Schistosoma mansoni and cytotoxicity to mammalian cells. Int. J. Mol. Sci. 2018, 19, 1802. [Google Scholar] [CrossRef] [PubMed]
- De Souza Oliveira, F.A.; Passarini, G.M.; de Medeiros, D.S.S.; de Azevedo Santos, A.P.; Fialho, S.N.; de Jesus Gouveia, A.; Latorre, M.; Freitag, E.M.; de Maria de Medeiros, P.S.; Teles, C.B.G.; et al. Antiplasmodial and antileishmanial activities of compounds from Piper tuberculatum Jacq fruits. Rev. Soc. Bras. Med. Trop. 2018, 51, 382–386. [Google Scholar] [CrossRef]
- Kim, N.; Do, J.; Bae, J.S.; Jin, H.K.; Kim, J.H.; Inn, K.S.; Oh, M.S.; Lee, J.K. Piper longumine inhibits neuroinflammation via regulating NF-kappaB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. J. Pharmacol. Sci. 2018, 137, 195–201. [Google Scholar] [CrossRef]
- Gamboa, F.; Muñoz, C.C.; Numpaque, G.; Sequeda-Castañeda, L.G.; Gutierrez, S.J.; Tellez, N. Antimicrobial activity of Piper marginatum Jacq and Ilex guayusa Loes on microorganisms associated with periodontal disease. Int. J. Microbiol. 2018, 2018, 4147383. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, N.; Kim, S.-H.; Park, M.; Woo, H.; Kim, H.; Yang, J.; Rhee, K.-J.; Kim, J. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agent Cancer 2014, 9, 43. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Park, M.; Lee, M.H.; Woo, H.J.; Kim, H.W.; Yang, J.Y.; Rhee, K.J.; Kim, J.B. Piperine treatment suppresses Helicobacter pylori toxin entry in to gastric epithelium and minimizes β-catenin mediated oncogenesis and IL-8 secretion in vitro. Am. J. Transl. Res. 2016, 8, 885–898. [Google Scholar]
- Durant-Archibold, A.A.; Santana, A.I.; Gupta, M.P. Ethnomedical uses and pharmacological activities of most prevalent species of genus Piper in Panama: A review. J. Ethnopharmacol. 2018, 217, 63–82. [Google Scholar] [CrossRef]
- Choudhary, N.; Singh, V. A census of P. longum’s phytochemicals and their network pharmacological evaluation for identifying novel drug-like molecules against various diseases, with a special focus on neurological disorders. PLoS ONE 2018, 13, e0191006. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L.(Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Acharya, S.G.; Momin, A.H.; Gajjar, A.V. Review of piperine as a bio-enhancer. Am. J. PharmTech Res. 2012, 2, 32–44. [Google Scholar]
- Damanhouri, Z.A.; Ahmad, A. A review on therapeutic potential of Piper nigrum L. (Black Pepper): The King of Spices. Med. Aromat. Plants 2014, 3, 161. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper (Piper nigrum) and its bioactive compound, piperine. In Molecular Targets and Therapeutic Uses of Spices: Modern Uses for Ancient Medicine; World Scientific: Singapore, 2009; pp. 25–64. [Google Scholar]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch. Carbohydr. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, A.; Wu, H.; Tan, L.; Long, Y.; Gou, Y.; Sun, S.; Sang, L. Influence of temperature, light and plant growth regulators on germination of black pepper (Piper nigrum L.) seeds. Afr. J. Biotechnol. 2010, 9. [Google Scholar] [CrossRef]
- Hao, C.; Rui, F.A.N.; Ribeiro, M.C.; Tan, L.; Wu, H.; Yang, J.; Zheng, W.; Huan, Y. Modeling the potential geographic distribution of black pepper (Piper nigrum) in Asia using GIS tools. J. Integr. Agric. 2012, 11, 593–599. [Google Scholar] [CrossRef]
- Sen, S.; Gode, A.; Ramanujam, S.; Ravikanth, G.; Aravind, N.A. Modeling the impact of climate change on wild Piper nigrum (Black Pepper) in Western Ghats, India using ecological niche models. J. Plant Res. 2016, 129, 1033–1040. [Google Scholar] [CrossRef]
- Mathew, P.J.; Jose, J.C.; Nair, G.M.; Mathew, P.M.; Kumar, V. Assessment and conservation of intraspecific variability in Piper nigrum (‘Black Pepper’) occurring in the Western Ghats of Indian Peninsula. In Proceedings of the III WOCMAP Congress on Medicinal and Aromatic Plants—Volume 2: Conservation, Cultivation and Sustainable Use of Medicinal and Aromatic Plants, ISHS Acta Horticulturae; 2003; Volume 676, pp. 119–126. Available online: https://www.actahort.org/books/676/676_14.htm (accessed on 1 February 2005).
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/ (accessed on 1 February 2019).
- Ravindran, P.N. Black Pepper: Piper nigrum; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0203303873. [Google Scholar]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Howard, R.A. Notes on the Piperaceae of the Lesser Antilles. J. Arnold Arbor. 1973, 54, 377–411. [Google Scholar] [CrossRef]
- Da Silva, J.K.; da Trindade, R.; Alves, N.S.; Figueiredo, P.L.; Maia, J.G.S.; Setzer, W.N. Essential oils from Neotropical Piper species and their biological activities. Int. J. Mol. Sci. 2017, 18, 2571. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Xiang, C.-P.; Han, J.-X.; Li, X.-C.; Li, Y.-H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.-Y.; Li, H.-Z.; Yang, C.-R. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from Piper species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef]
- Thin, D.B.; Chinh, H.V.; Luong, N.X.; Hoi, T.M.; Dai, D.N.; Ogunwande, I.A. Chemical analysis of essential oils of Piper laosanum and Piper acre (Piperaceae) from Vietnam. J. Essent. Oil Bear. Plants 2018, 21, 181–188. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar]
- De Almeida, R.R.P.; Souto, R.N.P.; Bastos, C.N.; da Silva, M.H.L.; Maia, J.G.S. Chemical variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Navickiene, H.M.; Morandim, A.D.; Alécio, A.C.; Regasini, L.O.; Bergamo, D.C.; Telascrea, M.; Cavalheiro, A.J.; Lopes, M.N.; Bolzani, V.D.; Furlan, M.; et al. Composition and antifungal activity of essential oils from Piper aduncum, Piper arboreum and Piper tuberculatum. Quím. Nov. 2006, 29, 467–470. [Google Scholar] [CrossRef]
- Vila, R.; Milo, B.; Tomi, F.; Casanova, J.; Ferro, E.A.; Cañigueral, S. Chemical composition of the essential oil from the leaves of Piper fulvescens, a plant traditionally used in Paraguay. J. Ethnopharmacol. 2001, 76, 105–107. [Google Scholar] [CrossRef]
- Rali, T.; Wossa, S.; Leach, D.; Waterman, P. Volatile chemical constituents of Piper aduncum L and Piper gibbilimbum C. DC (Piperaceae) from Papua New Guinea. Molecules 2007, 12, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Valardes, A.C.F.; Alves, C.C.F.; Alves, J.M.; De Deus, I.P.B.; De Oliveira Filho, J.G.; Dos Santos, T.C.L.; Dias, H.J.; Crotti, A.E.M.; Miranda, M.L.D. Essential oils from Piper aduncum inflorescences and leaves: Chemical composition and antifungal activity against Sclerotinia sclerotiorum. An. Acad. Bras. Ciênc. 2018, 90, 2691–2699. [Google Scholar]
- Radice, M.; Pietrantoni, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; Venturi, G.; Fortuna, C. Inhibitory effect of Ocotea quixos (Lam.) Kosterm. and Piper aduncum L. essential oils from Ecuador on West Nile virus infection. Plant Biosyst. 2018, 1–8. [Google Scholar] [CrossRef]
- Almeida, C.A.; Azevedo, M.M.B.; Chaves, F.C.M.; Roseo De Oliveira, M.; Rodrigues, I.A.; Bizzo, H.R.; Gama, P.E.; Alviano, D.S.; Alviano, C.S. Piper essential oils inhibit Rhizopus oryzae growth, biofilm formation, and rhizopuspepsin activity. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5295619. [Google Scholar] [CrossRef]
- Villamizar, L.H.; das Graças Cardoso, M.; de Andrade, J.; Teixeira, M.L.; Soares, M.J. Linalool, a Piper aduncum essential oil component, has selective activity against Trypanosoma cruzi trypomastigote forms at 4°C. Mem. Inst. Oswaldo Cruz 2017, 112, 131–139. [Google Scholar] [CrossRef]
- Corral, A.C.T.; de Queiroz, M.N.; de Andrade-Porto, S.M.; Morey, G.A.M.; Chaves, F.C.M.; Fernandes, V.L.A.; Ono, E.A.; Affonso, E.G. Control of Hysterothylacium sp. (Nematoda: Anisakidae) in juvenile pirarucu (Arapaima gigas) by the oral application of essential oil of Piper aduncum. Aquaculture 2018, 494, 37–44. [Google Scholar] [CrossRef]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.R.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; dos Santos, A.L.; da Fonseca Filho, H.D. Encapsulation of Piper aduncum and Piper hispidinervum essential oils in gelatin nanoparticles: A possible sustainable control tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.I.; Gupta, M.P. Potential of Panamanian aromatic flora as a source of novel essential oils. Biodivers. Int. J. 2018, 2, 405–413. [Google Scholar]
- Souto, R.N.P.; Harada, A.Y.; Andrade, E.H.A.; Maia, J.G.S. Insecticidal activity of Piper essential oils from the Amazon against the fire ant Solenopsis saevissima (Smith) (Hymenoptera: Formicidae). Neotrop. Entomol. 2012, 41, 510–517. [Google Scholar] [CrossRef]
- Dos Santos, A.L.; da Silva Novaes, A.; dos S. Polidoro, A.; de Barros, M.E.; Mota, J.S.; Lima, D.B.M.; Krause, L.C.; Cardoso, C.A.L.; Jacques, R.A.; Camkramão, E.B. Chemical characterisation of Piper amalago (Piperaceae) essential oil by comprehensive two-dimensional gas chromatography coupled with rapid-scanning quadrupole mass spectrometry (GC×GC/qMS) and their antilithiasic activity and acute toxicity. Phytochem. Anal. PCA 2018, 29, 432–445. [Google Scholar]
- Burfield, T. Natural Aromatic Materials: Odours & Origins, 2nd ed.; The Atlantic Institute of Aromatherapy: Tampa, FL, USA, 2017. [Google Scholar]
- Kumaratunge, K.G.A.; Arambewela, L.S.R.; Ekanayake, S.; Dias, K. Preliminary studies on Piper betle L. (Betel vine). In Proceedings of the 55th Annual Sessions, Sri Lanka Association for the Advancement of Science, Colombo, Sri Lanka, December 1999; Sri Lanka Association for the Advancement of Science: Colombo, Sri Lanka, 1999; p. 216. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Betel leaf oil. Perfum. Flavor. 2005, 30, 52–57. [Google Scholar]
- Basak, S.; Guha, P. Modelling the effect of essential oil of betel leaf (Piper betle L.) on germination, growth, and apparent lag time of Penicillium expansum on semi-synthetic media. Int. J. Food Microbiol. 2015, 215, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Han, B.H.; Park, J.H.; Cantoria, M.C. Studies on the constituents of Philippine Piper betle leaves. Arch. Pharm. Res. 1986, 9, 93–97. [Google Scholar] [CrossRef]
- Arambewela, L.; Kumaratunga, K.G.A.; Dias, K. Studies on Piper betle of Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2005, 33, 133–139. [Google Scholar] [CrossRef]
- Tawatsin, A.; Asavadachanukorn, P.; Thavara, U.; Wongsinkongman, P.; Bansidhi, J.; Boonruad, T.; Chavalittumrong, P.; Soonthornchareonnon, N.; Komalamisra, N.; Mulla, M.S. Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). Southeast Asian J. Trop. Med. Public Health 2006, 37, 915–931. [Google Scholar] [PubMed]
- Satyal, P.; Setzer, W.N. Chemical composition and biological activities of Nepalese Piper betle L. Int. J. Holist. Aromather. 2012, 1, 23–26. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Cubeb oil. Perfum. Flavor. 2016, 41, 54–57. [Google Scholar]
- Magalhães, L.G.; De Souza, J.M.; Wakabayashi, K.A.L.; Da S. Laurentiz, R.; Vinhólis, A.H.C.; Rezende, K.C.S.; Simaro, G.V.; Bastos, J.K.; Rodrigues, V.; Esperandim, V.R.; et al. In vitro efficacy of the essential oil of Piper cubeba L. (Piperaceae) against Schistosoma mansoni. Parasitol. Res. 2012, 110, 1747–1754. [Google Scholar] [CrossRef]
- Narayanan, C.S. Chemistry of Black Pepper. In Black Pepper—Piper nigrum; Ravindran, P.N., Ed.; Harwood: Amsterdam, The Netherlands, 2005; pp. 147–166. [Google Scholar]
- Liu, H.; Zheng, J.; Liu, P.; Zeng, F. Pulverizing processes affect the chemical quality and thermal property of black, white, and green pepper (Piper nigrum L.). J. Food Sci. Technol. 2018, 55, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Progress in essential oil. Perfum. Flavour. 1995, 20, 49–59. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Pepper oil. Perfum. Flavor. 2002, 27, 48–56. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Black and white pepper oil. Perfum. Flavor. 2010, 35, 48–57. [Google Scholar]
- Bagheri, H.; Abdul Manap, M.Y.B.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; De Heluani, C.S.; De Lampasona, M.P.; Catalan, C.A.N. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum). J. Agric. Food Chem. 2009, 57, 5358–5364. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Vila, R.; Tomi, F.; Cañigueral, S.; Casanova, J.; Proença Da Cunha, A.; Adzet, T. Essential oils from four Piper species. Phytochemistry 1998, 49, 2019–2023. [Google Scholar] [CrossRef]
- Nikolić, M.; Stojković, D.; Glamočlija, J.; Ćirić, A.; Marković, T.; Smiljković, M.; Soković, M. Could essential oils of green and black pepper be used as food preservatives? J. Food Sci. Technol. 2015, 52, 6565–6573. [Google Scholar] [CrossRef]
- Vinturelle, R.; Mattos, C.; Meloni, J.; Nogueira, J.; Nunes, M.J.; Vaz, I.S.; Rocha, L.; Lione, V.; Castro, H.C.; Das Chagas, E.F. In vitro evaluation of essential oils derived from Piper nigrum (Piperaceae) and Citrus limonum (Rutaceae) against the tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Biochem. Res. Int. 2017, 2017, 5342947. [Google Scholar] [CrossRef]
- Ao, P.; Hu, S.; Zhao, A. Essential oil analysis and trace element study of the roots of Piper nigrum L. Zhongguo Zhong Yao Za Zhi 1998, 23, 42–43, 63. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Long pepper oil. Perfum. Flavor. 2015, 40, 42–44. [Google Scholar]
- Dos Santos, P.R.D.; de Lima Moreira, D.; Guimarães, E.F.; Coelho Kaplan, M.A. Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic forest. Phytochemistry 2001, 58, 547–551. [Google Scholar] [CrossRef]
- Stashenko, E.; Martínez, J.R. The Expression of Biodiversity in the Secondary Metabolites of Aromatic Plants and Flowers Growing in Colombia; InTech: London, UK, 2018; pp. 59–86. [Google Scholar]
- Monzote, L.; García, M.; Montalvo, A.M.; Scull, R.; Miranda, M. Chemistry, cytotoxicity and antileishmanial activity of the essential oil from Piper auritum. Mem. Inst. Oswaldo Cruz 2010, 105, 168–173. [Google Scholar] [CrossRef]
- Schindler, B.; Heinzmann, B.M. Piper gaudichaudianum Kunth: Seasonal characterization of the essential oil chemical composition of leaves and reproductive organs. Braz. Arch. Biol. Technol. 2017, 60, e17160441. [Google Scholar] [CrossRef]
- Krinski, D.; Foerster, L.A.; Deschamps, C. Ovicidal effect of the essential oils from 18 Brazilian Piper species: Controlling Anticarsia gemmatalis (Lepidoptera, Erebidae) at the initial stage of development. Acta Sci. Agron 2018, 40, e35273. [Google Scholar] [CrossRef]
- Benitez, N.P.; Melendez Leon, E.M.; Stashenko, E.E. Essential oil composition from two species of Piperaceae family grown in Colombia. J. Chromatogr. Sci. 2009, 47, 804–807. [Google Scholar] [CrossRef]
- Delgado, W.A. Composicion quimica del acetite esencial de los frutos de Piper hispidum Kunt. Rev. Prod. Nat. 2007, 1, 5–8. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction–gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Tankam, J.M.; Ito, M. Inhalation of the essential oil of Piper guineense from Cameroon shows sedative and anxiolytic-like effects in mice. Biol. Pharm. Bull. 2013, 36, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, O.A.; Adeniyi, B.A.; Ajayi, O.; König, W.A. Essential oil composition of Piper guineense and its antimicrobial activity. Another chemotype from Nigeria. Phytother. Res. 2005, 19, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Oyemitan, I.A.; Olayera, O.A.; Alabi, A.; Abass, L.A.; Elusiyan, C.A.; Oyedeji, A.O.; Akanmu, M.A. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J. Ethnopharmacol. 2015, 166, 240–249. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepper. Adv. Pharmacol. Sci. 2013, 2013, 926047. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Carreira, L.M.M.; da Silva, M.H.L.; da Silva, J.D.; Bastos, C.N.; Sousa, P.J.C.; Guimarães, E.F.; Maia, J.G.S. Variability in essential oil composition of Piper marginatum sensu lato. Chem. Biodivers. 2008, 5, 197–208. [Google Scholar] [CrossRef]
- Autran, E.S.; Neves, I.A.; da Silva, C.S.B.; Santos, G.K.N.; Câmara, C.A.G.D.; Navarro, D.M.A.F. Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour. Technol. 2009, 100, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Sales, V.; Monteiro, Á.B.; de Araújo Delmondes, G.; do Nascimento, E.P.; de Figuêiredo, F.R.S.D.N.; de Souza Rodrigues, C.K.; de Lacerda, J.F.E.; Fernandes, C.N.; Barbosa, M.O.; Brasil, A.X.; et al. Antiparasitic activity and essential oil chemical analysis of the Piper tuberculatum Jacq fruit. Iran. J. Pharm. Res. 2018, 17, 268–275. [Google Scholar] [PubMed]
- Ordaz, G.; D’Armas, H.; Yanez, D.; Moreno, S. Chemical composition of essential oils from leaves of Helicteres guazumifolia (Sterculiaceae), Piper tuberculatum (Piperaceae), Scoparia dulcis (Arecaceae) and Solanum subinerme (Solanaceae) from Sucre, Venezuela. Rev. Biol. Trop. 2011, 59, 585–595. [Google Scholar]
- De Araujo, C.A.; Gomes da Camara, C.A.; de Moraes, M.M.; de Vasconcelos, G.J.N.; Silva Pereira, M.R.; Zartman, C.E. First record of the chemical composition of essential oil of Piper bellidifolium, Piper durilignum, Piper acutilimbum and Piper consanguineum from the Brazilian Amazon forest. Acta Amaz. 2018, 48, 330–337. [Google Scholar] [CrossRef]
- Velaz, J. Evaluaciδn de la Composiciδn Química y Actividad Biolδgica del Acetite Esencial Proveniente de las Hojas de Piper barbatum Kunth. (Cordoncillo). Thesis, Universidad Politécnica Salesiana, Quito, Ecuador, 2018. Available online: https://dspace.ups.edu.ec/handle/123456789/16382 (accessed on 31 December 2018).
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H.; Sirat, H.M. Chemical compositions, antioxidant and antimicrobial activities of essential oils of Piper caninum Blume. Int. J. Mol. Sci. 2011, 12, 7720–7731. [Google Scholar] [CrossRef]
- Do Carmo, D.F.M.; Amaral, A.C.F.; MacHado, G.M.C.; Leon, L.L.; De Andrade Silva, J.R. Chemical and biological analyses of the essential oils and main constituents of Piper species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef]
- Marques, A.M.; Barreto, A.L.S.; Batista, E.M.; Curvelo, J.A.D.R.; Velozo, L.S.M.; Moreira, D.D.L.; Guimarães, E.F.; Soares, R.M.A.; Kaplan, M.A.C. Chemistry and biological activity of essential oils from Piper claussenianum (Piperaceae). Nat. Prod. Commun. 2010, 5, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- D’Armas, H.; Montesinos, K.; Jaramillo, C.; León, R. Composición química de los aceites esenciales de las hojas de ocho plantas medicinales cultivadas en Ecuador. Rev. Cuba. Plantas Med. 2017, 22. Available online: http://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/428 (accessed on 5 April 2019).
- Leal, S.M.; Pino, N.; Stashenko, E.E.; Martínez, J.R.; Escobar, P. Antiprotozoal activity of essential oils derived from Piper spp. grown in Colombia. J. Essent. Oil Res. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Schultz Branquinho, L.; Alencar Santos, J.; Lima Cardoso, C.A.; da Silva Mota, J.; Lanza Junior, U.; Leite Kassuya, C.A.; Arena, A.C. Anti-inflammatory and toxicological evaluation of essential oil from Piper glabratum leaves. J. Ethnopharmacol. 2017, 198, 372–378. [Google Scholar] [CrossRef]
- Marques, A.M.; Peixoto, A.C.C.; Provance, D.W.; Kaplan, M.A.C.; Kaplan, M.A.C. Separation of volatile metabolites from the leaf-derived essential oil of Piper mollicomum Kunth (Piperaceae) by high-speed countercurrent chromatography. Molecules 2018, 23, 3064. [Google Scholar] [CrossRef]
- Bernuci, K.; Iwanaga, C.; Fernandez-Andrade, C.; Lorenzetti, F.; Torres-Santos, E.; Faiões, V.; Gonçalves, J.; do Amaral, W.; Deschamps, C.; Scodro, R.; et al. Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef] [PubMed]
- Hoff Brait, D.R.; Mattos Vaz, M.S.; da Silva Arrigo, J.; de Carvalho, L.N.B.; de Araújo, F.H.S.; Miron Vani, J.; da Silva Mota, J.; Lima Cardoso, C.A.; Oliveira, R.J.; Negrão, F.J. Toxicological analysis and anti-inflammatory effects of essential oil from Piper vicosanum leaves. Regul. Toxicol. Pharmacol. 2015, 73, 3699–3705. [Google Scholar] [CrossRef]
- Do Nascimento, L. Óleo Essencial de Piper aleyreanum C.DC. (Piperaceae) Reduz a Nocicepcao Inflamatoria e Neuropatica em Camunfongos: Papel dos Receptores TRPA1. Ph.D. Thesis, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, Brazil, 2018. [Google Scholar]
- Ciccio, J.F. Essential oil components in leaves and stems of Piper bisasperatum (Piperaceae). Rev. Biol. Trop. 1997, 44–45, 35–38. [Google Scholar]
- Clemes, S.M.; Santos, T.G.; Rebelo, R.A.; Laps, R.R.; Pescador, R. Seasonality and hydrodistillation time effects on the yield and chemical composition of leaves essential oil of Piper mikanianum (Kunth) Steudel. Eclet. Quim. 2015, 40, 117–125. [Google Scholar] [CrossRef]
- Ávila, M.C.; Cuca, L.E.; Cerón, J.A. Chemical composition and insecticidal properties of essential oils of Piper septuplinervium and P. subtomentosum (Piperaceae). Nat. Prod. Commun. 2014, 9, 1527–1530. [Google Scholar]
- Mundina, M.; Vila, R.; Tomi, F.; Gupta, M.P.; Adzet, T.; Casanova, J.; Cañigueral, S. Leaf essential oils of three panamanian Piper species. Phytochemistry 1998, 47, 1277–1282. [Google Scholar] [CrossRef]
- Woguem, V.; Maggi, F.; Fogang, H.; Tapondjoua, L.; Womeni, H.; Luana, Q.; Bramuccic, M.; Vitali, L.; Petrelli, D.; Lupidi, G. Antioxidant, antiproliferative and antimicrobial activities of the volatile oil from the wild pepper Piper capense used in Cameroon as a culinary spice. Nat. Prod. Commun. 2013, 8, 1791–1796. [Google Scholar] [CrossRef]
- Tirillini, B.; Velasquez, E.; Pellegrino, R. Chemical composition and antimicrobial activity of essential oil of Piper angustifolium. Planta Med. 1996, 62, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.; Rajudin, E.; Ahmad, F.; Sirat, H.M.; Arbain, D. Essential oil composition of Piper majusculum Ridl. from Indonesia. J. Mater. Environ. Sci. 2016, 7, 1921–1924. [Google Scholar]
- Salleh, W.M.N.H.W.; Ahmad, F.; Khong, H.Y. Chemical composition of Piper stylosum Miq. and Piper ribesioides Wall. essential oils, and their antioxidant, antimicrobial and tyrosinase inhibition activities. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 13, 488–497. [Google Scholar]
- Salleh, W.M.N.H.W.; Hashim, N.A.; Ahmad, F.; Yen, K.H. Anticholinesterase and antityrosinase activities of ten Piper species from Malaysia. Adv. Pharm. Bull. 2014, 4, 527–531. [Google Scholar]
- Uy, M.M.; Garcia, K.I. Evaluation of the antioxidant properties of the leaf extracts of Philippine medicinal plants Casuarina equisetifolia Linn, Cyperus brevifolius (Rottb) Hassk, Drymoglossum piloselloides Linn, Ixora chinensis Lam, and Piper abbreviatum Opiz. AAB Bioflux 2015, 7, 71–79. [Google Scholar]
- Valadeau, C.; Pabon, A.; Deharo, E.; Albán-Castillo, J.; Estevez, Y.; Lores, F.A.; Rojas, R.; Gamboa, D.; Sauvain, M.; Castillo, D.; et al. Medicinal plants from the Yanesha (Peru): Evaluation of the leishmanicidal and antimalarial activity of selected extracts. J. Ethnopharmacol. 2009, 123, 413–422. [Google Scholar] [PubMed]
- Martínez, J.; Rosa, P.T.; Ming, L.C.; Marques, M.O.; Angela, M.; Meireles, A. Extraction of volatile oil from Piper aduncum leaves with supercritical carbon dioxide. In Proceedings of the 6th International Symposium on Supercritical Fluids, ISASF, Nancy, France, 28–30 April 2003; pp. 65–70. [Google Scholar]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.; Ohlyan, R.; Kandale, A.; Walia, A.; Puri, S. Introduction, phytochemistry, traditional uses and biological activity of genus Piper: A review. Int. J. Curr. Pharm. Rev. Res. 2011, 2, 130–144. [Google Scholar]
- Facundo, V.A.; Pollli, A.R.; Rodrigues, R.V.; Militao, J.S.L.T.; Stabelli, R.G.; Cardoso, C.T. Fixed and volatile chemical constituents from stems and fruits of Piper tuberculatum Jacq. and from roots of P. hispidum H. B. K. Acta Amaz. 2008, 38, 743–748. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C.; Bates, R.B.; Nakkiew, P.; Jackes, B.R.; Chen, L.; McFerrin, M.B.; Meehan, E.J. Antibacterial hydroxycinnamic esters from Piper caninum from Paluma, North Queensland, Australia. The crystal and molecular structure of (+)-bornyl coumarate. Planta Med. 1999, 65, 747–749. [Google Scholar] [CrossRef]
- Torres-Pelayo, V.; del Socorro Fernández, M.; Hernández, O.; Molina-Torres, J.; Lozada-García, J. A Phytochemical and ethno-pharmacological review of the genus Piper: As a potent bio-insecticide. Res. J. Biol. 2014, 2, 104–114. [Google Scholar]
- Da Silva Arrigo, J.; Balen, E.; Júnior, U.L.; da Silva Mota, J.; Iwamoto, R.D.; Barison, A.; Sugizaki, M.M.; Leite Kassuya, C.A. Anti-nociceptive, anti-hyperalgesic and anti-arthritic activity of amides and extract obtained from Piper amalago in rodents. J. Ethnopharmacol. 2016, 179, 101–109. [Google Scholar] [CrossRef]
- Mahanta, P.K.; Ghanim, A.; Gopinath, K.W. Chemical constituents of Piper sylvaticum (Roxb) and Piper boehmerifolium (Wall). J. Pharm. Sci. 1974, 63, 1160–1161. [Google Scholar] [CrossRef]
- Ding, D.-D.; Wang, Y.-H.; Chen, Y.-H.; Mei, R.-Q.; Yang, J.; Luo, J.-F.; Li, Y.; Long, C.-L.; Kong, Y. Amides and neolignans from the aerial parts of Piper bonii. Phytochemistry 2016, 129, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Chen, D.M.; Qiu, B.Y.; Sheng, L.; Wang, Y.H.; Hu, G.W.; Zhao, F.W.; Ma, L.J.; Wang, H.; Huang, Q.Q.; et al. Cytotoxic amide alkaloids from Piper boehmeriaefolium. J. Nat. Prod. 2011, 74, 45–49. [Google Scholar] [CrossRef]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P.; Wiench, B.; Efferth, T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J. Ethnopharmacol. 2013, 149, 245–253. [Google Scholar] [CrossRef]
- Kaou, A.M.; Mahiou-Leddet, V.; Canlet, C.; Debrauwer, L.; Hutter, S.; Azas, N.; Ollivier, E. New amide alkaloid from the aerial part of Piper capense L.f. (Piperaceae). Fitoterapia 2010, 81, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Tekwu, E.M.; Askun, T.; Kuete, V.; Nkengfack, A.E.; Nyasse, B.; Etoa, F.X.; Beng, V.P. Antibacterial activity of selected Cameroonian dietary spices ethno-medically used against strains of Mycobacterium tuberculosis. J. Ethnopharmacol. 2012, 142, 374–382. [Google Scholar] [CrossRef]
- Fern, K.; Fern, A.; Morris, R. Useful Tropical Plants Database. Available online: http://tropical.theferns.info/ (accessed on 12 March 2019).
- Daoudi, A.; El Hamsas, A.; Youbi, E.; Bagrel, D.; Aarab, L. In vitro anticancer activity of some plants used in Moroccan traditional medicine. J. Med. Plants Res. 2013, 7, 1182–1189. [Google Scholar]
- Ahmad, G.; Ahmad, Q.; Jahan, N.; Tajuddin. Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi J. Kidney Dis. Transplant. 2012, 23, 773–781. [Google Scholar] [CrossRef]
- Holdsworth, D.; Kerenga, K. A survey of medicinal plants in the Simbu Province, Papua New Guinea. Pharm. Biol. 1987, 25, 183–187. [Google Scholar]
- Uhegbu, F.; Imo, C.; Ugbogu, A. Effect of aqueous extract of Piper guineense seeds on some liver enzymes, antioxidant enzymes and some hematological parameters in albino rats. Int. J. Plant Sci. Ecol. 2015, 1, 167–171. [Google Scholar]
- Besong, E.E.; Balogun, M.E.; Djobissie, S.F.A.; Mbamalu, O.S.; Obimma, J.N. A Review of Piper guineense (African Black Pepper). Int. J. Pharm. Pharm. Res. 2016, 6, 368–384. [Google Scholar]
- Soladoye, M.O.; Amusa, N.A.; Raji-Esan, S.O.; Chukwuma, E.C.; Taiwo, A.A. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann. Biol. Res. 2010, 1, 261–273. [Google Scholar]
- Nwosu, M.O.; Okafor, J.I. Preliminary studies of the antifungal activities of some medicinal plants against Basidiobolus and some other pathogenic fungi. Mycoses 1995, 38, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, D.K. Traditional medicinal plants of Rarotonga, Cook islands. Part II. Pharm. Biol. 1991, 29, 71–79. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Itharat, A.; Lerdvuthisopon, N.; Piyabhan, P.; Khonsung, P.; Boonraeng, S.; Jaijoy, K. Anti-inflammatory, analgesic, and antipyretic activities of the ethanol extract of Piper interruptum Opiz. and Piper chaba Linn. ISRN Pharmacol. 2012, 2012, 480265. [Google Scholar] [CrossRef]
- Naz, T.; Mosaddik, A.; Rahman, M.M.; Muhammad, I.; Haque, M.E.; Cho, S.K. Antimicrobial, antileishmanial and cytotoxic compounds from Piper chaba. Nat. Prod. Res. 2012, 26, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Taufiq-Ur-Rahman, M.; Shilpi, J.A.; Ahmed, M.; Hossain, C.F. Preliminary pharmacological studies on Piper chaba stem bark. J. Ethnopharmacol. 2005, 99, 203–209. [Google Scholar] [CrossRef]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobot. Res. Appl. 2006, 4, 223–231. [Google Scholar] [CrossRef]
- Majeed, M.; Prakasj, L. The medicinal uses of pepper. Int. Pepper News 2000, 25, 23–31. [Google Scholar]
- Xin, Y.M.; Qi, W.D.; Han, C.Y. Traditional Chinese Medicine for Treating Respiratory Cancer 2009. CN Patent 101455834 A, 17 June 2009. [Google Scholar]
- Chen, T.C. Observation of the medicine made by oneself in treating with 97 cases with gastric diseases. J. Pr. Med. Technol. 2008, 15, 593–594. [Google Scholar]
- Agbor, G.A.; Vinson, J.A.; Sortino, J.; Johnson, R. Antioxidant and anti-atherogenic activities of three Piper species on atherogenic diet fed hamsters. Exp. Toxicol. Pathol. 2012, 64, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.M.; Hama, J.R.; Alam, S.M. Synthesising a novel derivatives of piperine from black pepper (Piper nigrum L.). J. Food Meas. Charact. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Hiruma-Lima, C.A. Plantas Medicinais na Amazônia e na Mata Atlântica; Editora UNESP: São Paulo, Brazil, 2002; ISBN 85-7139-411-3. [Google Scholar]
- De Araújo, J.X.; De Oliveira, M.C.; Vasconcelos, L.E.; Gray, A.I. Cepharanone B from Piper tuberculatum. Biochem. Syst. Ecol. 1999, 27, 325–327. [Google Scholar] [CrossRef]
- Brú, J.; Guzman, J.D. Folk medicine, phytochemistry and pharmacological application of Piper marginatum. Braz. J. Pharmacogn. 2016, 26, 767–779. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; Ottesen, A.R. Duke’s Handbook of Medicinal Plants of Latin America; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cho-Ngwa, F.; Monya, E.; Azantsa, B.K.; Manfo, F.P.T.; Babiaka, S.B.; Mbah, J.A.; Samje, M. Filaricidal activities on Onchocerca ochengi and Loa loa, toxicity and phytochemical screening of extracts of Tragia benthami and Piper umbellatum. BMC Complement. Altern. Med. 2016, 16, 326. [Google Scholar] [CrossRef]
- Roersch, C.M.F.B. Piper umbellatum L.: A comparative cross-cultural analysis of its medicinal uses and an ethnopharmacological evaluation. J. Ethnopharmacol. 2010, 131, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Calderón, Á.I.; Romero, L.I.; Ortega-Barría, E.; Solís, P.N.; Zacchino, S.; Gimenez, A.; Pinzón, R.; Cáceres, A.; Tamayo, G.; Guerra, C.; et al. Screening of Latin American plants for antiparasitic activities against malaria, Chagas disease, and leishmaniasis. Pharm. Biol. 2010, 48, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.L.; Lee, F.P.; Wu, C.C.; Duh, C.Y.; Ishikawa, T.; Chen, J.J.; Chen, Y.C.; Seki, H.; Chen, I.S. New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens. Planta Med. 2005, 71, 535–542. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V. Ethnomedical field study in northern Peruvian Andes with particular reference to divination practices. J. Ethnopharmacol. 2003, 85, 243–256. [Google Scholar] [CrossRef]
- Svetaz, L.; Zuljan, F.; Derita, M.; Petenatti, E.; Tamayo, G.; Cáceres, A.; Cechinel Filho, V.; Giménez, A.; Pinzón, R.; Zacchino, S.A.; et al. Value of the ethnomedical information for the discovery of plants with antifungal properties. A survey among seven Latin American countries. J. Ethnopharmacol. 2010, 127, 137–158. [Google Scholar] [CrossRef]
- Facundo, V.A.; da Silveira, A.S.P.; Morais, S.M. Constituents of Piper alatabaccum Trel & Yuncker (Piperaceae). Biochem. Syst. Ecol. 2005, 33, 753–756. [Google Scholar]
- Bosquiroli, L.S.S.; Demarque, D.P.; Rizk, Y.S.; Cunha, M.C.; Marques, M.C.S.; De Matos, M.F.C.; Kadri, M.C.T.; Carollo, C.A.; Arruda, C.C.P. In vitro anti-Leishmania infantum activity of essential oil from Piper angustifolium. Braz. J. Pharmacogn. 2015, 25, 124–128. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.Á. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef]
- Lans, C.; Harper, T.; Georges, K.; Bridgewater, E. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement. Altern. Med. 2001, 1, 10. [Google Scholar] [CrossRef]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, G.; Nagarajan, S. Wound healing activity of Curcuma aromatica and Piper betle. Fitoterapia 1990, 61, 458–459. [Google Scholar]
- Prabhu, M.S.; Platel, K.; Saraswathi, G.; Srinivasan, K. Effect of orally administered betel leaf (Piper betle Linn.) on digestive enzymes of pancreas and intestinal mucosa and on bile production in rats. Indian J. Exp. Biol. 1995, 33, 752–756. [Google Scholar]
- Dasgupta, N.; De, B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004, 88, 219–224. [Google Scholar] [CrossRef]
- Venugopalan, A.; Sharma, A.; Venugopalan, V.; Gautam, H.K. Comparative study on the antioxidant activities of extracts from Piper betle leaves. Biomed. Pharmacol. J. 2008, 1, 115–120. [Google Scholar]
- Ahmad, F.B.; Ismail, G. Medicinal plants used by Kadazandusun communities around Crocker Range. ASEAN Rev. Biodivers. Environ. Conserv. 2003, 1, 1–10. [Google Scholar]
- Chakraborty, D.; Shah, B. Antimicrobial, antioxidative and antihemolytic of Piper betel leaf extracts. Int. J. Pharm. Pharm. Sci. 2011, 3, 192–199. [Google Scholar]
- Arawwala, L.; Arambewela, L.; Ratnasooriya, W. Gastro protective effect of Piper betel Linn. leaves grown in Srilanka. J. Ayurveda Integr. Med. 2014, 5, 38–42. [Google Scholar] [CrossRef]
- Shukla, R.; Sachan, S.; Mishra, A.; Kumar, S. A scientific review on commonly chewing plants of Asians: Piper betel Linn. J. Harmon. Res. Pharm. 2015, 4, 1–10. [Google Scholar]
- Dwivedi, V.; Tripathi, S. Review study on potential activity of Piper betle. J. Pharmacogn. Phytochem. 2014, 3, 93–98. [Google Scholar]
- Curvelo, J.A.R.; Marques, A.M.; Barreto, A.L.S.; Romanos, M.T.V.; Portela, M.B.; Kaplan, M.A.C.; Soares, R.M.A. A novel nerolidol-rich essential oil from Piper claussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014, 63, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Garavito, G.; Rincón, J.; Arteaga, L.; Hata, Y.; Bourdy, G.; Gimenez, A.; Pinzón, R.; Deharo, E. Antimalarial activity of some Colombian medicinal plants. J. Ethnopharmacol. 2006, 107, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Solís, P.N.; Olmedo, D.; Nakamura, N.; Calderón, Á.I.; Hattori, M.; Gupta, M.P. A new larvicidal lignan from Piper fimbriulatum. Pharm. Biol. 2005, 43, 378–381. [Google Scholar] [CrossRef]
- Calderón, Á.I.; Romero, L.I.; Ortega-Barría, E.; Brun, R.; Correa A, M.D.; Gupta, M.P. Evaluation of larvicidal and in vitro antiparasitic activities of plants in a biodiversity plot in the Altos de Campana National Park, Panama. Pharm. Biol. 2006, 44, 487–498. [Google Scholar] [CrossRef]
- Bastos, M.L.A.; Houly, R.L.S.; Conserva, L.M.; Andrade, V.S.; Rocha, E.M.M.; Lyra Lemos, R.P. Antimicrobial and wound healing activities of Piper hayneanum. J. Chem. Pharm. Res. 2011, 3, 213–222. [Google Scholar]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Calderón, Á.I.; Vázquez, Y.; Solís, P.N.; Caballero-George, C.; Zacchino, S.; Gimenez, A.; Pinzón, R.; Cáceres, A.; Tamayo, G.; Correa, M.; et al. Screening of Latin American plants for cytotoxic activity. Pharm. Biol. 2006, 44, 130–140. [Google Scholar] [CrossRef]
- Michel, J.L.; Chen, Y.; Zhang, H.; Huang, Y.; Krunic, A.; Orjala, J.; Veliz, M.; Soni, K.K.; Soejarto, D.D.; Caceres, A.; et al. Estrogenic and serotonergic butenolides from the leaves of Piper hispidum Swingle (Piperaceae). J. Ethnopharmacol. 2010, 29, 220–226. [Google Scholar] [CrossRef]
- Santana, A.I.; Vila, R.; Cañigueral, S.; Gupta, M.P. Chemical composition and biological activity of essential oils from different species of Piper from Panama. Planta Med. 2016, 82, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.M.; Cáceres, A.; Álvarez, L.; Morales, J.; Apel, M.A.; Henriques, A.T.; Salamanca, E.; Giménez, A.; Vásquez, Y.; Gupta, M.P. Chemical composition of essential oils of Piper jacquemontianum and Piper variabile from Guatemala and bioactivity of the dichloromethane and methanol extracts. Braz. J. Pharmacogn. 2011, 21, 587–593. [Google Scholar] [CrossRef]
- Mesa, A.M.; Toro, J.F.; Cardona, F.; Blair, S. Antiplasmodial and cytotoxic activity of ethanol extracts of species of the genus Piper. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 154–162. [Google Scholar]
- López, A.; Dong, S.M.; Towers, G.H.N. Antifungal activity of benzoic acid derivatives from Piper lanceaefolium. J. Nat. Prod. 2002, 65, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Brown-Joel, Z.O.; Colleran, E.S.; Stone, M.S. Inflammatory sebotropic reaction associated with kava kava ingestion. JAAD Case Rep. 2018, 4, 437–439. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some Drugs and Herbal Products; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2016; Volume 108, pp. 7–419. [Google Scholar]
- Silva, D.R.; Endo, E.H.; Filho, B.P.D.; Nakamura, C.V.; Svidzinski, T.I.E.; De Souza, A.; Young, M.C.M.; Tânia, U.N.; Cortez, D.A.G. Chemical composition and antimicrobial properties of Piper ovatum Vahl. Molecules 2009, 14, 1171–1182. [Google Scholar] [CrossRef]
- Otero, R.; Núñez, V.; Barona, J.; Fonnegra, R.; Jiménez, S.L.; Osorio, R.G.; Saldarriaga, M.; Díaz, A. Snakebites and ethnobotany in the northwest region of Colombia—Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol. 2000, 73, 233–241. [Google Scholar] [CrossRef]
- Fortin, H.; Vigor, C.; Lohézic-Le Dévéhat, F.; Robin, V.; Le Bossé, B.; Boustie, J.; Amoros, M. In vitro antiviral activity of thirty-six plants from La Réunion Island. Fitoterapia 2002, 73, 346–350. [Google Scholar] [CrossRef]
- Felipe, D.F.; Filho, B.P.D.; Nakamura, C.V.; Franco, S.L.; Cortez, D.A.G. Analysis of neolignans compounds of Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck by HPLC. J. Pharm. Biomed. Anal. 2006, 41, 1371–1375. [Google Scholar] [CrossRef]
- Koroishi, A.M.; Foss, S.R.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. In vitro antifungal activity of extracts and neolignans from Piper regnellii against dermatophytes. J. Ethnopharmacol. 2008, 117, 270–277. [Google Scholar] [CrossRef]
- Muharini, R.; Liu, Z.; Lin, W.; Proksch, P. New amides from the fruits of Piper retrofractum. Tetrahedron Lett. 2015, 56, 2521–2525. [Google Scholar] [CrossRef]
- Taylor, P.; Arsenak, M.; Abad, M.J.; Fernández, Á.; Milano, B.; Gonto, R.; Ruiz, M.C.; Fraile, S.; Taylor, S.; Estrada, O.; et al. Screening of Venezuelan medicinal plant extracts for cytostatic and cytotoxic activity against tumor cell lines. Phyther. Res. 2013, 27, 530–539. [Google Scholar] [CrossRef]
- Rukachaisirikul, T.; Siriwattanakit, P.; Sukcharoenphol, K.; Wongvein, C.; Ruttanaweang, P.; Wongwattanavuch, P.; Suksamrarn, A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004, 93, 173–176. [Google Scholar] [CrossRef]
- Tuntiwachwuttikul, P.; Phansa, P.; Pootaeng-on, Y.; Taylor, W.C. Chemical constituents of the roots of Piper sarmentosum. Chem. Pharm. Bull. (Tokyo) 2006, 54, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Duh, C.Y.; Huang, H.Y.; Chen, I.S. Cytotoxic constituents of Piper sintenense. Helv. Chim. Acta 2003, 86, 2058–2064. [Google Scholar] [CrossRef]
- Estevez, Y.; Castillo, D.; Pisango, M.T.; Arevalo, J.; Rojas, R.; Alban, J.; Deharo, E.; Bourdy, G.; Sauvain, M. Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. J. Ethnopharmacol. 2007, 114, 254–259. [Google Scholar] [CrossRef]
- Cícero Bezerra Felipe, F.; Trajano Sousa Filho, J.; de Oliveira Souza, L.E.; Alexandre Silveira, J.; Esdras de Andrade Uchoa, D.; Rocha Silveira, E.; Deusdênia Loiola Pessoa, O.; de Barros Viana, G.S. Piplartine, an amide alkaloid from Piper tuberculatum, presents anxiolytic and antidepressant effects in mice. Phytomedicine 2007, 14, 605–612. [Google Scholar] [CrossRef]
- Burci, L.M.; Pereira, I.T.; da Silva, L.M.; Rodrigues, R.V.; Facundo, V.A.; Militão, J.S.L.T.; Santos, A.R.S.; Marques, M.C.A.; Baggio, C.H.; Werner, M.F.D.P. Antiulcer and gastric antisecretory effects of dichloromethane fraction and piplartine obtained from fruits of Piper tuberculatum Jacq. in rats. J. Ethnopharmacol. 2013, 148, 165–174. [Google Scholar] [CrossRef]
- Restrepo, J.; Colmenares, A.J.; Mora, L.E.; Sánchez, R.A. Extraction, chemical composition and antimicrobial activity of the essential oils of pipilongo (Piper tuberculatum) using supercritical carbon dioxide. Rev. Cienc. 2013, 17, 45–56. [Google Scholar]
- Quílez, A.; Berenguer, B.; Gilardoni, G.; Souccar, C.; de Mendonça, S.; Oliveira, L.F.S.; Martín-Calero, M.J.; Vidari, G. Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010, 128, 583–589. [Google Scholar]
- De Las Heras, B.; Slowing, K.; Benedí, J.; Carretero, E.; Ortega, T.; Toledo, C.; Bermejo, P.; Iglesias, I.; Abad, M.J.; Gómez-Serranillos, P.; et al. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J. Ethnopharmacol. 1998, 61, 161–166. [Google Scholar] [CrossRef]
- Agra, M.F.; De Freitas, P.F.; Barbosa-Filho, J.M. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Braz. J. Pharmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef]
- Agra, M.F.; Silva, K.N.; Basílio, I.J.L.D.; De Freitas, P.F.; Barbosa-Filho, J.M. Survey of medicinal plants used in the region Northeast of Brazil. Braz. J. Pharmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Tintino, S.R.; Souza, C.E.S.; Guedes, G.M.M.; Costa, J.I.V.; Duarte, F.M.; Chaves, M.C.O.; Silva, V.A.; Pessôa, H.L.F.; Lima, M.A.; Garcia, C.A.; et al. Modulatory antimicrobial activity of Piper arboreum extracts. Acta Bot. Croat. 2014, 73, 281–289. [Google Scholar] [CrossRef]
- Carrara, V.S.; Filho, L.C.; Garcia, V.A.S.; Faiões, V.S.; Cunha-Júnior, E.F.; Torres-Santos, E.C.; Cortez, D.A.G. Supercritical fluid extraction of pyrrolidine alkaloid from leaves of Piper amalago L. Evid.-Based Complement. Altern. Med. 2017, 2017, 7401748. [Google Scholar] [CrossRef]
- Ma, J.; Jones, S.H.; Marshall, R.; Johnson, R.K.; Hecht, S.M. A DNA-damaging oxoaporphine alkaloid from Piper caninum. J. Nat. Prod. 2004, 67, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Sudmoon, R. Ethnobotany and species specific molecular markers of some medicinal sakhan (Piper, Piperaceae). J. Med. Plants Res. 2012, 6, 1168–1175. [Google Scholar]
- Facundo, V.A.; Morais, S.M.; Braz, R. Chemical constituents of Ottionia corcovadensis Miq. from Amazon Forest—H-1 and C-13 chemical shift assignments. Quim. Nova 2004, 27, 79–83. [Google Scholar]
- Shi, Y.N.; Shi, Y.M.; Yang, L.; Li, X.C.; Zhao, J.H.; Qu, Y.; Zhu, H.T.; Wang, D.; Cheng, R.R.; Yang, C.R.; et al. Lignans and aromatic glycosides from Piper wallichii and their antithrombotic activities. J. Ethnopharmacol. 2015, 162, 87–96. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Bora, J.; Gajurel, P.R. Antioxidant activities and phenolic content of Piper wallichii (Miq.) Hand.-Mazz. Int. J. Food Prop. 2014, 17, 309–320. [Google Scholar] [CrossRef]
- Huyan, T.; Tang, R.; Li, J.; Liu, Y.; Yang, H.; Li, Q. Chemical constituents of Piper wallichii (Miq.) Hand.-Mazz. and inhibitory effects on Tca83 cells. Biomed. Res. 2016, 27, 220–224. [Google Scholar]
- Raimundo, J.M.; Trindade, A.P.F.; Velozo, L.S.M.; Kaplan, M.A.C.; Sudo, R.T.; Zapata-Sudo, G. The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur. J. Pharmacol. 2009, 606, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Andrade, E.H.A.; Maia, J.G.S. Composition and cytotoxic and antioxidant activities of the oil of Piper aequale Vahl. Lipids Health Dis. 2016, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.K.S.; Ballico, L.J.; Rocha Lapa, F.; Gonçalves, H.P.; de Souza, L.M.; Iacomini, M.; Werner, M.F.D.P.; Baggio, C.H.; Pereira, I.T.; da Silva, L.M.; et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J. Ethnopharmacol. 2012, 142, 274–282. [Google Scholar] [CrossRef]
- Reddy, S.; Siva, B.; Poornima, B.; Kumar, D.; Tiwari, A.; Ramesh, U.; Babu, K. New free radical scavenging neolignans from fruits of Piper attenuatum. Pharmacogn. Mag. 2015, 11, 235–241. [Google Scholar] [PubMed]
- Kim, Y.J.; Deok, J.; Kim, S.; Yoon, D.H.; Sung, G.H.; Aravinthan, A.; Lee, S.; Lee, M.N.; Hong, S.; Kim, J.H.; et al. Anti-inflammatory effect of Piper attenuatum methanol extract in LPS-stimulated inflammatory responses. Evid.-Based Complement. Altern. Med. 2017, 2017, 4606459. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-Y.; Yang, J.; Zhang, P.-H.; Li, X.-N.; Luo, J.-F.; Long, C.-L.; Wang, Y.-H. Amides, isoquinoline alkaloids and dipeptides from the aerial parts of Piper mullesua. Nat. Prod. Bioprospect. 2018, 8, 419–430. [Google Scholar] [CrossRef]
- Manandhar, N.P. Plants and People of Nepal; Timber Press: Portland, OR, USA, 2002; ISBN 00368075. [Google Scholar]
- Rocha e Silva, L.F.; da Silva Pinto, A.C.; Pohlit, A.M.; Quignard, E.L.J.; Vieira, P.P.R.; Tadei, W.P.; Chaves, F.C.M.; Samonek, J.F.; Lima, C.A.J.; Costa, M.R.F.; et al. In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phyther. Res. 2011, 25, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; Maia, F.C.; Alexandre, P.; Verônica, C.; Pedro, W.; Martin, A. Piper peltatum: Biomass and 4-nerolidylcatechol production. Planta Med. 2010, 76, 1473–1476. [Google Scholar]
- Ahmad, F.; Bakar, S.; Ibrahim, Z.; Read, R. Constituents of the leaves of Piper caninum. Planta Med. 1997, 63, 193–194. [Google Scholar]
- Gurib-Fakim, A. Constituents of the essential oils from Piper sylvestre growing in Mauritius. Planta Med. 1994, 60, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Wolff, F.R.; Broering, M.F.; Jurcevic, J.D.; Zermiani, T.; Bramorski, A.; de Carvalho Vitorino, J.; Malheiros, A.; Santin, J.R. Safety assessment of Piper cernuum Vell. (Piperaceae) leaves extract: Acute, sub-acute toxicity and genotoxicity studies. J. Ethnopharmacol. 2019, 230, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Avella, E.; Díaz, P.; de Díaz, A. Constituents from Piper divaricatum. Planta Med. 1994, 60, 195. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.-J.; Wang, Y.-F.; Sun, Q.; Hu, H.-B. Chemical composition, antioxidant, antimicrobial and anti-inflammatory activities of the stem and leaf essential oils from Piper flaviflorum from Xishuangbanna, SW China. Nat. Prod. Commun. 2014, 9, 1011–1014. [Google Scholar] [CrossRef]
- Shi, Y.N.; Liu, F.F.; Jacob, M.R.; Li, X.C.; Zhu, H.T.; Wang, D.; Cheng, R.R.; Yang, C.R.; Xu, M.; Zhang, Y.J. Antifungal amide alkaloids from the aerial parts of Piper flaviflorum and Piper sarmentosum. Planta Med. 2017, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Péres, V.F.; Moura, D.J.; Sperotto, A.R.M.; Damasceno, F.C.; Caramão, E.B.; Zini, C.A.; Saffi, J. Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Piper gaudichaudianum Kunth leaves. Food Chem. Toxicol. 2009, 47, 2389–2395. [Google Scholar] [CrossRef]
- Sperotto, A.R.M.; Moura, D.J.; Péres, V.F.; Damasceno, F.C.; Caramão, E.B.; Henriques, J.A.P.; Saffi, J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Ling, P.; Hao, C.Y.; Li, F.P.; Huang, L.F.; Wu, B.D.; Wu, H.S. Construction of a cDNA library and preliminary analysis of expressed sequence tags in Piper hainanense. Genet. Mol. Res. 2015, 14, 12733–12745. [Google Scholar] [CrossRef]
- Do Nascimento, J.C.; David, J.M.; Barbosa, L.C.; De Paula, V.F.; Demuner, A.J.; David, J.P.; Conserva, L.M.; Ferreira, J.C.; Guimarães, E.F. Larvicidal activities and chemical composition of essential oils from Piper klotzschianum (Kunth) C. DC. (Piperaceae). Pest Manag. Sci. 2013, 69, 1267–1271. [Google Scholar]
- Salleh, W.M.N.H.; Kammil, M.F.; Ahmad, F.; Sirat, H.M. Antioxidant and anti-inflammatory activities of essential oil and extracts of Piper miniatum. Nat. Prod. Commun. 2015, 10, 2005–2008. [Google Scholar]
- Rukachaisirikul, T.; Prabpai, S.; Kongsaeree, P.; Suksamrarn, A. (+)-Bornyl piperate, a new monoterpene ester from Piper aff. pedicellatum roots. Chem. Pharm. Bull. (Tokyo) 2004, 52, 760–761. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Borah, S.C.; Das, M.R.; Boruah, M.P. In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: Green chemistry approach. Colloids Surf. B Biointerfaces 2013, 102, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liao, C.H.; Chen, I.S. Lignans, an amide and anti-platelet activities from Piper philippinum. Phytochemistry 2007, 68, 2101–2111. [Google Scholar] [CrossRef]
- McFerren, M.A.; Cordova, D.; Rodriguez, E.; Rauh, J.J. In vitro neuropharmacological evaluation of piperovatine, an isobutylamide from Piper piscatorum (Piperaceae). J. Ethnopharmacol. 2002, 83, 201–207. [Google Scholar] [CrossRef]
- McFerren, M.A.; Rodriguez, E. Piscicidal properties of piperovatine from Piper piscatorum (Piperaceae). J. Ethnopharmacol. 1998, 60, 183–187. [Google Scholar] [CrossRef]
- Larionova, M.; Spengler, I.; Nogueiras, C.; Quijano, L.; Ramírez-Gualito, K.; Cortés-Guzmán, F.; Cuevas, G.; Calderón, J.S. A C-glycosylflavone from Piper ossanum, a compound conformationally controlled by CH/π and other weak intramolecular interactions. J. Nat. Prod. 2010, 73, 1623–1627. [Google Scholar] [CrossRef]
- Zhang, D.D.; Yang, J.; Luo, J.F.; Li, X.N.; Long, C.L.; Wang, Y.H. New aporphine alkaloids from the aerial parts of Piper semiimmersum. J. Asian Nat. Prod. Res. 2017, 20, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sanubol, A.; Chaveerach, A.; Tanee, T.; Sudmoon, R. Pre-clinical evaluation of extracts and essential oils from betel-like scent Piper species identifies potential cancer treatments. Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 89–102. [Google Scholar] [CrossRef]
- Odonne, G.; Bourdy, G.; Castillo, D.; Estevez, Y.; Lancha-Tangoa, A.; Alban-Castillo, J.; Deharo, E.; Rojas, R.; Stien, D.; Sauvain, M. Ta’ta’, Huayani: Perception of leishmaniasis and evaluation of medicinal plants used by the Chayahuita in Peru. Part II. J. Ethnopharmacol. 2009, 126, 149–158. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, J.J.; Chang, Y.L.; Teng, C.M.; Lin, W.Y.; Wu, C.C.; Chen, I.S. A new aristolactam alkaloid and anti-platelet aggregation constituents from Piper taiwanense. Planta Med. 2004, 70, 174–177. [Google Scholar] [PubMed]
- Upadhya, V.; Pai, S.R.; Ankad, G.M.; Hegde, H.V. Pharmacognostic screening of Piper trichostachyon fruits and its comparative analysis with Piper nigrum using chromatographic techniques. Pharmacogn. Mag. 2016, 12, S152–S158. [Google Scholar]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phyther. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol. 2018, 64, 57–64. [Google Scholar] [CrossRef]
- Himabindu, D.; Arunkumar, H. Effect of black pepper (Piper nigrum L.) on the keeping quality of spiced cottage cheese. Res. Rev. J. Food Dairy Technol. 2017, 5, 30–36. [Google Scholar]
- Nakatani, N.; Inatani, R.; Ohta, H.; Nishioka, A. Chemical constituents of peppers (Piper spp.) and application to food preservation: Naturally occurring antioxidative compounds. Environ. Health Perspect. 1986, 67, 135–142. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Kim, S.-H.; Kim, M.J.; Yang, H.J.; Rhyu, M.-R.; Park, J.-H. Piperine, a component of black pepper, decreases eugenol-induced cAMP and calcium levels in non-chemosensory 3T3-L1 cells. FEBS Open Bio 2015, 5, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Bhosale, R.; Singhal, R. Microencapsulation of black pepper oleoresin. Food Chem. 2006, 94, 105–110. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Ozdemir, N.; Hill, L.E.; Gomes, C.L. Synthesis and characterization of nano-encapsulated black pepper oleoresin using hydroxypropyl beta-cyclodextrin for antioxidant and antimicrobial applications. J. Food Sci. 2013, 78, N1913–N1920. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Karsha, P.V.; Lakshmi, O.B. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J. Nat. Prod. Resour. 2010, 1, 213–215. [Google Scholar]
- Ravindran, P.N.; Kallupurackal, J.A. Black pepper. In Handbook of Herbs and Spices, 2nd ed.; Woodhead: Cambridge, UK, 2012; Volume 1, pp. 86–115. [Google Scholar]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Akthar, M.S.; Birhanu, G.; Demisse, S. Antimicrobial activity of Piper nigrum L. and Cassia didymobotyra L. leaf extract on selected food borne pathogens. Asian Pac. J. Trop. Dis. 2014, 4, S911–S919. [Google Scholar] [CrossRef]
- Roy, A.; Guha, P. Formulation and characterization of betel leaf (Piper betle L.) essential oil based nanoemulsion and its in vitro antibacterial efficacy against selected food pathogens. J. Food Process. Preserv. 2018, 42, e13617. [Google Scholar] [CrossRef]
- Nouri, L.; Nafchi, A.M. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef]
- Pauli, A. Antimicrobial properties of essential oil constituents. Int. J. Aromather. 2001, 11, 126–133. [Google Scholar] [CrossRef]
- Basak, S.; Guha, P. Use of predictive model to describe sporicidal and cell viability efficacy of betel leaf (Piper betle L.) essential oil on Aspergillus flavus and Penicillium expansum and its antifungal activity in raw apple juice. LWT-Food Sci. Technol. 2017, 80, 510–516. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.-P.; Zhang, X.; Pan, D.-D.; Sun, Y.-Y.; Cao, J.-X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Hu, Y.-Y.; Chen, W.-X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef]
- Ahmad, N.; Abbasi, B.H.; Fazal, H. Effect of different in vitro culture extracts of black pepper (Piper nigrum L.) on toxic metabolites-producing strains. Toxicol. Ind. Health 2016, 32, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, W.; Dou, Z.-M.; Chen, R.; Hu, Y.; Chen, W.; Chen, H. Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. Technol. 2017, 54, 2067–2076. [Google Scholar] [CrossRef]
- Mbaya, A.W.; Ogwiji, M. In-vivo and in-vitro activities of medicinal plants on ecto, endo and haemoparasitic infections: A review. Curr. Clin. Pharmacol. 2014, 9, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Waako, P.J.; Gumede, B.; Smith, P.; Folb, P.I. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J. Ethnopharmacol. 2005, 99, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kaou, A.M.; Mahiou-Leddet, V.; Hutter, S.; Aïnouddine, S.; Hassani, S.; Yahaya, I.; Azas, N.; Ollivier, E. Antimalarial activity of crude extracts from nine African medicinal plants. J. Ethnopharmacol. 2008, 116, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sawangjaroen, N.; Subhadhirasakul, S.; Phongpaichit, S.; Siripanth, C.; Jamjaroen, K.; Sawangjaroen, K. The in vitro anti-giardial activity of extracts from plants that are used for self-medication by AIDS patients in southern Thailand. Parasitol. Res. 2005, 95, 17–21. [Google Scholar] [CrossRef]
- Leesombun, A.; Boonmasawai, S.; Nishikawa, Y. Effects of Thai Piperaceae plant extracts on Neospora caninum infection. Parasitol. Int. 2017, 66, 219–226. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.C.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Rahuman, A.A.; Mohanakrishnan, D.; Bagavan, A.; Elango, G.; Zahir, A.A.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; et al. Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J. Ethnopharmacol. 2012, 141, 796–802. [Google Scholar] [CrossRef]
- Varela, M.T.; Dias, R.Z.; Martins, L.F.; Ferreira, D.D.; Tempone, A.G.; Ueno, A.K.; Lago, J.H.G.; Fernandes, J.P.S. Gibbilimbol analogues as antiparasitic agents—Synthesis and biological activity against Trypanosoma cruzi and Leishmania (L.) infantum. Bioorg. Med. Chem. Lett. 2016, 26, 1180–1183. [Google Scholar] [CrossRef]
- Houël, E.; Gonzalez, G.; Bessière, J.M.; Odonne, G.; Eparvier, V.; Deharo, E.; Stien, D. Therapeutic switching: From antidermatophytic essential oils to new leishmanicidal products. Mem. Inst. Oswaldo Cruz 2015, 110, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ceole, L.F.; Cardoso, M.D.G.; Soares, M.J. Nerolidol, the main constituent of Piper aduncum essential oil, has anti-Leishmania braziliensis activity. Parasitology 2017, 144, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Montes, R.; Scull, R.; Sánchez, A.; Cos, P.; Monzote, L.; Setzer, W.N. Chemodiversity associated with cytotoxicity and antimicrobial activity of Piper aduncum var. ossanum. Chem. Biodivers. 2016, 13, 1715–1719. [Google Scholar] [CrossRef]
- Da Silva Carrara, V.; Serra, L.Z.; Cardozo-Filho, L.; Cunha-Júnior, E.F.; Torres-Santos, E.C.; Cortez, D.A.G. HPLC analysis of supercritical carbon dioxide and compressed propane extracts from Piper amalago L. with antileishmanial activity. Molecules 2011, 17, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla Carmona, M.; Valerio Campos, I.; Sánchez Porras, R.; Bagnarello Madrigal, V.; Martínez Esquivel, L.; González Paniagua, A.; Alpizar Cordero, J.; Cordero Villalobos, M.; Rodríguez Chaves, D. Anti-leishmanial activity in plants from a Biological Reserve of Costa Rica. Rev. Biol. Trop. 2014, 62, 1129–1140. [Google Scholar] [CrossRef]
- Sarkar, A.; Sen, R.; Saha, P.; Ganguly, S.; Mandal, G.; Chatterjee, M. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol. Res. 2008, 102, 1249–1255. [Google Scholar] [CrossRef]
- Misra, P.; Kumar, A.; Khare, P.; Gupta, S.; Kumar, N.; Dube, A. Pro-apoptotic effect of the landrace Bangla Mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. J. Med. Microbiol. 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Shakya, S.; Soni, V.K.; Dangi, A.; Kumar, N.; Bhattacharya, S.M. The n-hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi. Int. Immunopharmacol. 2009, 9, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Al-Adhroey, A.H.; Nor, Z.M.; Al-Mekhlafi, H.M.; Amran, A.A.; Mahmud, R. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules 2010, 16, 107–118. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mondal, S.; Basak, S.; Das, P.; Saha, A.; Bera, T. In Vitro susceptibilities of wild and drug resistant Leishmania donovani amastigotes to piperolactam A loaded hydroxypropyl-β-cyclodextrin nanoparticles. Acta Trop. 2016, 158, 97–106. [Google Scholar] [CrossRef]
- Leesombun, A.; Boonmasawai, S.; Shimoda, N.; Nishikawa, Y. Effects of extracts from Thai Piperaceae plants against infection with Toxoplasma gondii. PLoS ONE 2016, 11, e0156116. [Google Scholar] [CrossRef]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P.J. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J. Ethnopharmacol. 2009, 123, 475–482. [Google Scholar] [CrossRef]
- Thiengsusuk, A.; Chaijaroenkul, W.; Na-Bangchang, K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol. Res. 2013, 112, 1475–1481. [Google Scholar] [CrossRef]
- Esperandim, V.R.; da Silva Ferreira, D.; Sousa Rezende, K.C.; Magalhães, L.G.; Medeiros Souza, J.; Pauletti, P.M.; Januário, A.H.; da Silva de Laurentz, R.; Bastos, J.K.; Símaro, G.V.; et al. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Med. 2013, 79, 1653–1655. [Google Scholar] [CrossRef]
- Chinchilla, M.; Valerio, I.; Sánchez, R.; Mora, V.; Bagnarello, V.; Martínez, L.; Gonzalez, A.; Vanegas, J.C.; Apestegui, Á. In vitro antimalarial activity of extracts of some plants from a biological reserve in Costa Rica. Rev. Biol. Trop. 2012, 60, 881–891. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Mockenhaupt, F.P.; Bienzle, U.; Gupta, M.P.; Eich, E. In vitro antiplasmodial activity of Central American medicinal plants. Trop. Med. Int. Health 1999, 4, 611–615. [Google Scholar] [CrossRef]
- Hamedt, A.L.; Ortiz, I.C.; García-Huertas, P.A.; Sáenz, J.; de Araujo, A.C.; De Mattos, J.C.P.; Rodríguez-Gazquez, M.A.; Triana-Chávez, O. Cytotoxic, mutagenic and genotoxic evaluation of crude extracts and fractions from Piper jericoense with trypanocidal action. Acta Trop. 2014, 131, 92–97. [Google Scholar] [CrossRef]
- García-Huertas, P.; Olmo, F.; Sánchez-Moreno, M.; Dominguez, J.; Chahboun, R.; Triana-Chávez, O. Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense. Exp. Parasitol. 2018, 189, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dayany da Silva, A.M.; Freitas, V.P.; Conserva, G.A.; Alexandre, T.R.; Purisco, S.U.; Tempone, A.G.; Melhem, M.S.; Kato, M.J.; Guimarães, E.F.; Lago, J.H. Bioactivity-guided isolation of laevicarpin, an antitrypanosomal and anticryptococcal lactam from Piper laevicarpu (Piperaceae). Fitoterapia 2016, 111, 24–28. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Singh, M.; Gupta, N.; Saxena, R.; Puri, A.; Verma, A.K.; Saxena, R.P.; Dubey, C.B.; Saxena, K.C. Management of giardiasis by an immuno-modulatory herbal drug Pippali rasayana. J. Ethnopharmacol. 1994, 44, 143–146. [Google Scholar] [CrossRef]
- Tripathi, D.M.; Gupta, N.; Lakshmi, V.; Saxena, K.C.; Agrawal, A.K. Antigiardial and immunostimulatory effect of Piper longum on giardiasis due to Giardia lamblia. Phyther. Res. 1999, 13, 561–565. [Google Scholar] [CrossRef]
- Singh, S.K.; Bimal, S.; Narayan, S.; Jee, C.; Bimal, D.; Das, P.; Bimal, R. Leishmania donovani: Assessment of leishmanicidal effects of herbal extracts obtained from plants in the visceral leishmaniasis endemic area of Bihar, India. Exp. Parasitol. 2011, 127, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Gangwar, M.; Nath, G. Osmoregulatory and tegumental ultrastructural damages to protoscoleces of hydatid cysts Echinococcus granulosus induced by fungal endophytes. J. Parasit. Dis. 2014, 38, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.T.; Lima, M.L.; Galuppo, M.K.; Tempone, A.G.; de Oliveira, A.; Lago, J.H.G.; Fernandes, J.P.S. New alkenyl derivative from Piper malacophyllum and analogues: Antiparasitic activity against Trypanosoma cruzi and Leishmania infantum. Chem. Biol. Drug Des. 2017, 90, 1007–1011. [Google Scholar] [CrossRef]
- Chouhan, G.; Islamuddin, M.; Want, M.Y.; Ozbak, H.A.; Hemeg, H.A.; Sahal, D.; Afrin, F. Leishmanicidal activity of Piper nigrum bioactive fractions is interceded via apoptosis in vitro and substantiated by Th1 immunostimulatory potential in vivo. Front. Microbiol. 2015, 6, 1368. [Google Scholar] [CrossRef]
- De Souza Chagas, A.C.; De Barros, L.D.; Cotinguiba, F.; Furlan, M.; Giglioti, R.; De Sena Oliveira, M.C.; Bizzo, H.R. In vitro efficacy of plant extracts and synthesized substances on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2012, 110, 295–303. [Google Scholar] [CrossRef]
- Carvalho, C.O.; Chagas, A.C.S.; Cotinguiba, F.; Furlan, M.; Brito, L.G.; Chaves, F.C.M.; Stephan, M.P.; Bizzo, H.R.; Amarante, A.F.T. The anthelmintic effect of plant extracts on Haemonchus contortus and Strongyloides venezuelensis. Vet. Parasitol. 2012, 183, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.N.N.A.; Furuta, T.; Kojima, S.; Takane, K.; Ali Mohd, M. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 1999, 64, 249–254. [Google Scholar] [CrossRef]
- Bagatela, B.S.; Lopes, A.P.; Fonseca, F.L.A.; Andreo, M.A.; Nanayakkara, D.N.P.; Bastos, J.K.; Perazzo, F.F. Evaluation of antimicrobial and antimalarial activities of crude extract, fractions and 4-nerolidylcathecol from the aerial parts of Piper umbellata L. (Piperaceae). Nat. Prod. Res. 2013, 27, 2202–2209. [Google Scholar] [CrossRef]
- Esperandim, V.R.; da Silva Ferreira, D.; Rezende, K.C.S.; Cunha, W.R.; Saraiva, J.; Bastos, J.K.; e Silva, M.L.A.; de Albuquerque, S. Evaluation of the in vivo therapeutic properties of (-)-cubebin and (-)-hinokinin against Trypanosoma cruzi. Exp. Parasitol. 2013, 133, 442–446. [Google Scholar] [CrossRef]
- Pecková, R.; Doležal, K.; Sak, B.; Květoňová, D.; Kváč, M.; Wisnu, N.; Foitová, I. Effect of Piper betle on Giardia intestinalis infection in vivo. Exp. Parasitol. 2018, 184, 39–45. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Tayeboon, G.S.; Niknam, F.; Sharifi-Rad, M.; Mohajeri, M.; Salehi, B.; Iriti, M.; Sharifi-Rad, M. Veronica persica Poir. extract—Antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell. Mol. Biol. 2018, 64, 50–56. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed cell death, from a cancer perspective: An overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Valussi, M.; Flaviana Bezerra Morais-Braga, M.; Nalyda Pereira Carneiro, J.; Linkoln Alves Borges Leal, A.; Douglas Melo Coutinho, H.; Vitalini, S.; Kręgiel, D.; Antolak, H.; Sharifi-Rad, M.; et al. Tagetes spp. essential oils and other extracts: Chemical characterization and biological activity. Molecules 2018, 23, 2847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Indian J. Biochem. Biophys. 2014, 51, 358–364. [Google Scholar] [PubMed]
- Ovadje, P.; Ma, D.; Tremblay, P.; Roma, A.; Steckle, M.; Guerrero, J.-A.; Arnason, J.T.; Pandey, S. Evaluation of the efficacy & biochemical mechanism of cell death induction by Piper longum extract selectively in in-vitro and in-vivo models of human cancer cells. PLoS ONE 2014, 9, e113250. [Google Scholar]
- Iwamoto, L.H.; Vendramini-Costa, D.B.; Monteiro, P.A.; Ruiz, A.L.T.G.; Sousa, I.M.D.O.; Foglio, M.A.; de Carvalho, J.E.; Rodrigues, R.A.F. Anticancer and anti-inflammatory activities of a standardized dichloromethane extract from Piper umbellatum L. leaves. Evid.-Based Complement. Altern. Med. 2015, 2015, 948737. [Google Scholar] [CrossRef] [PubMed]
- De Souza Grinevicius, V.M.A.; Kviecinski, M.R.; Mota, N.S.R.S.; Ourique, F.; Castro, L.S.E.P.W.; Andreguetti, R.R.; Correia, J.F.G.; Wilhem Filho, D.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Sriwiriyajan, S.; Tedasen, A.; Lailerd, N.; Boonyaphiphat, P.; Nitiruangjarat, A.; Deng, Y.; Graidist, P. Anticancer and cancer prevention effects of piperine-free Piper nigrum extract on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Prev. Res. 2016, 9, 74–82. [Google Scholar] [CrossRef]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. J. Pharm. Pharmacol. 2009, 61, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singhal, V.; Roshan, R.; Sharma, A.; Rembhotkar, G.W.; Ghosh, B. Piperine inhibits TNF-α induced adhesion of neutrophils to endothelial monolayer through suppression of NF-κB and IκB kinase activation. Eur. J. Pharmacol. 2007, 575, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Shah, A.J.; Gilani, A.H. Blood pressure lowering and vasomodulator effects of piperine. J. Cardiovasc. Pharmacol. 2008, 52, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Doucette, C.D.; Hilchie, A.L.; Liwski, R.; Hoskin, D.W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013, 24, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Lee, K.; Park, W.-H.; Kim, H.; Hong, H. Piperine inhibits platelet-derived growth factor-BB-induced proliferation and migration in vascular smooth muscle cells. J. Med. Food 2015, 18, 208–215. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Si, L.; Yang, R.; Lin, R.; Yang, S. Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Abu, N.; Ho, W.Y.; Yeap, S.K.; Akhtar, M.N.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. The flavokawains: Uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 102. [Google Scholar] [CrossRef]
- Kuo, Y.-F.; Su, Y.-Z.; Tseng, Y.-H.; Wang, S.-Y.; Wang, H.-M.; Chueh, P.J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef]
- Sakai, T.; Eskander, R.N.; Guo, Y.; Kim, K.J.; Mefford, J.; Hopkins, J.; Bhatia, N.N.; Zi, X.; Hoang, B.H. Flavokawain B, a kava chalcone, induces apoptosis in synovial sarcoma cell lines. J. Orthop. Res. 2012, 30, 1045–1050. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Liu, Z.; Simoneau, A.R.; Xie, J.; Zi, X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer 2010, 127, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 2015, 9, 1401–1417. [Google Scholar]
- Rossette, M.C.; Moraes, D.C.; Sacramento, E.K.; Romano-Silva, M.A.; Carvalho, J.L.; Gomes, D.A.; Caldas, H.; Friedman, E.; Bastos-Rodrigues, L.; De Marco, L. The in vitro and in vivo antiangiogenic effects of flavokawain B. Phyther. Res. 2017, 31, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.; Akhtar, M.N.; Mohd Ali, N.; Yeap, S.K.; Quah, C.K.; Loh, W.-S.; Alitheen, N.B.; Zareen, S.; Ul-Haq, Z.; Shah, S.A.A. Design, synthesis and docking studies of flavokawain B type chalcones and their cytotoxic effects on MCF-7 and MDA-MB-231 cell lines. Molecules 2018, 23, 616. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Costa-Lotufo, L.V.; Montenegro, R.C.; Alves, A.; Madeira, S.V.F.; Pessoa, C.; Moraes, M.E.A.; Moraes, M.O. The contribution of natural products as source of new anticancer drugs: Studies carried out at the national experimental oncology laboratory from the Federal University of Ceará. Rev. Virtual Quím. 2010, 2, 47–58. [Google Scholar] [CrossRef]
- Golovine, K.V.; Makhov, P.B.; Teper, E.; Kutikov, A.; Canter, D.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate 2013, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.-H.; Chen, X.-X.; Wang, H.; Jiang, Q.-W.; Pan, S.-S.; Qiu, J.-G.; Mei, X.-L.; Xue, Y.-Q.; Qin, W.-M.; Zheng, F.-Y. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid. Med. Cell. Longev. 2014, 2014, 906804. [Google Scholar] [CrossRef]
- Thongsom, S.; Suginta, W.; Lee, K.J.; Choe, H.; Talabnin, C. Piperlongumine induces G2/M phase arrest and apoptosis in cholangiocarcinoma cells through the ROS-JNK-ERK signaling pathway. Apoptosis 2017, 22, 1473–1484. [Google Scholar] [CrossRef]
- Karki, K.; Hedrick, E.; Kasiappan, R.; Jin, U.-H.; Safe, S. Piperlongumine induces reactive oxygen species (ROS)-dependent downregulation of specificity protein transcription factors. Cancer Prev. Res. 2017, 10, 467–477. [Google Scholar] [CrossRef]
- Machado, F.D.S.; Munari, F.M.; Scariot, F.J.; Echeverrigaray, S.; Aguzzoli, C.; Pich, C.T.; Kato, M.J.; Yamaguchi, L.; Moura, S.; Henriques, J.A.P. Piperlongumine Induces Apoptosis in Colorectal Cancer HCT 116 Cells Independent of Bax, p21 and p53 Status. Anticancer Res. 2018, 38, 6231–6236. [Google Scholar] [CrossRef] [PubMed]
- Da Nóbrega, F.; Ozdemir, O.; Nascimento Sousa, S.; Barboza, J.; Turkez, H.; de Sousa, D. Piplartine analogues and cytotoxic evaluation against glioblastoma. Molecules 2018, 23, 1382. [Google Scholar] [CrossRef]
- Hamdy, N.A.; Gamal-Eldeen, A.M. New pyridone, thioxopyridine, pyrazolopyridine and pyridine derivatives that modulate inflammatory mediators in stimulated RAW 264.7 murine macrophage. Eur. J. Med. Chem. 2009, 44, 4547–4556. [Google Scholar] [CrossRef]
- Tumer, T.B.; Onder, F.C.; Ipek, H.; Gungor, T.; Savranoglu, S.; Tok, T.T.; Celik, A.; Ay, M. Biological evaluation and molecular docking studies of nitro benzamide derivatives with respect to in vitro anti-inflammatory activity. Int. Immunopharmacol. 2017, 43, 129–139. [Google Scholar] [CrossRef]
- Tumer, T.B.; Yılmaz, B.; Ozleyen, A.; Kurt, B.; Tok, T.T.; Taskin, K.M.; Kulabas, S.S. GR24, a synthetic analog of strigolactones, alleviates inflammation and promotes Nrf2 cytoprotective response: In vitro and in silico evidences. Comput. Biol. Chem. 2018, 76, 179–190. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef]
- Bui, T.T.; Piao, C.H.; Song, C.H.; Shin, H.S.; Shon, D.-H.; Chai, O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell. Immunol. 2017, 322, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Laksmitawati, D.R.; Widyastuti, A.; Karami, N.; Afifah, E.; Rihibiha, D.D.; Nufus, H.; Widowati, W. Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J. Pharmacol. 2017, 12, 35–40. [Google Scholar] [CrossRef]
- Reddy, P.S.; Gupta, R.K.; Reddy, S.M. Analgesic and anti-inflammatory activity of hydroalcoholic extract of Piper betle leaves in experimental animals. Int. J. Basic Clin. Pharmacol. 2016, 5, 979–985. [Google Scholar] [CrossRef]
- Finato, A.C.; Fraga-Silva, T.F.; Prati, A.U.C.; de Souza Júnior, A.A.; Mazzeu, B.F.; Felippe, L.G.; Pinto, R.A.; de Assis Golim, M.; Arruda, M.S.P.; Furlan, M. Crude leaf extracts of Piperaceae species downmodulate inflammatory responses by human monocytes. PLoS ONE 2018, 13, e0198682. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Sarwar, A.H.M.G.; Umar, K.; Ahmad, N.; Sajad, M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell. Immunol. 2013, 284, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.-G.; Lee, M.Y.; Lee, Y.-M.; Bae, J.-S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Clouatre, D.L. Kava kava: Examining new reports of toxicity. Toxicol. Lett. 2004, 150, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Gaus, W.; Loew, D. Kava extracts: Safety and risks including rare hepatotoxicity. Phytomedicine 2003, 10, 440–446. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-κB and AP-1 activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef]
- Alshammari, A.; Patel, J.; Al-Hashemi, J.; Cai, B.; Panek, J.; Huck, O.; Amar, S. Kava-241 reduced periodontal destruction in a collagen antibody primed Porphyromonas gingivalis model of periodontitis. J. Clin. Periodontol. 2017, 44, 1123–1132. [Google Scholar] [CrossRef]
- Huck, O.; You, J.; Han, X.; Cai, B.; Panek, J.; Amar, S. Reduction of articular and systemic inflammation by kava-241 in Porphyromonas gingivalis-induced arthritis murine model. Infect. Immun. 2018, 86, e00356-18. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Rasool, M.; Amjad Kamal, M.; A. Damanhouri, G.; Zubair Alam, M.; Alam, Q.; Mushtaq, G. Inflammatory process in Alzheimer’s and Parkinson’s diseases: Central role of cytokines. Curr. Pharm. Des. 2016, 22, 541–548. [Google Scholar]
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef]
- Mathew, G.; Unnikrishnan, M.K. Multi-target drugs to address multiple checkpoints in complex inflammatory pathologies: Evolutionary cues for novel “first-in-class” anti-inflammatory drug candidates: A reviewer’s perspective. Inflamm. Res. 2015, 64, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef]
- Ferreres, F.; Oliveira, A.P.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Piper betle leaves: Profiling phenolic compounds by HPLC/DAD–ESI/MSn and anti-cholinesterase activity. Phytochem. Anal. 2014, 25, 453–460. [Google Scholar] [CrossRef]
- Yeo, E.T.Y.; Wong, K.W.L.; See, M.L.; Wong, K.Y.; Gan, S.Y.; Chan, E.W.L. Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J. Ethnopharmacol. 2018, 217, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Vaibhav, K.; Tabassum, R.; Khan, A.; Ishrat, T.; Khan, M.M.; Ahmad, A.; Islam, F.; Safhi, M.M.; Islam, F. Anti-apoptotic and anti-inflammatory effect of piperine on 6-OHDA induced Parkinson’s rat model. J. Nutr. Biochem. 2013, 24, 680–687. [Google Scholar] [CrossRef]
- Kinzler, E.; Krömer, J.; Lehmann, E. Effect of a special kava extract in patients with anxiety-, tension-, and excitation states of non-psychotic genesis. Double blind study with placebos over 4 weeks. Arzneimittelforschung 1991, 41, 584–588. [Google Scholar]
- Volz, H.-P.; Kieser, M. Kava-kava extract WS 1490 versus placebo in anxiety disorders-a randomized placebo-controlled 25-week outpatient trial. Pharmacopsychiatry 1997, 30, 1–5. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Patahuddin, H.; Mohamad, A.S.; Israf, D.A.; Sulaiman, M.R. In vivo anti-nociceptive and anti-inflammatory activities of the aqueous extract of the leaves of Piper sarmentosum. J. Ethnopharmacol. 2010, 128, 42–48. [Google Scholar] [CrossRef]
- Amran, A.A.; Zakaria, Z.; Othman, F.; Das, S.; Al-Mekhlafi, H.M.; Nordin, N.-A.M.M. Changes in the vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and c-reactive protein following administration of aqueous extract of Piper sarmentosum on experimental rabbits fed with cholesterol diet. Lipids Health Dis. 2011, 10, 2. [Google Scholar] [CrossRef]
- Hafizah, A.H.; Zaiton, Z.; Zulkhairi, A.; Ilham, A.M.; Anita, M.M.N.N.; Zaleha, A.M. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J. Zhejiang Univ. Sci. B 2010, 11, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Elhussein, S.A.A.; Khan, M.M.; Khan, N. Anti-acetylcholinesterase activity of Piper sarmentosum by a continuous immobilized-enzyme Assay. APCBEE Procedia 2012, 2, 199–204. [Google Scholar] [CrossRef]
- Li, Q.; Qu, F.-L.; Gao, Y.; Jian effects in r g, Y.-P.; Rahman, K.; Lee, K.-H.; Han, T.; Qin, L.-P. Piper sarmentosum Roxb. produces antidepressant-like odents, associated with activation of the CREB-BDNF-ERK signaling pathway and reversal of HPA axis hyperactivity. J. Ethnopharmacol. 2017, 199, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Salem, A.M.; Sabry, G.M.; Husein, A.A.; Kotob, S.E. Possible Therapeutic uses of Salvia triloba and Piper nigrum in Alzheimer’s Disease–induced rats. J. Med. Food 2013, 16, 437–446. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta (1–42) rat model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Li, W.; Koike, K.; Nikaido, T.; Wang, M.-W. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J. Asian Nat. Prod. Res. 2007, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xian, Y.-F.; Ip, S.-P.; Che, C.-T. Involvement of serotonergic system in the antidepressant-like effect of piperine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1144–1147. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Huang, Z.; Ip, S.-P.; Xian, Y.-F.; Che, C.-T. Role of 5-HT1A and 5-HT1B receptors in the antidepressant-like effect of piperine in the forced swim test. Neurosci. Lett. 2011, 504, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Huang, Z.; Zhong, X.-M.; Xian, Y.-F.; Ip, S.-P. Piperine reverses the effects of corticosterone on behavior and hippocampal BDNF expression in mice. Neurochem. Int. 2014, 74, 36–41. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.-H.; Liu, H.; Qu, H.-D. Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int. J. Mol. Med. 2015, 36, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bové, J.; Vila, M. Mitochondria and programmed cell death in Parkinson’s disease: Apoptosis and beyond. Antioxid. Redox Signal. 2012, 16, 883–895. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Dehay, B.; Jackson-Lewis, V.; Rabinovitch, P.S.; Przedborski, S.; Vila, M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann. Neurol. 2010, 68, 184–192. [Google Scholar]
- Xilouri, M.; Stefanis, L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev. Mol. Med. 2011, 13, e8. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Beal, M.F.; Thomas, B. Autophagy in neurodegenerative disorders: Pathogenic roles and therapeutic implications. Trends Neurosci. 2010, 33, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, M.; Wang, X.; Wang, Y.; Duan, C.; Gao, G.; Lu, L.; Wu, X.; Wang, X.; Yang, H. Piperine induces autophagy by enhancing protein phosphotase 2A activity in a rotenone-induced Parkinson’s disease model. Oncotarget 2016, 7, 60823–60843. [Google Scholar]
- Wang, H.; Liu, J.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Protection effect of piperine and piperlonguminine from Piper longum L. alkaloids against rotenone-induced neuronal injury. Brain Res. 2016, 1639, 214–227. [Google Scholar] [CrossRef]
- Chen, W.-S.; An, J.; Li, J.-J.; Hong, L.; Xing, Z.-B.; Li, C.-Q. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 2017, 42, 44–48. [Google Scholar]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Lu, Y.; Tian, H.; Duan, C.; Lu, L.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 2018, 14, 845–861. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdelmonsif, D.A.; Abdallah, O.Y. Oral brain-targeted microemulsion for enhanced piperine delivery in Alzheimer’s disease therapy: In vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech 2018, 19, 3698–3711. [Google Scholar] [CrossRef] [PubMed]
- Anissian, D.; Ghasemi-Kasman, M.; Khalili-Fomeshi, M.; Akbari, A.; Hashemian, M.; Kazemi, S.; Moghadamnia, A.A. Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int. J. Biol. Macromol. 2018, 107, 973–983. [Google Scholar] [CrossRef]
- Han, J.G.; Gupta, S.C.; Prasad, S.; Aggarwal, B.B. Piperlongumine chemosensitizes tumor cells through interaction with cysteine 179 of IkappaBalpha kinase, leading to suppression of NF-kappaB-regulated gene products. Mol. Cancer Ther. 2014, 13, 2422–2435. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Gu, S.M.; Lee, H.P.; Ham, Y.W.; Son, D.J.; Kim, H.Y.; Oh, K.W.; Han, S.-B.; Yun, J.; Hong, J.T. Piperlongumine improves lipopolysaccharide-induced amyloidogenesis by suppressing NF-KappaB pathway. Neuromol. Med. 2018, 20, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.M.; Yun, J.; Son, D.J.; Kim, H.Y.; Nam, K.T.; Kim, H.D.; Choi, M.G.; Choi, J.S.; Kim, Y.M.; Han, S.-B. Piperlongumine attenuates experimental autoimmune encephalomyelitis through inhibition of NF-kappaB activity. Free Radic. Biol. Med. 2017, 103, 133–145. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, B.; Meng, X.; Yao, J.; Fang, J. Synthesis of piperlongumine analogues and discovery of nuclear factor erythroid 2-related factor 2 (Nrf2) activators as potential neuroprotective agents. J. Med. Chem. 2015, 58, 5242–5255. [Google Scholar] [CrossRef]
- Go, J.; Seo, J.Y.; Park, T.-S.; Ryu, Y.-K.; Park, H.-Y.; Noh, J.-R.; Kim, Y.-H.; Hwang, J.H.; Choi, D.-H.; Hwang, D.Y. Piperlongumine activates Sirtuin1 and improves cognitive function in a murine model of Alzheimer’s disease. J. Funct. Foods 2018, 43, 103–111. [Google Scholar] [CrossRef]
- Go, J.; Park, T.; Han, G.; Park, H.; Ryu, Y.; Kim, Y.; Hwang, J.H.; Choi, D.; Noh, J.; Hwang, D.Y. Piperlongumine decreases cognitive impairment and improves hippocampal function in aged mice. Int. J. Mol. Med. 2018, 42, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; LaPorte, E.; Schweitzer, I. Kava: A comprehensive review of efficacy, safety, and psychopharmacology. Aust. N. Z. J. Psychiatry 2011, 45, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, A.; Siegl, S.; Fendt, M.; Jansen, S.; Soppa, U.; Brandenburg, L.-O.; Pufe, T.; Weis, J.; Wruck, C.J. Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer’s disease. Redox Biol. 2017, 12, 843–853. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. The Pharmacology, Pharmacokinetics, Efficacy, and Adverse Events Associated With Kava. J. Clin. Pharmacol. 2018, 58, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Aaboe, K.; Knop, F.K.; Vilsboll, T.; Vølund, A.; Simonsen, U.; Deacon, C.F.; Madsbad, S.; Holst, J.J.; Krarup, T. KATP channel closure ameliorates the impaired insulinotropic effect of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Geinisman, Y.; Detoledo-Morrell, L.; Morrell, F.; Heller, R.E. Hippocampal markers of age-related memory dysfunction: Behavioral, electrophysiological and morphological perspectives. Prog. Neurobiol. 1995, 45, 223–252. [Google Scholar] [CrossRef]
- Watkins, L.L.; Connor, K.M.; Davidson, J.R.T. Effect of kava extract on vagal cardiac control in generalized anxiety disorder: Preliminary findings. J. Psychopharmacol. 2001, 15, 283–286. [Google Scholar] [PubMed]
- Ballinger, C.B. Psychiatric aspects of the menopause. Br. J. Psychiatry 1990, 156, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.G.; Bancroft, J.; Johnstone, E.C. Affective symptoms in women attending a menopause clinic. Br. J. Psychiatry 1994, 164, 513–516. [Google Scholar] [CrossRef]
- Gath, D.; Iles, S. Depression and the menopause. BMJ Br. Med. J. 1990, 300, 1287. [Google Scholar] [CrossRef]
- Sherwin, B.B. Impact of the changing hormonal milieu on psychological functioning. In Treatment of the Postmenopausal Woman, 3rd ed.; Lobo, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 217–226. [Google Scholar]
- Cagnacci, A.; Volpe, A.; Arangino, S.; Malmusi, S.; Draetta, F.P.; Matteo, M.L.; Maschio, E.; Vacca, A.M.B.; Melis, G.B. Depression and anxiety in climacteric women: Role of hormone replacement therapy. Menopause 1997, 4, 206–211. [Google Scholar] [CrossRef]
- Cagnacci, A.; Arangino, S.; Renzi, A.; Zanni, A.L.; Malmusi, S.; Volpe, A. Kava-kava administration reduces anxiety in perimenopausal women. Maturitas 2003, 44, 103–109. [Google Scholar] [CrossRef]
- Geier, F.P.; Konstantinowicz, T. Kava treatment in patients with anxiety. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lehrl, S. Clinical efficacy of kava extract WS® 1490 in sleep disturbances associated with anxiety disorders: Results of a multicenter, randomized, placebo-controlled, double-blind clinical trial. J. Affect. Disord. 2004, 78, 101–110. [Google Scholar] [CrossRef]
- Sarris, J.; Kavanagh, D.J.; Byrne, G.; Bone, K.M.; Adams, J.; Deed, G. The Kava Anxiety Depression Spectrum Study (KADSS): A randomized, placebo-controlled crossover trial using an aqueous extract of Piper methysticum. Psychopharmacology (Berl.) 2009, 205, 399–407. [Google Scholar] [CrossRef]
- Sarris, J.; Stough, C.; Teschke, R.; Wahid, Z.T.; Bousman, C.A.; Murray, G.; Savage, K.M.; Mouatt, P.; Ng, C.; Schweitzer, I. Kava for the treatment of generalized anxiety disorder RCT: Analysis of adverse reactions, liver function, addiction, and sexual effects. Phyther. Res. 2013, 27, 1723–1728. [Google Scholar] [CrossRef]
- Sarris, J.; Stough, C.; Bousman, C.A.; Wahid, Z.T.; Murray, G.; Teschke, R.; Savage, K.M.; Dowell, A.; Ng, C.; Schweitzer, I. Kava in the treatment of generalized anxiety disorder: A double-blind, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2013, 33, 643–648. [Google Scholar] [CrossRef]
- Savage, K.M.; Stough, C.K.; Byrne, G.J.; Scholey, A.; Bousman, C.; Murphy, J.; Macdonald, P.; Suo, C.; Hughes, M.; Thomas, S. Kava for the treatment of generalised anxiety disorder (K-GAD): Study protocol for a randomised controlled trial. Trials 2015, 16, 493. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Fomeshi, M.; Azizi, M.G.; Esmaeili, M.R.; Gol, M.; Kazemi, S.; Ashrafpour, M.; Moghadamnia, A.A.; Hosseinzadeh, S. Piperine restores streptozotocin-induced cognitive impairments: Insights into oxidative balance in cerebrospinal fluid and hippocampus. Behav. Brain Res. 2018, 337, 131–138. [Google Scholar] [CrossRef]
| Compound | Brazil [44] | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil [45] | Brazil | Brazil | Brazil [46] | Brazil [42] | Brazil [47] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Humulene | 5.5 | 8.5–10.6 | ||||||||||||||||||||||||
| (E)-Nerolidol | 80.6–82.5 | 79.2–81.2 | 10.3 | 5.9 | 14.3–16.7 | 25.2 | ||||||||||||||||||||
| (E)-β-Ocimene | 6.4 | 5.0 | 11.6 | 13.4 | 13.9 | 4.1 | ||||||||||||||||||||
| (Z)-cadin-4-en-7-ol | 7.5–12.2 | |||||||||||||||||||||||||
| (Z)-β-Ocimene | 7.0 | |||||||||||||||||||||||||
| 1,8-Cineole | 42.0–42.5 | 57.2 | 8.7 | 55.8 | ||||||||||||||||||||||
| Asaricin | 9.2–10.5 | 5.6 | 15.8 | 14.9 | 80.1 | 73.4 | ||||||||||||||||||||
| Bicyclogermacrene | 3.8–6.0 | 11.3 | 20.9 | |||||||||||||||||||||||
| Camphene | 10.9 | |||||||||||||||||||||||||
| Camphor | 17.1 | |||||||||||||||||||||||||
| Dillapiole | 76 | 94.8 | 49.5 | 79.0 | 92 | 6.3 | 52.4 | |||||||||||||||||||
| Germacrene D | 6.9 | |||||||||||||||||||||||||
| Limonene | ||||||||||||||||||||||||||
| Linalool | 31.8 | 9.3–13.4 | 13.4 | |||||||||||||||||||||||
| Longipinanol | 2.4–5.6 | 11.1–13.6 | ||||||||||||||||||||||||
| Myristicin | 12.4 | |||||||||||||||||||||||||
| Piperitone | 22.7 | 24.9 | 11.0 | 16.3 | 34.0 | 11.8 | ||||||||||||||||||||
| Safrole | 13.3 | 10.8 | 10.5 | 6.2 | ||||||||||||||||||||||
| Spathulenol | 0–5.6 | 10.6 | 5.3 | 6.3 | ||||||||||||||||||||||
| Terpinen-4-ol | 15.0 | 16.8 | 6.7 | 6.3 | ||||||||||||||||||||||
| Valencene | 6.9 | 9.7 | ||||||||||||||||||||||||
| Viridiflorol | 7.4 | 4.4 | ||||||||||||||||||||||||
| α-Pinene | 8.0–8.9 | 14.2 | 6.4 | |||||||||||||||||||||||
| α-Selinene | 4.7 | |||||||||||||||||||||||||
| α-Terpinene | 4.8 | |||||||||||||||||||||||||
| α-Terpineol | 5.9 | |||||||||||||||||||||||||
| β-Caryophyllene | 6 | 9.3 | 5.1–6.7 | 5.4 | 5.8 | |||||||||||||||||||||
| β-Phellandrene | 6.8 | 6.6 | ||||||||||||||||||||||||
| β-Pinene | 3.5 | 6.6–7.0 | 9.0 | |||||||||||||||||||||||
| γ-Cadinene | 5.5 | |||||||||||||||||||||||||
| γ-Terpinene | 8.3 | 8.2 | 9.0 |
| Compound | Cuba | Cuba | Cuba | Costa Rica | Bolivia | Panama [48] | Equador [43] | Papua New Guinea [40] |
|---|---|---|---|---|---|---|---|---|
| α-Humulene | 5.1 | |||||||
| (E)-β-Ocimene | 7.5 | |||||||
| 1-epi-Cubenol | 6.2 | |||||||
| 1,8-Cineole | 40.5 | |||||||
| Aromadendrene | 13.4 | |||||||
| Asaricin | 12.9 | |||||||
| Camphene | 6.1 | 5.4–7.4 | ||||||
| Camphor | 8.3 | 9.4–13.9 | ||||||
| Dillapiole | 82.2 | 48.2 | 43.3 | |||||
| Germacrene D | 8.2 | |||||||
| Limonene | 6.7 | 5.0 | ||||||
| Linalool | 8.6 | |||||||
| Piperitone | 12.9 | 19.0–20.1 | 6.7 | |||||
| Sabinene | 18.4 | |||||||
| Viridiflorol | 13.0–18.8 | |||||||
| α-Pinene | 39.3 | 9.0 | 8.8 | |||||
| β-Caryophyllene | 6.7 | 17.4 | 4.8 | 8.2 | ||||
| β-Pinene | 7.1 |
| Compound | % | % | % | % | % | % | % [49] | % | % | % | * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-β-Ocimene | 3.0 | ||||||||||
| Dillapiole | 82.2 | 86.9 | 91.1 | 91.1 | 88.1 | 86.9 | 64.4 | 85.9 | 73.0 | 50.8 | 56.3 |
| Piperitone | 3.3 | 13.9 | 7.0 | ||||||||
| Terpinen-4-ol | 7.3 | ||||||||||
| γ-Terpinene | 6.5 | ||||||||||
| β-Caryophyllene | 2.5 | ||||||||||
| Germacrene D | 2.7 | ||||||||||
| Bicyclogermacrene | 2.0 | ||||||||||
| Terpinolene | 2.0 |
| Compound | Cuba [41] | Equador [36] | Bolivia [36] | China [34] |
|---|---|---|---|---|
| (E)-β-Ocimene | 10.4 | |||
| 1,8-Cineole | 40 | |||
| Camphene | 5.9 | |||
| Camphor | 17.1 | |||
| Dillapiole | 45.9 | |||
| Piperitone | 23.7 | 8.5 | ||
| Terpinen-4-ol | 3.1 | |||
| Viridiflorol | 14.5 | |||
| γ-Terpinene | 2.4 | |||
| β-Caryophyllene | 2.6 | |||
| Eugenol | 29.1 | |||
| Spathulenol | 8.3 | |||
| Propiopiperone | 7.1 | |||
| Germacrene D | 5.8 | |||
| Bicyclogermacrene | 3.9 | |||
| Methyleugenol | 3.8 |
| Compound | Brazil [32] | Brazil [42] |
|---|---|---|
| (E)-β-Ocimene | 11.1 | |
| Piperitone | 23.4 | |
| Terpinen-4-ol | 23.4 | |
| γ-Terpinene | 12.0 | |
| α-Terpinene | 6.8 | |
| (Z)-β-Ocimene | 5.6 | |
| Linalool | 41.2 | |
| (E)-Nerolidol | 6.1 | |
| β-Caryophyllene | 7.2 | |
| α-Humulene | 6.9 | |
| Myristicin | 6.5 |
| Compound | Costa Rica | Brazil [50] | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil |
|---|---|---|---|---|---|---|---|---|---|
| Borneol | 5.7 | ||||||||
| Bicyclogermacrene | 13.0 | 19.4 | 20.8 | 15.0 | 27.9 | ||||
| Camphene | 8.9 | ||||||||
| Cubenol | 6.2 | ||||||||
| Germacrene A | 6.5–9.7 | ||||||||
| Germacrene D | 28.9–29.4 | 11.7 | 9.9 | ||||||
| Limonene | 6.2 | 6.8 | |||||||
| Methyl geraniate | 7.8 | ||||||||
| Myrcene | 6.8 | ||||||||
| p-Cymene | 9.4 | ||||||||
| Sabinene | 8.2 | 6.7 | |||||||
| Spathulenol | 5.6 | 9.1 | 19.2 | ||||||
| α-Amorphene | 25.7 | ||||||||
| α-Cadinol | 7.6 | ||||||||
| α-Phellandrene | 1.7–8.1 | ||||||||
| α-Pinene | 3.7 | 6.7 | 11.7 | 14.8 | 30.5 | ||||
| β-Caryophyllene | 15.9–23.3 | 3.0 | |||||||
| β-Elemene | 11.5–24.6 | ||||||||
| β-Phellandrene | 12.3 | 15.9 | 33.1 | 39.3 | |||||
| γ-Muurolene | 3.6 | 5.9 | 7.3 |
| Compound | Malaysia | India | India a | India a | India [54] | India c [55] | India [56] | India [57] | India [58] | Thailand | Vietnam | Nepal [59] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Isoeugenol | 5.2 b | 28.3 | 72.0 b | |||||||||
| Chavicyl acetate | 8.1 | |||||||||||
| Eugenyl acetate | 31.4 | |||||||||||
| Allyl-pyrocatechol | 8.7–10.8 | 2.1 | ||||||||||
| Allyl-pyrocatechyl diacetate | 3.6 | 6.2 | ||||||||||
| Allyl-pyrocatechyl monoacetate | 8.5 | |||||||||||
| Aromadendrene | 0.89–1.35 | |||||||||||
| Bicyclogermacrene | 1.0 | |||||||||||
| Chavibetol | 69.0 | 4.2–7.2 | 2.0 | 22.0 | 53.1 | 26.0 | 80.5 | |||||
| Chavibetyl acetate | 14.7–20.7 | 15.5 | 12.5 | 11.7 | ||||||||
| Chavicol | 6.0 | 47.8–48.8 | 1.1 | 11.8 | 2.0 | 3.2 | 0.4 | |||||
| Estragole | 15.8 | |||||||||||
| Eugenol | 63.6 | 9.0 | 63.4 | 0.4 | ||||||||
| Eugenyl acetate | 8.3 | 18.7 | 2.2 | 14.1 | 31.8 | |||||||
| Germacrene D | 2.9 | |||||||||||
| Isoeugenyl acetate | 12.2 b | |||||||||||
| Ledene | 1.0 | |||||||||||
| Linalool | 1.8 | 0.9 | ||||||||||
| Methyl eugenol | 6.9 | 0.7 | 0.4 | |||||||||
| p-Menth-3-en-9-ol | 1.5 | |||||||||||
| Sabinene | 2.6 | |||||||||||
| Safrole | 39.9 | 48.7 | ||||||||||
| Terpinen-4-ol | 6.3 | |||||||||||
| Viridiflorol | 1.5 | |||||||||||
| α-Amorphene | 2.5 | |||||||||||
| α-Humulene | 1.0 | tr | ||||||||||
| β-Bergamotene | 0.7–1.3 | |||||||||||
| β-Caryophyllene | 2.4 | 1.2 | 11.3 | 4.2 | 3.1 | 0.4 | ||||||
| β-Cubebene | 13.6 | |||||||||||
| β-Pinene | 1.7 | |||||||||||
| γ-Cadinene | 1.6 | 2.4 | ||||||||||
| γ-Muurolene | 5.2 | |||||||||||
| γ-Terpinene | 1.9 |
| Molecule | Percentage |
|---|---|
| (E)-Asarone | 0.9–3.7 |
| (E)-Nerolidol | 0.1–3.6 |
| (E)-α-Bergamotene | <0.1–0.2 |
| (E)-β-Farnesene | <0.1–0.2 |
| (E)-β-Ocimene | <0.1–0.1 |
| cis-Calamenene | 1.0–3.8 |
| cis-Sabinene hydrate | <0.1–0.4 |
| 1-epi-Cubenol | 0.3–3.5 |
| 1,8-Cineole | 0.3–0.8 |
| allo-Aromadendrene | 0.2–11.0 |
| Apiole | <0.1–0.2 |
| Borneol | <0.1–0.3 |
| Cadina-1, 4-diene | <0.1–0.2 |
| Caryophyllene oxide | <0.1–0.1 |
| Cubebol | 5.6–30.9 |
| Cuminaldehyde | <0.1–0.2 |
| Cyclosativene | <0.1–0.2 |
| Dillapiole | <0.1–0.2 |
| epi-Cubebol | <0.1–4.6 |
| Germacrene D | 0.1–11.1 |
| Globulol | <0.1–3.5 |
| Ledol | <0.1–0.2 |
| Limonene | 0.1–4.4 |
| Linalool | <0.1–1.0 |
| Myrcene | <0.1–1.7 |
| Myristicin | <0.1–0.1 |
| p-Cymen-8-ol | 0.1–0.3 |
| p-Cymene | <0.1–1.1 |
| Sabinene | 0.7–29.6 |
| Safrole | <0.1–0.1 |
| τ-Muurolol | <0.1–0.3 |
| Terpinen-4-ol | <0.1–2.7 |
| Terpinolene | <0.1–0.3 |
| α-Cadinol | 0.2–1.0 |
| α-Copaene | 3.8–14.3 |
| α-Cubebene | 1.5–5.7 |
| α-Humulene | 0.5–0.9 |
| α-Muurolene | 0.6–1.7 |
| α-Pinene | 0.3–7.9 |
| α-Terpinene | <0.1–1.3 |
| α-Terpineol | 0.1–2.8 |
| α-Thujene | <0.1–2.5 |
| β-Bisabolene | 1.5–2.0 |
| β-Caryophyllene | 1.1–9.5 |
| β-Cubebene | 0.2–11.1 |
| β-Elemene | 1.0–9.4 |
| β-Phellandrene | <0.1–0.8 |
| β-Pinene | <0.1–0.2 |
| γ-Cadinene | 0.1–0.3 |
| γ-Muurolene | <0.1–11.5 |
| γ-Terpinene | 0.1–0.7 |
| δ-Cadinene | <0.1–9.5 |
| δ-Elemene | 0.1–0.3 |
| Molecule | India | India | India | Indonesia | Brazil a [61] |
|---|---|---|---|---|---|
| trans-Muurola-4(14),5-diene | 0.8 | ||||
| (E)-Nerolidol | 0.7 | ||||
| trans-Sabinene hydrate | 0.5 | ||||
| (Z,Z)-Farnesol | 0.6 | ||||
| cis-Calamenene | 0.7 | ||||
| cis-Sabinene hydrate | 0.9 | 0.7 | |||
| 1-epi-Cubenol | 0.4 | ||||
| 1,8-Cineole | 1.3 | 11.9 | |||
| 4-epi-Cubenol | 1.9 | ||||
| Terpinen-4-ol | 0.9 | 1.0 | 6.4 | ||
| allo-Aromadendrene | 2.3 | 3.1 | 4.1 | ||
| Bicyclogermacrene | 1.5 | 1.5 | |||
| Camphor | 5.6 | ||||
| Caryophyllene oxide | 1.3 | 0.6 | |||
| Cedrol | 0.3 | ||||
| Citronellyl acetate | 0.5 | ||||
| Cubebol | 23.6 | 4.7 | 13.3 | ||
| Germacrene D | 1.5 | 2.6 | 4.7 | 7.5 | |
| Guaiol | 1.0 | ||||
| Isoborneol | 3.6 | ||||
| Limonene | 2.0 | 0.5 | |||
| Linalool | 1.5 | 4.9 | 3.2 | 5.0 | |
| Linalool oxide | 1.4 | ||||
| Methyl eugenol | 2.3 | ||||
| Myrcenol | 0.6 | ||||
| p-Cymene | 0.4 | 1.0 | 0.4 | 4.4 | |
| Sabinene | 19.4 | 9.6 | 20.0 | ||
| Sativene | 8.7 | ||||
| Spathulenol | 0.5 | 27.1 | |||
| Terpinolene | 0.2 | ||||
| α-Cadinol | 0.9 | ||||
| α-Copaene | 0.9 | 8.8 | 7.4 | 4.9 | |
| α-Cubebene | 4.1 | 3.9 | |||
| α-Gurjunene | 0.7 | ||||
| α-Humulene | 0.3 | 0.9 | 2.0 | ||
| α-Muurolene | 0.6 | 0.6 | |||
| α-Pinene | 2.2 | 4.1 | 1.9 | ||
| α-Terpineol | 1.7 | 0.3 | 4.1 | ||
| α-Thujene | 4.5 | 1.9 | 3.3 | ||
| β-Bisabolene | 3.1 | ||||
| β-Bisabolol | 1.0 | ||||
| β-Caryophyllene | 0.4 | 3.7 | 5.3 | ||
| β-Copaene | 3.3 | ||||
| β-Cubebene | 5.6 | 18.3 | 18.9 | ||
| β-Elemene | 7.3 | 0.6 | 1.4 | ||
| β-Eudesmol | 2.4 | ||||
| β-Phellandrene | 5.9 | 1.7 | |||
| β-Pinene | 18.2 | 0.7 | 0.6 | 5.8 | |
| β-Selinene | 0.6 | ||||
| γ-Amorphene | 2.0 | ||||
| γ-Cadinene | 0.9 | ||||
| γ-Muurolene | 2.4 | ||||
| γ-Terpinene | 0.7 | 0.1 | 3.4 | ||
| δ-3-Carene | 0.3 | 5.3 | |||
| δ-Cadinene | 4.7 | 0.2 | |||
| δ-Elemene | 0.1 | ||||
| δ-Terpineol | 0.6 |
| Compounds | Black | White |
|---|---|---|
| (E)-β-Farnesene | tr.-3.3 | |
| Limonene | 16.4–24.4 | 22.6 |
| p-Cymene | 1.0 | |
| Sabinene | 0.1–13.8 | |
| α-Copaene | 0.1–3.9 | |
| α-Cubebene | 0.2–1.6 | |
| α-Humulene | 1.0 | |
| α-Phellandrene | 4.5 | |
| α-Pinene | 1.1–16.2 | 4.0 |
| β-Bisabolene | 0.1–5.2 | |
| β-Caryophyllene | 9.4–30.9 | 23.4 |
| β-Myrcene | 2.7 | |
| β-Pinene | 4.9–14.3 | 9.3 |
| δ-3-Carene | tr.-15.5 | 25.2 |
| δ-Elemene | 2.1 |
| Compound | Malaysia | Malaysia White, Green, Black | Indian Fresh and Dried Black | India Ground Black | Indian Cultivars | Cuba |
|---|---|---|---|---|---|---|
| (E)-Nerolidol | tr.-7.1 | tr.-7.1 | ||||
| 6-Hydroxypiperitol | 0.6 | |||||
| ar-Turmerone | 0.7 | |||||
| Caryophyllene oxide | 4.1 | 0.4–3.7 | 6.8–9.9 | 29.3 | ||
| Caryophyllenol | 0.5 | |||||
| Eugenol | 1.1–41.0a | |||||
| Humulene epoxide II | 1.4 | |||||
| Isocaryophyllene oxide | 1.6 | |||||
| Isospathulenol | 3.1 | |||||
| Limonene | 8.7 | 2.9–14.3 | 18.8–20.1 | 22.7–26.2 | 8.3–23.8 | 3.7 |
| Linalool | 0.6 | |||||
| Myrcene | 9.1 | |||||
| Myrtenol | 0.5 | |||||
| p-Cymen-8-ol | 1.4 | |||||
| p-Cymene | tr.-13.0 | 11.0–13.2 | 0.8 | |||
| Piperitenone oxide | 0.0–2.0 | |||||
| Sabinene | 2.4 | 0.8–12.1 | 16.7–24.5 | 0.0–27.5 | ||
| Terpinen-4-ol | tr.-8.9 | tr.-8.9 | ||||
| trans-Sabinol | 0.5 | |||||
| Verbenone | 2.0 | |||||
| α-Copaene | 3.8 | 3.6–4.5 | 3.1 | |||
| α-Humulene | 2.8 | 0.8 | ||||
| α-Pinene | 0.3 | 0.3–3.8 | 5.4–11.2 | 3.4–4.6 | 1.7–14.6 | 3.1 |
| α-Selinene | 1.1 | |||||
| α-Terpinene | 7.3 | |||||
| β-Bisabolene | 4.4–7.2 | |||||
| β-Caryophyllene | 39.7 | 3.5–70.4 | 6.7–10.8 | 1.6–2.1 | 6.4–52.9 | 6.9 |
| β-Elemene | 1.6 | |||||
| β-Eudesmol | tr.-9.7 | |||||
| β-Pinene | 0.7–5.9 | 14.2–15.2 | 10.5–14.4 | 0–23.9 | ||
| β-Selinene | 1.1 | |||||
| δ-Cadinene | 3.9 | |||||
| δ-3-Carene | 10.9 | 1.7–13.8 | 0.2-8.0 | 0–23.4 | ||
| δ-Elemene | 2.5 | 1.6 |
| Compound | West Africa [69] | India [68] | Malaysia [67] | China Black [63] | China White [63] | China Green [63] | China Black [34] | China Red [34] | China White [34] | Black Commercial [70] | Green Commercial [70] | Commercial [71] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Elemene | 4.3 | |||||||||||
| Caryophylla-4(12),8(13)-dien-5β-ol | 10.0 | |||||||||||
| Caryophyllene oxide | 3.9 | 1.3–9.3 | 4.1–6.7 | 1.5–4.54 | 4.7–13.5 | 9.0 | ||||||
| β-Selinene | 5.1–8.0 | |||||||||||
| γ-Selinene | 6.2 | 5.6 | ||||||||||
| Isospathulenol | 4.9 | 7.6 | ||||||||||
| Limonene | 18.8 | 15.0 | 7.2–13.3 | 12.9–14.9 | 9.7–11.7 | 19.0 | ||||||
| Linalool | 12.1 | |||||||||||
| p-Cymene | 4.6–5.2 | |||||||||||
| Sabinene | 16.5 | 5.9 | 13.8 | 7.5 | ||||||||
| α-Copaene | 3.7 | 4.2 | ||||||||||
| α-Humulene | 2.6–5.9 | 5.5 | 6.3 | |||||||||
| α-Phellandrene | 0.2–6.2 | |||||||||||
| α-Pinene | 4.5 | 8.0 | 5.2 | 4.3–5.8 | 3.4–5.6 | 10.0 | 5.5 | |||||
| α-Selinene | 5.3 | |||||||||||
| β-Bisabolene | 3.9 | 4.7 | ||||||||||
| β-Caryophyllene | 15.4 | 29.9 | 18.6 | 15.1–16.3 | 8.9–15.9 | 12.9–18.4 | 42.0–51.8 | 55.2 | 58.9 | 30.3 | 26.2 | |
| β-Pinene | 15.4 | 7.9 | 9.7 | 6.6–8.9 | 9.3–11.0 | 7.7–8.2 | 7.4 | 24.0 | ||||
| δ-3-Carene | 4.4 | 8.6 | 12.3–19.4 | 16.0–22.1 | 14.5–18.9 | 5.3 | 19.7 | |||||
| δ-Elemene | 7.3–8.3 | 4.2–5.4 | 8.0–8.4 | 4.1 |
| Compound | Sample 1 (1978) | Sample 2 (1997) | Sample 3 (Indonesian) | Sample 4 (2008 Bangladesh—Leaves and Inflorescence-Rich Spike) | Sample 5 (2011) |
|---|---|---|---|---|---|
| trans-Cadinα-1(6),4-diene | 5.4 | ||||
| (E)-Cinnamyl acetate | 5.9 | ||||
| (E)-Nerolidol | 1.3 | ||||
| (E)-β-Farnesene | 6.8 | ||||
| (Z)-β-Farnesene | 3.7 | 1.1 | |||
| 1-Pentadecene | 7.1 | ||||
| 1-Tridecene | 1.5 | ||||
| 1,8-Cineole | 0.5 | ||||
| 14-Hydroxy-isocaryophyllene | 1.9 | ||||
| 7-epi-α-Selinene | 3.0 | ||||
| 8-Heptadecene | 7.4 | ||||
| Apiole | 3.9 | ||||
| ar-Curcumene | 4.8 | ||||
| Caryophyllene oxide | 3.7 | 1.5 | 5.4 | ||
| Cubebol | 4.0 | ||||
| Cubenol | 1.4 | ||||
| Eugenol | 33.1 | ||||
| Germacrene B | 1.8 | ||||
| Germacrene D | 10.3 | 4.9 | 16.5 | ||
| Globulol | 2.6 | 1.5 | |||
| Heptadecane | 1.3 | 5.7 | 9.6 | 4.7 | |
| Heptadecene | 2.3 | ||||
| Muurola-4(10(14)-dien-1β-ol | 1.7 | ||||
| Myristicin | 3.0 | ||||
| Nonadecane isomers | 5.9 | ||||
| Pentadecane isomers | 1.3 | 19.6 | 6.6 | 15.8 | |
| Selina-3,11-diene + ar-curcumene | 13.8 | ||||
| Selina-4,11-diene | 1.5 | ||||
| Spathulenol | 3.0 | 6.6 | |||
| Tridecane | 4.7 | ||||
| Zingiberene | 5.0 | ||||
| α-Copaene | 1.6 | 2.6 | |||
| α-Humulene | 2.0 | 2.9 | |||
| α-Terpineol + borneol | 2.6 | ||||
| β-Bisabolene | 11.2 | 3.3 | 5.9 | ||
| β-Caryophyllene | 17.0 | 10.2 | 9.3 | ||
| β-Caryophyllene + terpinen-4-ol | 10.0 | ||||
| β-Elemene | 2.4 | ||||
| β-Selinene | 12.4 | 3.9 | |||
| δ-Cadinene | 10.3 |
| Compound | Panama [32,48] | Brazil [32] | Brazil [32] | Brazil [32] | Brazil var latifolium [32] | Brazil [74] |
|---|---|---|---|---|---|---|
| trans-Cadinα-1(6),4-diene | 9.6 | |||||
| (E)-Nerolidol | 5.2 | |||||
| (E)-β-Ocimene | 1.4 | |||||
| (Z)-β-Ocimene | 2.3 | |||||
| 1-epi-Cubenol | 10.4 | |||||
| 10-epi-γ-Eudesmol | 4.4 | |||||
| Bicyclogermacrene | 12.1 | 49.5 | ||||
| Bulnesol | 8.1 | |||||
| Caryophyllene oxide | 10.2 | 5.9 | ||||
| Germacrene A | 3.3 | |||||
| Germacrene D | 5.3 | 9.6 | 72.9 | 3.6 | ||
| Octanal | 5.5 | |||||
| Sabinene | 4.0 | |||||
| Spathulenol | 8.4 | 7.9 | ||||
| α-Cadinol | 5.4 | |||||
| α-Humulene | 1.2 | |||||
| α-Copaene | 7.4 | 5.6 | ||||
| α-Eudesmol | 12.2 | |||||
| α-Muurolene | 4.2 | |||||
| α-Pinene | 4.3 | |||||
| β-Bisabolene | 12.6 | |||||
| β-Caryophyllene | 4.4 | 25.1 | ||||
| β-Pinene | 6.6 | |||||
| γ-Elemene | 6.8 | |||||
| γ-Eudesmol | 14.6 | |||||
| δ-Cadinene | 25.8 | |||||
| δ-Elemene | 3.1 |
| Compounds | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | Sample 8 | Sample 9 (var cernuum) |
|---|---|---|---|---|---|---|---|---|---|
| trans-Dihydroagarofuran | 28.7 | 33.8 | 30.0–36.7 | ||||||
| cis-Dihydroagarofuran | 32.3 | ||||||||
| (Z)-α-Bisabolene | 5.7 | ||||||||
| cis-β-Guaiene | 8.2 | ||||||||
| 10-epi-γ-Eudesmol | 13.5 | 12.2 | |||||||
| 4-epi-cis-Dihydroagarofuran | 10.8 | 11.2–13.4 | |||||||
| Bicyclogermacrene | 21.9 | 25.1 | 19.9 | ||||||
| Camphene | 6.3 | 8.7 | 5.3 | ||||||
| Caryophyllene oxide | 7.7 | 5.1 | |||||||
| Elemol | 5.9–9.2 | 6.7 | |||||||
| epi-Cubebol | 13.1 | ||||||||
| Germacrene D | 6.7 | 9.3 | 12.7 | ||||||
| Spathulenol | 7.2 | 9.6 | 11.5 | ||||||
| Valeranone | 9.1 | ||||||||
| α-Muurolol | 5.8 | ||||||||
| α-Pinene | 7.2 | 10.0 | 11.8 | 11.4 | 2.6–5.4 | 10.2 | |||
| β-Caryophyllene | 20.7 | 22.2 | 8.3 | 16.3 | 6.9 | 5.9–8.7 | |||
| β-Elemene | 7.2 | 11.6 | 30.0 | 10.1 | |||||
| β-Pinene | 6.2 | 7.9 | 7.4 | ||||||
| γ-Eudesmol | 8.3–13.3 | ||||||||
| γ-Muurolene | 7.6 | ||||||||
| τ-Muurolol | 6.2 |
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Nerolidol | 10.2 | 5.7 | ||||||||||
| (Z)-α-Bisabolene | 39.3 | 23.2 | ||||||||||
| (Z)-β-Farnesene | 7.0 | |||||||||||
| (Z)-β-Ocimene | 10.0 | |||||||||||
| Atractylone | 5.1 | |||||||||||
| Bicyclogermacrene | 7.4 | 27.6 | 9.4 | 7.9 | 6.7 | 34.7 | 13.2 | |||||
| Caryophyllene oxide | 6.1 | |||||||||||
| Curzerene | 13.8 | 28.7 | ||||||||||
| Germacrene D | 6.7 | 10.2 | 12.6 | 30.2 | 18.5 | 24.5 | 8.5 | 15.2 | 43.0 | |||
| Hinesol | 6.4 | 6.4 | ||||||||||
| Limonene | 6.4 | 19.4 | ||||||||||
| p-Cymene | 11.7 | 5.1 | ||||||||||
| Spathulenol | 11.8 | 15.0 | 40.6 | 9.3 | 35.2 | |||||||
| α-Cadinol | 12.2 | 7.0 | 5.8 | 6.4 | ||||||||
| α-Eudesmol | 8.0 | |||||||||||
| α-Pinene | 9.7 | |||||||||||
| α-Selinene | 6.1 | 6.9 | ||||||||||
| β-Bisabolene | 8.1 | 5.5 | ||||||||||
| β-Caryophyllene | 11.7 | 15.5 | 7.4 | 5.1 | ||||||||
| β-Elemene | 21.8 | 13.8 | ||||||||||
| β-Pinene | 14.8 | 10.5 | ||||||||||
| β-Selinene | 6.4 | |||||||||||
| δ-Cadinene | 5.4 | 8.5 | ||||||||||
| δ-Elemene | 7.6 |
| Compounds | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil CT 1 [78] | Brazil CT 2 [78] | Brazil | Brazil | Brazil Inflorescence | Brazil Fol Fresh | Brazil Fructus | Brazil Leaves and Branches |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Nerolidol | 5.3–22.4 | 22.4 | 22.1 | 5.0 | |||||||||||
| trans-β-Guaiene | 6.9 | ||||||||||||||
| 1-epi-Cubenol | 24.2 | ||||||||||||||
| allo-Aromadendrene | 7.7 | ||||||||||||||
| Aromadendrene | 15.6 | ||||||||||||||
| Bicyclogermacrene | 7.4 | 7.4 | 13.2 | 5.1 | 6.4 | ||||||||||
| Cadalene | 33.7 | ||||||||||||||
| Caryophyllene oxide | 8.5 | ||||||||||||||
| Dillapiole | 83.1–87.5 | 61.6–69.2 | 85.2–87.8 | ||||||||||||
| Germacrene B | 4.7–6.9 | ||||||||||||||
| Hinesol | 6.4 | ||||||||||||||
| Ishwarane | 10.0 | ||||||||||||||
| Limonene | 0.5 | ||||||||||||||
| Myristicin | 6.2–9.3 | 6.9–11.9 | |||||||||||||
| Selin-11-en-4α-ol | 8.5 | ||||||||||||||
| Viridiflorene | 8.1 | ||||||||||||||
| Viridiflorol | 27.5 | ||||||||||||||
| α-Cadinol | 7.0 | ||||||||||||||
| α-Humulene | 13.3–37.5 | 16.5 | 23.4 | 21.3 | 13.3 | 29.2 | 8.2–13.3 | 13.3–29.2 | |||||||
| α-Pinene | 12.2 | 9.7 | |||||||||||||
| α-Selinene | 8.9–16.6 | 16.6 | 8.9 | 16.6–8.9 | |||||||||||
| β-Caryophyllene | 10.4–19.3 | 8.9 | 15.6 | 7.5 | 8.5 | 17.8 | 12.1 | 19.3 | 12.1–19.3 | ||||||
| β-Pinene | 5.6–7.0 | 7.0 | 13.8 | ||||||||||||
| β-Selinene | 3.7–15.7 | 10.5 | 6.6 | 15.7 | 15.7–3.7 | ||||||||||
| γ-Elemene | 5.4 | ||||||||||||||
| δ-3-Carene | 5.9 | ||||||||||||||
| δ-Cadinene | 45.3 | 6.9 |
| Compounds | Brazil | Brazil | Brazil | Brazil | Brazil [44] | Brazil [44] | Brazil [47] | Brazil [78] | Brazil | Brazil [74] | Cuba | Colombia [79] | Venezuela | Panama [48] | Cuba [80] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E,E)-α-Farnesene | 5.6 | ||||||||||||||
| (E)-Nerolidol | 23.6 | ||||||||||||||
| 14-Hydroxy-α-muurolene | 5.0 | ||||||||||||||
| 9-epi-Caryophyllene | 4.3 | ||||||||||||||
| Bicyclogermacrene | 11.5 | ||||||||||||||
| Camphene | 15.6 | ||||||||||||||
| Caryophyllene oxide | 5.9 | 5.0 | 5.4 | 7.8 | |||||||||||
| Curzerene | 12.9 | 4.9 | |||||||||||||
| Dillapiole | 57.7 | ||||||||||||||
| Elemol | 7.6 | 3.6 | |||||||||||||
| Germacrene B | 6.1 | 4.5 | 5.2 | ||||||||||||
| Germacrene D | 9.7 | 7.1 | |||||||||||||
| Guaiene | 3.4 | ||||||||||||||
| Humulene epoxide II | 3.6 | ||||||||||||||
| Khusimene | 12.1 | ||||||||||||||
| Ledol | 8.8 | ||||||||||||||
| Limonene | 6.9 | 16.3 | |||||||||||||
| Linalool | 32.5 | 9.6 | |||||||||||||
| Myristicin | 2.0 | ||||||||||||||
| p-Cymene | 12.1 | ||||||||||||||
| Piperitone | 10.0 | ||||||||||||||
| Safrole | 91.4 | ||||||||||||||
| Spathulenol | 6.2 | 4.1 | 5.0 | ||||||||||||
| Terpinolene | 7.3 | ||||||||||||||
| Viridiflorol | 6.3 | ||||||||||||||
| α-Cadinol | 6.9 | ||||||||||||||
| α-Copaene | 7.3 | 28.7–36.2 | |||||||||||||
| α-Eudesmol | 8.1 | ||||||||||||||
| α-Guaiene | 11.5 | ||||||||||||||
| α-Humulene | 9.5 | ||||||||||||||
| α-Pinene | 5.2 | 9.0 | 7.1–13.9 | 6.6 | 1.4 | 3.4 | 15.3 | ||||||||
| α-Selinene | 9.0 | ||||||||||||||
| α-Terpinene | 14.4 | ||||||||||||||
| α-Terpineol | 8.5 | ||||||||||||||
| β-Caryophyllene | 5.4 | 10.5 | 5.3 | 22.0 | 6.3 | 5.1 | 6.2 | 4.3 | |||||||
| β-Elemene | 5.1 | 8.1 | |||||||||||||
| β-Eudesmol | 17.5 | ||||||||||||||
| β-Phellandrene | 9.7 | ||||||||||||||
| β-Pinene | 19.7 | 7.5–13.3 | 12.0 | 3.6 | 14.5 | ||||||||||
| β-Selinene | 5.1 | 8.1 | 14.8 | ||||||||||||
| γ-Cadinene | 25.1 | 13.2 | 3.0 | ||||||||||||
| γ-Elemene | 10.9 | 2.8 | |||||||||||||
| γ-Eudesmol | 9.3 | ||||||||||||||
| γ-Muurolene | 4.0 | ||||||||||||||
| γ-Terpinene | 30.4 | ||||||||||||||
| δ-3-Carene | 7.4 | 9.1 | 6.9 | ||||||||||||
| δ-Cadinene | 6.3 | 4.0 |
| Compounds | Nigeria [84] | Nigeria [85] | Nigeria [83] | Cameroon [82] | S. Tomé e Príncipe [69] | Colombia [79] |
|---|---|---|---|---|---|---|
| (E)-Nerolidol | 23.6 | |||||
| (E)-α-Bisabolene | 4.3 | |||||
| β-Bisabolene | 5.4 | |||||
| 1,8-Cineole | 17.2 | |||||
| 3,5-Dimethoxytoluene | 10.9 | |||||
| Bicyclogermacrene | 6.0 | |||||
| Camphene | 4.8 | |||||
| Camphor | 3.2 | |||||
| Caryophyllene oxide | 5.4 | |||||
| Curzerene | 4.9 | |||||
| Dillapiole | 44.8 | |||||
| Germacrene B | 5.7 | 4.5 | ||||
| Ishwarane | 1.4 | |||||
| Limonene | 5.8 | |||||
| Linalool | 6.1 | 41.8 | ||||
| Myrcene | 4.4 | |||||
| Myristicin | 9.8 | |||||
| Sabinene | 3.3 | |||||
| α-Cubebene | 3.4 | |||||
| α-Phellandrene | 8.2 | |||||
| α-Pinene | 5.3 | 13.6 | 5.8 | |||
| β-Caryophyllene | 6.9 | 5.1 | ||||
| β-Elemene | 5.1 | |||||
| β-Pinene | 41.2 | 23.2 | 9.2 | |||
| β-Sesquiphellandrene | 20.9 | |||||
| γ-Terpinene | 5.7 | |||||
| δ-3-Carene | 5.7 |
| Compounds | Brazil [86]: 22 Samples | Brazil [32]: 24 Samples | Brazil [44] | Panama [48] | Colombia [75] |
|---|---|---|---|---|---|
| (E)-Anethole | 0.1–26.4 | 13.6–26.4 | |||
| (E)-Asarone | 0.2–10.8 | 6.4–10.8 | |||
| (E)-β-Ocimene | 0.4–15.2 | 5.2–15.2 | 8.3 | ||
| (Z)-Anethole | 3.0–8.4 | 6.0–8.4 | |||
| (Z)-Asarone | 1.7–8.8 | 8.8–30.4 | |||
| (Z)-β-Ocimene | 0.2–8.6 | 5.2–8.6 | 6.0 | ||
| 2- Methoxy-4,5-(methylenedioxy)propiophenone | 1.1–26.3 | 26.3 | |||
| 3,4-Methylenedoxypropiophenone | 7.3– 40.7 | ||||
| Bicyclogermacrene | 0.2–11.7 | 6.4–11.7 | 4.0 | ||
| Crocatone | 21.9 | ||||
| Elemicin | 0.1–6.5 | 6.5 | |||
| Elemol | 9.7 | ||||
| Exalataxin | 7.9 | 7.9 | |||
| Germacrene D | 0.5–10.4 | 5.5–10.4 | |||
| Isoosmorhizol | 15.8–46.8 | 15.8–46.8 | |||
| Isosafrole | 34.4 | ||||
| Methoxy-4,5-(methylenedioxy)propiophenone isomer 5 | 2.0–21.9 | ||||
| Methyl eugenol | 0.1–5.9 | 5.9 | 5.4 | ||
| Myristicin | 0.2–16.0 | 5.0–16.0 | |||
| Myristicin derivative | 10.7 | ||||
| Nothosmorhizol | 2.9–24.5 | 11.2–24.5 | |||
| p-Menthα-1(7),8-diene | 0.1–39.0 | 5.2–39.0 | |||
| Patchouli alcohol | 16.0 | ||||
| Propriopiperone | 7.3–40.7 | 13.2 | |||
| Safrole | 0.2–63.9 | 5.7–63.9 | 4.6 | ||
| Spathulenol | 0.3–6.6 | 6.6 | |||
| α-Acoradiene | 5.1 | ||||
| α-Copaene | 0.1–11.4 | ||||
| α-Pinene | 0.1–7.3 | 5.0 | |||
| β-Caryophyllene | 1.2–13.6 | 6.0–13.6 | 6.3 | 11.0 | |
| γ-Elemene 8.5% | 5.6–11.4 | ||||
| γ-Terpinene | 0.1–14.4 | 6.5–14.4 | 10.5 | ||
| δ-3-Carene | 11.3 | ||||
| τ-Muurolol | 5.0 | ||||
| α-Phellandrene | 11.0 | ||||
| Limonene | 8.0 | ||||
| β-Elemene | 4.0 |
| Compounds | Brazil (Fruit) [88] | Brazil (Fruit) [38] | Brazil (Stem) [32] | Brazil (Stem) [38] | Brazil (Leaf) [38] | Brazil (Leaf) [32] | Brazil (Leaf) [32] | Brazil (Leaf) [44] | Brazil (Leaf) [78] | Brazil (Leaf) a [32] | Brazil (Floral) [32] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Nerolidol | 12.7 | 6.5 | |||||||||
| (E)-β-Farnesene | 8.3 | 6.1 | |||||||||
| (E)-β-Ocimene | 12.5 | 14.5 | 8.6 | 9.0 | 9.8 | ||||||
| Caryophyllene oxide | 13.3 | 6.4 | |||||||||
| Germacrene D | 5.5 | 8.4 | |||||||||
| Limonene | 3.0 | 2.4 | 2.1 | 4.2 | 1.6 | 6.7 | |||||
| Spathulenol | 15.8 | ||||||||||
| Viridiflorol | 13.5 | ||||||||||
| α-Copaene | 1.3 | 5.4 | |||||||||
| α-Cadinol | 13.7 | ||||||||||
| α-Pinene | 26.5 | 28.7 | 17.3 | 17.3 | 10.4 | 10.4 | 9.4 | 8.4 | 28.7 | ||
| β-Bisabolene | 9.1 | ||||||||||
| β-Caryophyllene | 14.4 | 14.0 | 32.1 | 32.1 | 40.2 | 40.2 | 30.1 | 7.9 | 26.3 | 14.0 | |
| β-Elemene | 1.6 | 3.0 | |||||||||
| β-Pinene | 27.7 | 38.2 | 27.0 | 27.0 | 12.5 | 12.5 | 15.0 | 7.0 | 38.2 | ||
| τ-Cadinol | 6.3 | ||||||||||
| Sabinene | 2.7 | 0.8 |
| Compounds | P. acre Blume [35] | P. acutifolium Ruiz & Pav. [32] | P. acutilimbum C. DC. [90] | P. aequale Vahl [32] | P. aleyreanum C. DC. [32] | P. amplum Kunth [32] | P. augustum Rudge [32] | P. barbatum Kunth [91] | P. bellidifolium Yunck. [90] | P. bisasperatum Trel. [32] | P. bredemeyeri J. Jacq. [32] | P. cachimboense Yunck. [78] | P. caldense C. DC. [32] | P. callosum Ruiz & Pav.a [44] | P. caninum Blume [92] | P. durilignum C. DC. [90] | P. divaricatum G. Mey.b [32] | P. diospyrifolium Kunth [32] | P. demeraranum (Miq.) C. DC. [93] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Nerolidol | 22.7 | 20.3 | 3.9 | 6.2 | 18.2 | ||||||||||||||
| trans-Sesquisabinene hydrate | 8.2–20.9 | ||||||||||||||||||
| (E)-β-Ocimene | 8.1 | 5.8 | |||||||||||||||||
| cis-Eudesmα-6,11-diene | 21.1 | ||||||||||||||||||
| (Z)-β-Ocimene | 10.5 | 3.4 | |||||||||||||||||
| 1-epi-Cubenol | 5.6 | ||||||||||||||||||
| 1,8-Cineole | 3.7 | 8.9–9.6 | |||||||||||||||||
| 2-Undecanone | 2.0 | ||||||||||||||||||
| allo-Aromadendrene | 6.0 | ||||||||||||||||||
| Apiole | 5.7 | ||||||||||||||||||
| Aromadendrene | 13.3 | ||||||||||||||||||
| Benzyl benzoate | 3.5 | ||||||||||||||||||
| Bicyclogermacrene | 9.2 | 14.1 | 8.8 | ||||||||||||||||
| Caryophyllene oxide | 11.5 | 7.7 | |||||||||||||||||
| Cembratrienol 1 | 25.4 | ||||||||||||||||||
| Cembratrienol 2 | 8.6 | ||||||||||||||||||
| Cembrene | 11.7 | ||||||||||||||||||
| Dillapiole | 5.9 | ||||||||||||||||||
| Elemol | 7.2–10.2 | ||||||||||||||||||
| τ-Cadinol | 5.2 | ||||||||||||||||||
| Germacrene A | 13.2 | 13.2 | |||||||||||||||||
| Germacrene B | 6.9 | 6.2–6.7 | |||||||||||||||||
| Germacrene D | 5.5 | 9.5 | 21.7 | 6.3–27.6 | 4.9 | 11.1 | 6.3–6.5 | 5.2 | |||||||||||
| Globulol | 6.6 | ||||||||||||||||||
| Hisenol | 5.7 | ||||||||||||||||||
| Humulene epoxide I | 6.9 | ||||||||||||||||||
| Limonene | 6.7 | 8.6 | 13.0 | 2.8–7.0 | 10.7 | 6.7–8.5 | 19.3 | ||||||||||||
| Linalool | 10.3 | 7.0 | 5.1 | 23.4–29.7 | |||||||||||||||
| Longifolene | 5.4 | ||||||||||||||||||
| Methyeugenol | 7.6 | ||||||||||||||||||
| Sabinene | 19.5 | 18.4 | |||||||||||||||||
| Safrole | 53.8 | 17.1 | |||||||||||||||||
| Selin-11-en-4α-ol | 17.7 | ||||||||||||||||||
| Spathulenol | 6.7 | 4.7 | 25.4 | ||||||||||||||||
| Thujopsan-2β-ol | 7.4 | ||||||||||||||||||
| α-Cadinene | 6.7 | ||||||||||||||||||
| α-Cadinol | 19.0 | ||||||||||||||||||
| α-Copaene | 6.1 | 10.9 | 5.4–47.7 | ||||||||||||||||
| α-Humulene | 5.7 | ||||||||||||||||||
| α-Muurolol | 6.4 | 9.0 | |||||||||||||||||
| α-Phellandrene | 8.6 | 14.7 | 9.8–43.16 | ||||||||||||||||
| α-Pinene | 12.6–39.3 | 7.0 | 18.1 | 6.0–10.5 | 12.2 | 4.0 | 9.0–18.8 | 6.7 | |||||||||||
| β-Caryophyllene | 7.9 | 18.6 | 8.8 | 13.5 | 24.2 | 4.7–7.5 | 6.7 | 9.1 | 7.4–16.8 | 6.0 | |||||||||
| β-Elemene | 16.3 | 12.3 | 46.4 | 34.0 | 2.1 | 33.1 | |||||||||||||
| β-Eudesmol | 7.5 | 3.5–4.6 | |||||||||||||||||
| β-Longipinene | 6.2 | ||||||||||||||||||
| β-Phellandrene | 5.6 | ||||||||||||||||||
| β-Pinene | 15.6 | 14.4 | 7.7 | 8.9 | 5.0 | 19.9–25.3 | 6.7 | ||||||||||||
| γ-Amorphene | 6.6–6.9 | ||||||||||||||||||
| γ-Eudesmol | 7.5 | ||||||||||||||||||
| γ-Gurjunene | 6.9 | ||||||||||||||||||
| γ-Muurolene | 4.0 | 10.6 | |||||||||||||||||
| δ-Cadinene | 12.4 | 6.2 | 6.2–9.2 | ||||||||||||||||
| δ-Elemene | 19.0 | 8.2 | |||||||||||||||||
| β-Selinene | 5.0 |
| Compounds | P. darienense C. DC. [32] | P. crassinervium Kunth [32] | P. curtispicum C. DC. [32] | P. corrugatum Kuntze [32] | P. corcovadensis (Miq.) C. DC. [32] | P. angustifolium Lam. [32] | P. consanguineum (Kunth) Steud. [90] | P. claussenianum (Miq.) C. DC. [32,94] | P. carpunya Ruiz & Pav. [32,95] | P. carniconnectivum C. DC. Brazil [32] | P. lanceifolium Kuntha [79,96] | P. laosanum C. DC. Vietnam [35] | P. jacquemontianum Kunth [48] | P. ilheusense Yunck. [32] | P. humaytanum Yunck. [32] | P. hoffmanseggianum Schult. [74] | P. goesii Yunck. [74] | P. glabratum Kunth [97] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Nerolidol | 8.2 | 12.8 | 5.8 | 6.2 | 24.3–83.3 | 4.6 | 5.3 | |||||||||||
| β-Caryophyllene | 7.8–8.1 | 6.3 | 0.9–1.9 | 5.1–5.8 | 11.8 | 10.1 | 13.3 | 14.6 | ||||||||||
| (E)-β-Farnesene | 63.7 | |||||||||||||||||
| (E,E)-α-Farnesene | 7.2 | 14.1 | ||||||||||||||||
| (Z)-β-Ocimene | 1.1 | |||||||||||||||||
| 1,8-Cineole | 5.9 | 13.0 | ||||||||||||||||
| 10-epi-γ-Eudesmol | 16.0 | |||||||||||||||||
| 4-Butyl-1,2-methylenedioxybenzene | 30.6 | |||||||||||||||||
| Apiole | 9.8–11.7 | |||||||||||||||||
| Bicyclogermacrene | 5.1–9.2 | 6.7 | ||||||||||||||||
| Camphene | 3.4 | |||||||||||||||||
| Carvotacetone acetate | 8.2 | 8.2 | ||||||||||||||||
| Caryophyllene oxide | 13.1 | 21.3 | 3.4–5.9 | 16.6 | ||||||||||||||
| Dillapiole | 9.8–74.6 | |||||||||||||||||
| Elemicin | 16.4–24.4 | |||||||||||||||||
| epi-α-Selinene | 5.0 | |||||||||||||||||
| Germacrene B | 7.2 | |||||||||||||||||
| Germacrene D | 9.2–14.0 | 10.7–42.8 | 4.95 | 28.87 | ||||||||||||||
| Gleenol | 7.5 | |||||||||||||||||
| Globulol | 4.59 | |||||||||||||||||
| Guiaol | 5.5–5.8 | |||||||||||||||||
| Hinesol | 19.3 | |||||||||||||||||
| Isocaryophyllene | 5.2 | |||||||||||||||||
| Limonene | 6.3 | 26.6 | 26.6 | 12.2 | ||||||||||||||
| Linalool | 4.2 | 2.1–53.5 | 5.65 | 14.5 | ||||||||||||||
| Longiborneol | 12.0 | |||||||||||||||||
| Patchouli alcohol | 11.1 | |||||||||||||||||
| p-Cymene | 3.3 | 2.2 | 8.6 | 10.9 | 7.4 | |||||||||||||
| Piperitone | 2.6 | 51.0 | ||||||||||||||||
| Sabinene | 6.0 | |||||||||||||||||
| Safrole | 14.9 | |||||||||||||||||
| Sesquicineole | 5.0 | |||||||||||||||||
| Spathulenol | 9.8 | 23.8 | 9.8 | 5.1 | 6.3 | |||||||||||||
| Terpinolene | 17.4 | |||||||||||||||||
| α- Humulene | 2.5 | 2.6 | 3.3 | |||||||||||||||
| α-Cadinol | 4.9 | |||||||||||||||||
| α-Cubebene | 4.3 | |||||||||||||||||
| α-Curcumene | 12.0 | |||||||||||||||||
| α-Eudesmol | 8.3 | |||||||||||||||||
| α-Muurolol | 5.0 | |||||||||||||||||
| α-Phellandrene | 4.7 | 13.8 | ||||||||||||||||
| α-Pinene | 10.0–11.5 | 12.2 | 5.9 | 5.9 | 3.6 -13.7 | 9.6 | 9.7 | |||||||||||
| α-Selinene | 7.8 | |||||||||||||||||
| α-Terpinene | 7.8 | 12.1 | ||||||||||||||||
| α-Terpineol | 4.7 | |||||||||||||||||
| α-Terpinyl acetate | 4.3 | |||||||||||||||||
| β-Elemene | 2.5 | |||||||||||||||||
| β-Eudesmol | 10.1 | 8.1 | ||||||||||||||||
| β-Oplopenone | 6.0 | |||||||||||||||||
| β-Phellandrene | 8.2 | |||||||||||||||||
| β-Pinene | 11.6–15.2 | 26.6 | 6.3 | 5.4–15.8 | 10.1 | 13.0 | ||||||||||||
| β-Selinene | 0.7 | 4.8–7.8 | 15.8 | |||||||||||||||
| γ-Cadinene | 11.3 | 6.9 | ||||||||||||||||
| γ-Elemene | 0.5 | |||||||||||||||||
| γ-Eudesmol | 18.0 | 8.5 | ||||||||||||||||
| γ-Muurolene | 3.2 | 4.0 | ||||||||||||||||
| γ-Terpinene | 2.9 | 6.9 | ||||||||||||||||
| δ-Cadiene | 6.1 | 3.8 | 6.3 | |||||||||||||||
| δ-Elemene | 3.3 | 5.9 |
| Compounds | P. fulvescens C. DC. [39] | P. friedrichsthalii C. DC. [48] | P. fimbriulatum C. DC. [32,48] | P. obliquum Ruiz & Pav. [48] | P. multiplinervium C. DC. [48] | P. mollicomum Kuntha [74,98] | P. mosenii C. DC. [78,99] | P. longispicum C. DC. [32,48] | P. leptorum Kunth [32] | P. lucaeanum Kunth [32] | P. madeiranum Yunck. [32] | P. vicosanum Yunck. [100] | P. variabile C. DC. [32] | P. tectoniaefolium (Kunth) Kunth ex C. DC. [32] | P. solmsianum C. DC. [32,78] | P. reticulatum L. [32,48] | P. glabrescens (Miq.) C.DC. [32] | P. trigonum C. DC. [48] | P. gaudichaudianum (Kunth) Kunth ex Steud. [77] | P. imperiale (Miq.) C. DC. [32] | P. grande Vahl [48] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Anethole | 26.4 | ||||||||||||||||||||
| (E)-Isoelemecin | 53.5 | ||||||||||||||||||||
| (E)-Nerolidol | 5.5 | 7.5–23.2 | 13.7 | ||||||||||||||||||
| β-Caryophyllene | 3.7 | 4.3 | 11.3 | 27.6 | 8.6–16.8 | 45.2 | 45.2 | 5.0 | 11.2 | 9.2 | 7.1 | 23.4 | 25.5 | ||||||||
| (Z)-β-Farnesene | 5.7 | ||||||||||||||||||||
| 1,10-di-epi-Cubenol | 7.0 | ||||||||||||||||||||
| 1,8-Cineole | 10.4–15.0 | ||||||||||||||||||||
| Selin-11-en-4α-ol | 12.8 | ||||||||||||||||||||
| 2-Tridecanone | 4.3 | ||||||||||||||||||||
| Aromadendrene | 4.2 | ||||||||||||||||||||
| Asaricin | 39.2 | ||||||||||||||||||||
| Bicyclogermacrene | 7.4 | 32.5 | 19.7 | ||||||||||||||||||
| Camphene | 25.3 | 16.6 | |||||||||||||||||||
| Camphor | 39.9 | 28.4 | |||||||||||||||||||
| Caryophyllene oxide | 8.3 | 3.1 | 9.8 -12.1 | 5.5 | 10.9 | ||||||||||||||||
| Dillapiole | 2.8 | 6.7 | |||||||||||||||||||
| epi-α-Bisabolol | 5.4 | ||||||||||||||||||||
| Germacrene A | 8.5 | ||||||||||||||||||||
| Germacrene D | 9.6 | 12.8–32.9 | 3.9 | 3.7 | 3.3 | 19.7 | 5.5 | ||||||||||||||
| Germacrene D-4-ol | 11.1 | ||||||||||||||||||||
| Globulol | 6.1 | ||||||||||||||||||||
| Guaiol | 6.3 | ||||||||||||||||||||
| Humulene epoxide II | 2.3 | 6.3 | |||||||||||||||||||
| Ishawarane | 12.1 | ||||||||||||||||||||
| Limonene | 11.4 | 5.1 | 9.1–45.5 | 13.9 | 56.6 | ||||||||||||||||
| Linalool | 5.3 | 16.5 | |||||||||||||||||||
| Linalyl acetate | 5.3 | ||||||||||||||||||||
| Myrcene | 26.1 | ||||||||||||||||||||
| p-Cymene | 9.4 | 6.3 | 43.9 | ||||||||||||||||||
| Spathulenol | 5.4 | 4.3 | 10.6 | 3.8 | 5.2–7.5 | 6.1 | 11.1 | ||||||||||||||
| Terpinolene | 10.1 | ||||||||||||||||||||
| Viridiflorol | 5.8 | ||||||||||||||||||||
| α-Alaskene | 13.4 | ||||||||||||||||||||
| α-Bisabolol | 7.1 | ||||||||||||||||||||
| α-Bulnesene | 10.8 | ||||||||||||||||||||
| α-Cadinol | 5.8 | ||||||||||||||||||||
| α-Copaene | 3.3 | 5.6 | 3.4 | 3.4 | 6.0 | ||||||||||||||||
| α-Guaiene | 7.6 | ||||||||||||||||||||
| α-Humulene | 4.2 | 11.3 | |||||||||||||||||||
| α-Phellandrene | 11.8 | ||||||||||||||||||||
| α-Pinene | 10.2 | 7.1 | 6.3 | 30.0 | 6.1–7.2 | 12.9 | 7.8–22.7 | 26.0 | 6.3 | ||||||||||||
| α-Selinene | 12.0 | 5.5 | 15.5 | ||||||||||||||||||
| α-Zingiberene | 30.4 | ||||||||||||||||||||
| β-Bisabolene | 4.5 | 8.9 | |||||||||||||||||||
| β-Elemene | 16.1 | 8.4 | 5.2 | ||||||||||||||||||
| β-Pinene | 7.9 | 5.8 | 8.8 | 14.5 | |||||||||||||||||
| β-Selinene | 7.9 | 19.0 | |||||||||||||||||||
| β-Sesquiphellandrene | 11.1 | ||||||||||||||||||||
| γ-Elemene | 14.2 | ||||||||||||||||||||
| γ-Muurulene | 3.7 | ||||||||||||||||||||
| γ-Terpinene | 8.0 | ||||||||||||||||||||
| δ-3-Carene | 23.3–66.9 | ||||||||||||||||||||
| δ-Cadinene | 4.2 | 2.3 | 7.2 | ||||||||||||||||||
| δ-Elemene | 9.4 |
| Compounds | P. aereum Trel. | P. duckei C. DC. [93] | P. friedrichsthalii C.DC. | P. grande Vahl | P. heterophyllum Ruiz & Pav. | P. hostmannianum (Miq.) C.DC. | P. jacquemontianum Kunth | P. malacophyllum (C.Presl) C.DC. | P. mollicomum (Kunth) Kunth ex Steud. | P. nemorense C.DC. | P. ovatum Vahl | P. peltatum L. | P. pseudolindenii C.DC. | P. regnelli (Miq.) C.DC. and P. regnellii (Miq.) C. DC. var. regnellii | P. rivinoides Kunth | P. trigonum C.DC. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| trans-Calamenene | 5.4 | |||||||||||||||
| (E)-Nerolidol | 8.0 | 9.1 | 9.6 | 5.5 | 8.4 | |||||||||||
| trans-p-Menth-2-en-1-ol | 7.0 | |||||||||||||||
| (E)-β-Ocimene | 6.5 | 12.1–14.0 | ||||||||||||||
| cis-p-Menth-2-en-1-ol | 5.1 | |||||||||||||||
| (Z)-α-Bisabolene | 10.9 | |||||||||||||||
| (Z)-β-Ocimene | 12.1–14.1 | |||||||||||||||
| 1,8-Cineole | 5.8 | 39.0 | ||||||||||||||
| Asaricin | 8.8 | 27.4 | ||||||||||||||
| Bicyclogermacrene | 5.2 | 5.4 | 9.7 | 11.8 | ||||||||||||
| Camphene | 20.8–30.8 | |||||||||||||||
| Camphor | 32.8 | |||||||||||||||
| Caryophyllene oxide | 22.9 | |||||||||||||||
| Dehydroaromadendrane | 7.8 | |||||||||||||||
| Dillapiole | 7.7 | |||||||||||||||
| epi-Cubebol | 10.7 | |||||||||||||||
| Germacrene B | 13.4 | 5.4 | 6.7 | |||||||||||||
| Germacrene D | 14.7 | 9.6 | 6.8 | 10.8 | 10.3 | 9.0 | 45.6–51.4 | 19.7 | ||||||||
| Guaiol | 41.2 | |||||||||||||||
| Limonene | 12.2 | 6.3–11.4 | ||||||||||||||
| Linalool | 14.5–69.4 | 16.5 | 15.9 | |||||||||||||
| Myrcene | 15.5–52.6 | |||||||||||||||
| Myristicin | 20.3 | |||||||||||||||
| Myrtenic acid | 7.5 | |||||||||||||||
| o-Cymene | 6.2 | |||||||||||||||
| p-Cymene | 43.9 | 7.4 | 9.0 | |||||||||||||
| Piperitone | 5.6 | |||||||||||||||
| Selin-11-en-4α-ol | 12.8 | |||||||||||||||
| Spathulenol | 9.0 | 7.8 | 5.1 | |||||||||||||
| Terpinolene | 8.2 | |||||||||||||||
| α-Bisabolol | 9.9 | |||||||||||||||
| α-Cadinol | 9.2 | 5.8 | ||||||||||||||
| α-Chamigrene | 8.9–11.3 | |||||||||||||||
| α-Copaene | 5.7 | 5.2 | 6.0 | |||||||||||||
| α-Humulene | 7.0 | 10.0 | ||||||||||||||
| α-Muurolol | 5.8 | |||||||||||||||
| α-Phellandrene | 13.8 | 8.8–11.8 | ||||||||||||||
| α-Pinene | 13.4 | 6.3 | 9.3 | 9.6 | 5.0 | 7.1 | 23.1 | 32.9–73.2 | ||||||||
| α-Selinene | 12.0 | |||||||||||||||
| α-Terpinene | 13.9 | |||||||||||||||
| β-Aromadendrene | 8.3 | |||||||||||||||
| β-Bourbonene | 14.0 | |||||||||||||||
| β-Caryophyllene | 6.6 | 27.1 | 5.6 | 5.3 | 11.8 | 7.2–9.5 | 6.6–7.6 | 7.1 | ||||||||
| β-Copaene | 15.0 | |||||||||||||||
| β-Elemene | 15.0 | 8.4 | ||||||||||||||
| β-Phellandrene | 5.2 | |||||||||||||||
| β-Pinene | 14.5 | 6.2 | 10.1 | 7.9 | 14.2 | 6.7 | 13.3 | 5.2–24.7 | ||||||||
| β-Selinene | 7.9 | 5.9 | ||||||||||||||
| γ-Elemene | 6.8 | |||||||||||||||
| γ-Eudesmol | 17.9 | |||||||||||||||
| γ-Terpinene | 8.0 | 21.5 | ||||||||||||||
| δ-Cadinene | 7.3 | 7.2 |
| Compounds | P. aequale Vahl | P. aleyreanum C. DC. [101] | P. anonifolium (Kunth) Steud. | P. artanthe C.DC. | P. austrosinense Y.Q. Tseng [34] | P. barbatum Kunth | P. bisasperatum Trel. [102] | P. bogotense C.DC. | P. boehmeriifolium (Miq.) Wall. ex C. DC. [34] | P. brachypodon (Benth.) C. DC. | P. bredemeyeri J.Jacq. [75] | P. cryptopodon C. DC. | P. dactylostigmum Yunck. | P. demeraranum (Miq.) C.DC. | P. divaricatum G.Mey. [75] | P. flaviflorum C. DC. [34] | P. hainanense Hemsl. [34] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Asarone | 14.1 | ||||||||||||||||
| trans-Calamene | 6.4 | ||||||||||||||||
| (E)-Nerolidol | 10.4 | 6.6 | 48.4 | ||||||||||||||
| trans-Sabinene hydrate | 14.2 | ||||||||||||||||
| (Z)-Asarone | 4.7 | ||||||||||||||||
| (Z)-Isoelemicin | 6.2 | ||||||||||||||||
| cis-β-Guaiene | 29.3 | ||||||||||||||||
| Cadalene | 4.9 | ||||||||||||||||
| 1,8-Cineole | 18.0 | ||||||||||||||||
| Propiopiperone | 5.6 | ||||||||||||||||
| 2′-Methoxy-4′,5′-methylenedioxypropiophenone | 29.5 | ||||||||||||||||
| Eugenol | 54.3 | ||||||||||||||||
| 9-epi-β-Caryophyllene | 5.8 | ||||||||||||||||
| Apiole | 14.5 | 4.2 | 8.0 | ||||||||||||||
| Asaricin | 4.4 | ||||||||||||||||
| Asarone | 3.2 | ||||||||||||||||
| Bicyclogermacrene | 5.5 | 9.2 | 8.1 | 8.3–23.3 | |||||||||||||
| Bulnesol | 3.6 | ||||||||||||||||
| Camphene | 4,1–5.2 | ||||||||||||||||
| Caryophyllene oxide | 11.5 | 5.8 | 10.8 | 6.0 | 51.8 | ||||||||||||
| Crocatone | 10.9 | ||||||||||||||||
| Cubebol | 7.2 | 6.3 | |||||||||||||||
| epi-Cubebol | 8.9 | ||||||||||||||||
| epi-α-Bisabolol | 26.3 | ||||||||||||||||
| Germacrene B | 10.1–26.8 | ||||||||||||||||
| Germacrene D | 6.9 | 9.6 | 5.9 | 4.0 | 7.5–17.9 | ||||||||||||
| Guaiac alcohol | 4.64 | ||||||||||||||||
| Palmitic acid | 3.5 | ||||||||||||||||
| Ishwarane | 19.1 | ||||||||||||||||
| Limonene | 5.9–8.5 | 5.3 | 4.0 | 20.2–40.3 | |||||||||||||
| Linalool | 15.0 | ||||||||||||||||
| Methyl (E)-cinnamate | 40.7 | ||||||||||||||||
| Myristicin | 6.4 | ||||||||||||||||
| p-Cymene | 3.8 | 6.3 | |||||||||||||||
| Phytol | 4.5 | ||||||||||||||||
| Sabinene | 12.9–22.7 | ||||||||||||||||
| Selin-11-en-4α-ol | 20.0 | ||||||||||||||||
| Spathulenol | 5.2–6.7 | 6.3 | 5.7 | 6.9–8.4 | 4.6 | 53.8 | |||||||||||
| τ-Cadinol | 6.8 | ||||||||||||||||
| α-Bisabolol | 17.6 | ||||||||||||||||
| α-Bulnesene | 5.2 | ||||||||||||||||
| α-Cadinol | 9.5 | 21.7 | |||||||||||||||
| α-Cubebene | 5.1–6.7 | ||||||||||||||||
| α-Eudesmol | 33.5 | ||||||||||||||||
| α-Phellandrene | 13.7 | 6.0 | |||||||||||||||
| α-Pinene | 12.6 | 7.3–53.1 | 8.7 | 20.3 | 7.5 | 7.3 | 11.0 | ||||||||||
| α-Selinene | 11.9 | 8.0 | |||||||||||||||
| β-Atlantol | 5.9 | ||||||||||||||||
| β-Caryophyllene | 6.2–6.6 | 2.5–6.3 | 10.2 | 20.2 | 6.3 | 18.1–34.6 | 8.9 | 8.0 | 5.4 | ||||||||
| β-Elemene | 8.8–16.3 | 4.0 | |||||||||||||||
| β-Pinene | 15.6 | 5.1–9.0 | 17.2–22.9 | 32.3 | 6.0 | 7.7–14.4 | 5.0 | ||||||||||
| β-Selinene | 12.7 | 9.0 | |||||||||||||||
| γ-Muurolene | 4.3 | 5.9 | |||||||||||||||
| δ-Cadinene | 8.1 | 3.8 | |||||||||||||||
| δ-Cadinol | 8.1 -23,1 | ||||||||||||||||
| δ-Elemene | 19.0 | 8.2 | 11.7 | ||||||||||||||
| τ-Muurolol | 7.5 |
| Compounds | P. hancei Maxim. [34] | P. krukaffii Yunck. | P. laetispicum C. DC. [34] | P. manausense Yunck. | P. mikanianum (Kunth) Steud. [103] | P. obliquum Ruiz & Pav. a [36] | P. vitaceum Yunck. | P. wallichii [34] | P. xylosteoides (Miq.) Hand.-Mazz. | P. puberulum (Benth.) Maxim. [34] | P. senporeiense Yamam. [34] | P. septuplinervium (Miq.) C. DC. [104] | P. sarmentosum Roxb. [34] | P. brachypodon Yunck var hirsuticaule | P. obrutum Trel. & Yunck. | P. plurinervosum Yunck. | P. regnellii (Miq.) C.DC. | P. renitens (Miq.) Yunck. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (E)-Asarone | 11.6 | |||||||||||||||||
| (E)-Nerolidol | 20.6 | 8.2–8.5 | 5.8 | 6.4 | 13.7 | |||||||||||||
| trans-β-Guaiene | 7.8 | |||||||||||||||||
| (Z)-Asarone | 11.5 | 5.1 | ||||||||||||||||
| (Z)-Isoelemicin | 3.5 | 21.5 | 4.9 | 10.1 | 4.4 | |||||||||||||
| 1,8-Cineole | 31.6 | |||||||||||||||||
| Propiopiperone | 12.2 | 5.8 | 23.4 | 21.1 | ||||||||||||||
| Apiole | 25.3–34.1 | 64.9 | 2.7 | |||||||||||||||
| Aromadendrene | 5.0 | |||||||||||||||||
| Asaricin | 65.9 | 2.6 | 54.5 | |||||||||||||||
| Asarone | 28.5 | 3.6 | ||||||||||||||||
| Bicyclogermacrene | 7.8–41.0 | 5.3–14.3 | 7.2 | 6.2 | 6.6 | |||||||||||||
| Camphene | 5.6 | |||||||||||||||||
| Caryophyllene oxide | 5.2 | 5.7 | ||||||||||||||||
| Elemicin | 4.1 | 2.6 | 3.2 | |||||||||||||||
| epi-Cubebol | 9.0 | |||||||||||||||||
| Eudesm-7(11)-en-4-ol | 9.3 | |||||||||||||||||
| Germacrene B | 7.8 | 10.6 | ||||||||||||||||
| Germacrene D | 3.5–6.1 | 16.7 | 13.8 | |||||||||||||||
| Germacrene D-4-ol | 5.6 | |||||||||||||||||
| Gleenol | 6.8–9.4 | |||||||||||||||||
| Globulol | 3.8 | 9.4 | 6.1 | |||||||||||||||
| Guaiol | 6.2 | 13.9 | ||||||||||||||||
| Palmitic acid | 2.5 | 8.2 | ||||||||||||||||
| Isocaryophyllene | 6.8 | 2.4 | ||||||||||||||||
| Isospathulenol | 3.1 | |||||||||||||||||
| Limonene | 14.8 | 33.2 | 5.1 | |||||||||||||||
| Linalool | 15.8 | |||||||||||||||||
| Myrcene | 5.6 | 31.0 | ||||||||||||||||
| Myristicin | 26.7–40.6 | |||||||||||||||||
| p-Cymene | 2.6 | 12.8 | 12.4 | |||||||||||||||
| Phytol | 7.4 | 2.9 | ||||||||||||||||
| Safrole | 44.1–82.0 | 45.9 | 47.8–84.1 | |||||||||||||||
| Spathulenol | 7.8 | 15.0 | 18.8 | 12.3 | 7.7 | 11.1 | ||||||||||||
| Terpinolene | 11.5 | |||||||||||||||||
| Valencene | 8.0 | |||||||||||||||||
| Viridiflorol | 7.9 | |||||||||||||||||
| Zingiberene | 9.3 | |||||||||||||||||
| α-Cadinol | 5.1 | 4.4 | 8.5 | |||||||||||||||
| α-Eudesmol | 6.9 | 5.8 | ||||||||||||||||
| α-Guaiene | 5.9 | |||||||||||||||||
| α-Gurjunene | 4.7 | |||||||||||||||||
| α-Humulene | 9.6 | 6.4 | ||||||||||||||||
| α-Muurolol | 7.6 | |||||||||||||||||
| α-Pinene | 5.2–9.1 | 6.5–19.4 | 6.0–15.3 | 12.5 | ||||||||||||||
| α-Selinene | 7.1 | 19.1 | ||||||||||||||||
| α-Terpinene | 6.2 | 11.3 | ||||||||||||||||
| α-Thujene | 6.0 | 7.9 | ||||||||||||||||
| β-Caryophyllene | 8.8 | 5.9–8.5 | 10.5 | 7.0 | 5.0 | 9.8 | 6.6 | 23.4 | 6.9 | |||||||||
| β-Copaen-4α-ol | 9.4 | |||||||||||||||||
| β-Elemene | 1.7–8.2 | 4.6 | 6.4 | 7.6 | ||||||||||||||
| β-Eudesmol | 6.8 | 6.0 | ||||||||||||||||
| β-Phellandrene | 22.6 | |||||||||||||||||
| β-Pinene | 4.7–9.2 | 2.0 | 12.4 | |||||||||||||||
| β-Selinene | 7.99 | 23.8 | ||||||||||||||||
| β-Vetivone | 33.5 | |||||||||||||||||
| γ-Terpinene | 17.1 | 26.1 | ||||||||||||||||
| δ-Cadinene | 5.8–7.0 | 4.7 | 10.9 | |||||||||||||||
| δ-Elemene | 6.6 | |||||||||||||||||
| τ-Muurolol | 0.2–5.7 |
| Extract Type | Bacteria and Fungi | Main Results | References |
|---|---|---|---|
| Seeds: acetone and dichloromethane extracts | Gram positive (Staphylococcus aureus, Bacillus cereus, Staphylococcus faecalis); Gram negative (Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli) | MIC of 50–500 ppm showed excellent inhibition | [254] |
| EO | Vibrio cholerae, Staphylococcus albus, Clostridium diphthereae, Shigella dysenteriae, Streptomyces faecalis, Bacillus spp., Pseudomonas spp., Aspergillus parasiticus | EO inhibited bacterial and fungal growth | [255] |
| Encapsulated EO | Staphylococcus aureus and Escherichia coli | Antibacterial activity was four times greater with encapsulation when compared to free EO | [256] |
| Leaf: ethanol & methanol extract | Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, and Pseudomonas aeruginosa and fungi (Aspergillus spp. and Candida albicans) | Minimum bactericidal and fungicidal concentrations were between 12.5–50.0 mg/mL for all tested strains | [257] |
| EO | Four strains of E. coli isolated from pork | MIC was found to be 1.0 μL/mL. The diameter of inhibition zone values was with range from 17.12 to 26.13 mm diameter. EO treatment also caused the physical and morphological alterations in the cell wall and membrane of Escherichia coli. | [262] |
| Seeds: chloroform extract | Escherichia coli and Staphylococcus aureus | The extract increased pyruvic acid concentration in bacterial solutions and reduced the ATP level in bacterial cells. The extract destroyed the permeability of the cell membrane, which consequently caused metabolic dysfunction, inhibited energy synthesis, and triggered cell death. | [263] |
| Callus, shoots, and seeds: ethanol, hexane, and chloroform, extract | Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Candida albicans | The zone of inhibition for these tested strains ranged from 16–26 mm in diameter. | [264] |
| Seeds: petroleum ether extract | Listeria monocytogenes and Salmonella typhimurium | The MICs of extract against Listeria monocytogenes and Salmonella typhimurium were 0.625 and 1.25 mg/mL, respectively. Results also showed that the extract could destroy cell wall integrity, alter the permeability of the cell membrane, and inhibit the activity of intracellular enzymes, which would make the extract bactericidal. | [265] |
| Piper Species | Tested Product | Parasite Target | References |
|---|---|---|---|
| Piper aduncum L. | Extract | Leishmania amazonensis | [271] |
| Essential oil | Leishmania braziliensis | [275] | |
| Essential oil | Trypanosoma cruzi | [45] | |
| Essential oil | Plasmodium falciparum, Trypanosoma cruzi, Trypanosoma brucei, Leishmania amazonensis, Leishmania infantum | [41] | |
| Piper aduncum var. ossanum C.DC. | Essential oil | Plasmodium falciparum, Trypanosoma cruzi, Trypanosoma brucei Leishmania amazonensis, Leishmania infantum | [276] |
| Piper amalago L. | Extract | Leishmania amazonensis | [277] |
| Piper auritum Kunth | Essential oil | L. major, L. mexicana, Leishmania braziliensis and L. donovani | [76] |
| Extract | Leishmania spp. | [278] | |
| Piper betle L. | Extract | G. lamblia | [269] |
| Extract | L. donovani | [279] | |
| Extract | L. donovani | [280] | |
| Extract | Brugia malayi | [281] | |
| Extract | P. berghei | [282] | |
| Extract and fractions | L. donovani | [283] | |
| Extract | Toxoplasma gondii | [284] | |
| Extract | Neospora caninum | [270] | |
| Piper capense L.f. | Extract | Plasmodium falciparum | [268] |
| Piper chaba Hunt | Extract | Schistosoma mansoni | [285] |
| Extract | Plasmodium falciparum | [286] | |
| Piper claussenianum (Miq.) C. DC. | Essential oil | Leishmania amazonensis | [94] |
| Piper cubeba L.f. | Essential oil | Trypanosoma cruzi | [287] |
| Piper cumanense Kunth | Extract | Plasmodium falciparum | [170] |
| Piper demeraranum (Miq.) C. DC. | Essential oil | Leishmania amazonensis and L. guyanensis | [93] |
| Piper dennisii Trel | Extract | Leishmania amazonensis | [112] |
| Piper duckei C. DC. | Essential oil | Leishmania amazonensis and L. guyanensis | [93] |
| Piper friedrichsthalii C. DC. | Extract | P. berghei | [288] |
| Piper hispidum Sw. | Extract | Plasmodium falciparum | [289] |
| Extract | Leishmania amazonensis | [193] | |
| Essential oil | Leishmania amazonensis | [274] | |
| P. heptaphyllum (Aubl.) Marchand | Essential oil | Leishmania amazonensis | [274] |
| Piper jericoense Trel. & Yunck. | Extract and fractions | Trypanosoma cruzi | [290] |
| Extract and fractions | Trypanosoma cruzi | [291] | |
| Piper laevicarpum Yunck. | Extract | Trypanosoma cruzi | [292] |
| Piper longum L. | Ayurvedic herbal medicine preparation | Giardia lamblia | [293] |
| Extract | Giardia lamblia | [294] | |
| Extract | Blastocystis hominis | [269] | |
| Extract | Leishmania donovani | [295] | |
| Extract | Echinococcus granulosus | [296] | |
| Piper loretoanum Trel. | Extract | Leishmania amazonensis | [237] |
| Piper malacophyllum (C. Presl) C. DC. | Essential oil | Trypanosoma cruzi and Leishmania infantum | [297] |
| Piper nigrum L. | Fractions | Leishmania donovani | [298] |
| Extract | Plasmodium falciparum | [272] | |
| Piper sanguineispicum Trel. | Extract | Leishmania amazonensis | [237] |
| Piper strigosum Trel. | Extract | Leishmania amazonensis | [193] |
| Piper tuberculatum Jacq. | Extract | Rhipicephalus (Boophilus) microplus | [299] |
| Extract | Haemonchus contortus and Strongyloides venezuelensis | [300] | |
| Essential oil | Leishmania braziliensis, Leishmania infantum and Trypanosoma cruzi | [88] | |
| Piper umbellatum L. | Extract | Onchocerca ochengi and Loa loa | [149] |
| Piper Species Extract | Design/Model | Key Effects | References |
|---|---|---|---|
| Piper longumL. Hexane, Benzene, Acetone, Ethyl Acetate, Ethyl Alcohol, Chloroform, And Aqueous Extracts | in-vitro studies Du-145, A549, Thp-1, Igr-Ovi-1 Ovary and MCF-7 cells in-vivo studies Metal-induced Wistar rats | -growth inhibition for all extracts in THP-1 (76–90%) -growth inhibitory effect of hexane and benzene extracts (>80%) on all cell lines. -increased cell cycle inhibition (41, 63 and 43%, respectively) and sub-G1 DNA fraction population for hexane, benzene and acetone extracts in A549 cell line. -protective effect of aqueous, chloroform and ethyl alcohol extracts on liver (65, 71 and 64%), respectively against peroxidative damage. | [309] |
| Piper longumL. Ethanol Extract | in-vitro studies G-361, HT-29 And HCT116, OVCAR-3, BXPC-3 cells in-vivo studies Immunocompromised mice | -selectively induced cell death in cancer cells-colon (HCT116), pancreatic (BxPC-3), leukemia (T cell)-but was not effective for normal colon epithelial cells. -the growth of colon cancer tumors in ethanolic extract treated ımmunocompromised mice group of animal models was suppressed without any toxic effect. | [310] |
| Piper umbellatumL. Dichloromethane Extract | in-vitro studies UACC-62, -U251, MCF-7, NCI-H460, PC-3, NCI-ADR/RES, and OVCAR-3 cells in-vivo studies Carrageenan-induced paw edema and peritonitis models Balb/C mice | -total growth inhibition in several different human tumor cell lines. -the sizes of Ehrlich solid tumor Balb/C mice were reduced 38.7 and 52.2% in 200 and 400 mg/kg extract treatment groups, respectively, without toxicity. | [311] |
| Piper tuberculatumJacq. Crude Extract and Piplartine | in-vitro studies SF-295 and HCT-8 cells in-vivo studies Bal/C (Nu/Nu) mice | -cytotoxic activity in SF-295 cells (10 μg/mL) and in HCT-8 cell line (4.3 μg/mL). -inhibition of cell proliferation (24.6–54.8%) in Ehrlich solid tumor Balb/C (Nu/Nu) mice for (100–200 mg/kg/day) crude extract. | [10] |
| Piper nigrumL. Ethanolic Extract | in-vitro studies MCF-7 and HT-29 cells in-vivo studies Ehrlich carcinoma mice | -EC50 in MCF-7: 27.1±2.0 µg/mL and in HT-29: 80.5±6.6 µg/mL. -In Ehrlich carcinoma model of mice 60% decrease of tumor growth and %76 increase of survival time -increased rate of apoptosis. -increasing expression levels of Bax and p53 protein inhibition of Bcl-xL and cyclin A expressions. | [312] |
| Piper nigrumL. Supercritical Fluid Extraction SFE200 Extract/Piperine Higher Piperine Content | in-vitro studies MCF-7 cells in-vivo studies Ehrlich carcinoma mice | -higher cytotoxic effect in MCF-7 cells than conventional extract. -more significant tumor growth inhibition and increased survival time as compared to conventional extract treatment group of the mice. -decreased cell number founding at S phase. | [313] |
| Piper nigrumL. Piperine-free P. nigrum Extract (PFPE) | in-vitro studies Several cell line including MCF-7 in-vivo studies N-nitroso-N-methylurea–induced mammary tumorigenesis sprague-dawley rats | -higher cytotoxic effect of PFPE on MCF-7 breast cancer cell line (IC50:7.45 µg/mL), together with better selectivity. -PFPE induced apoptosis in MCF-7 cells in dose dependent manner. -upregulation of p53 and cyt C and downregulation of topoisomerase II. -100 mg/kg PFPE treatment result in decrease of tumor growth in rats with mammary tumorigenesis. | [314] |
| Piper Species Extract | Design/Model | Key Effects | References |
|---|---|---|---|
| Piper betle L. Hydroalcoholic Extract | in-vivo studies Carrageenan-induced paw edema (Wistar rats) Cotton pellet-induced granuloma (Albino mice) | -significant analgesic property in both animal groups. -decreasing in the pain by both central and peripheral mechanisms. -inhibition of the growth of paw at 50, 100, 200 mg/kg treatment groups in dose dependent manner. -reduction in the dry weights of granuloma. | [349] |
| Piper crocatum Ruiz & Pav. 96% Ethanol Extract | in-vitro studies LPS-induced RAW 264.7 Cells | -cytotoxic effect of extract in 150 μg/mL or higher concentration treatment group. -significant decrease in TNF-α level at 50 μg/mL treated cell group. -reduction in IL-1β level at 10, 50 and 75 ug/mL treatment groups. -significant reduction in IL-6 and NO levels at 50 μg/mL treatment group. | [348] |
| Piper nigrum L. Ethanol, Hexane Extracts | in-vivo studies Carrageenan-induced Paw edema model Swiss albino mice | -reduction of edema growth in 5–10 mg/kg of hexane extract treatment groups. -good inhibition profile for edema growth at 10 mg/kg of ethanol extract treatment group. | [346] |
| Piper nigrum L. Ethanol Extract | in-vivo studies OVA-induced allergic asthma model BALB/c mice | -decreasing in number of inflammation related cells. -regulation of balance of T cells responses by decreasing IL-1β, IL-4, IL-17A, and TNF-α cytokine levels. | [347] |
| Piper umbellatum L. Standardized Dichloromethane Extract | in-vivo studies Carrageenan-induced paw edema and peritonitis in Balb/C mice | -inhibitory effect on paw edema at 48 h without toxicity. | [311] |
| Piperaceae species Ethanolic-Crude Extract | in-vitro studies LPS-induced PBMC | -differential inhibitory effect on proinflammatory cytokines. | [350] |
| Piper Species Extract | Design/Model | Key Effects | References |
|---|---|---|---|
| Piper betle L. Aqueous and Ethanol Extracts | in-vitro studies SH-SY5Y cells | -inhibitory activity for both acetyl- and butyrylcholinesterase. -no effect of ethanolic extract on the mitochondrial function or the membrane integrity between 7.8–1000 µg/mL concentrations. -cell viability reduction about 20% by 125 µg/mL of aqueous extract. | [363] |
| Piper nigrum L. Methanol Extract | in-vivo studies AlCl3-induced AD model Sprague-Dawley rats | -marked improvement in the biochemical parameters (Ach, CRP, NF-ĸB, MCP-1) in brain of AD rats. | [374] |
| Piper nigrum L. Methanol Extract | in-vivo studies Aβ (1-42)-induced AD model Wistar rats | -significantly improved memory performance and exhibited remarkable antioxidant potential. | [375] |
| Piper nigrum L. Methanol Extract | in-vivo studies Aβ (1-42)-induced AD model Wistar rats | -significantly increased the swimming time. -increase in the number of open-arm entries. | [365] |
| Piper sarmentosumRoxb. Hexane, Dichloromethane, Ethyl acetate, and Methanol Extracts | in-vitro studies Aβ-induced BV-2 cells Aβ-induced microglia-mediated neurotoxicity in SH-SY5Y cells | -reduction in the secretion levels of Aβ-induced pro-inflammatory cytokines (IL-1β and TNF-α) by downregulating the mRNA expressions of pro-inflammatory cytokines in BV-2 cells with ethyl acetate and methanol extract. -protection of SH-SY5Y cells against microglia-mediated neurotoxicity through downregulation of phosphorylated tau proteins with ethyl acetate and methanol extract. | [364] |
| Piper sarmentosumRoxb. Ethanol Extract | in-vivo studies CUMS-treated ICR mice | -reduced immobility time in the forced swimming test and tail suspension test. -no influence on the locomotor activity in the open field test -up-regulation of BDNF protein levels. -increased CREB and ERK phosphorylation levels in the hippocampus on CUMS rats. | [373] |
| Design/Model | Key Effects | References |
|---|---|---|
| in-vivo studies 6-OHDA-induced PD Model of Wistar Rats | -reduction of neuronal cell apoptosis at a remarkable rate through inhibition of poly (ADP-ribose) polymerase activation and pro-apoptotic Bax levels as well as through the elevation of Bcl-2 levels. -decreasing in cytochrome-c release from mitochondria. -reduction in caspase-3 and caspase-9 activation. -reduction in lipid peroxidation and stimulated glutathione levels in striatum of rats. -depletion of inflammatory markers, TNF-α and IL-1β in 6-OHDA-induced PD model of rats. | [366] |
| in-vivo studies CUMS-treated mice | -significant reduction of immobility time in both forced swim and tail suspension tests. -significant amelioration on behavioural deficits of CUMS-treated mice. -significantly increased BDNF protein expression in the hippocampus and frontal cortex of both naive and CUMS-treated mice. | [380] |
| in-vivo studies Corticosterone-induced Depression Model in ICR mice | -suppression of corticosterone induced-depression like behavior in mice. -significant decrease in sucrose consumption. -increasing BDNF expression. | [380] |
| in-vivo studies MPTP-induced PD Model in C57BL/6 Mice | -neuroprotective effects against MPTP-induced mouse model of PD. -decreasing MPTP-induced deficits in motor coordination and cognitive functioning. -prevention MPTP-induced decreases in the number of tyrosine hydroxylase-positive cells in the substantia nigra. -reduction the number of activated microglia. -inhibition of expression of cytokine IL-1β and oxidative stress. -maintaining the balance of Bcl-2/Bax. | [381] |
| in-vitro studies Rotenone-induced SK-N-SH and Primary Rat Cortical Neuron Cells in-vivo studies Rotenone-induced PD Model in C57BL mice | -increasing cell viability and restored mitochondrial functioning (in-vitro studies). -reduction of rotenone-induced motor deficits of PD mice models. -recovery the loss of dopaminergic neurons in the substantia nigra of PD mice models. -increasing in rate of autophagy by inhibiting mTORC1 and activation of PP2A in PD mice model. | [386] |
| in-vivo studies Rotenone-induced PD Model in Wistar Rats | -abrogation of apoptosis. -stimulation of autophagy likely mitigating neuronal injury in the rotenone-induced rat model of PD. | [387] |
| in-vitro studies LPS-induced BV2 Microglia | -inhibition in LPS-induced TNF-α, IL-6, IL-1β, and PGE 2 production. -inhibition of LPS-induced NF-κB and activation of Nrf2. | [388] |
| in-vivo studies ICV-STZ-induced AD Model in Wistar rats | -cognitive enhancing effect. -hippocampal malonaldehyde decrement and keeping redox balance. | [420] |
| in-vivo studies ICV- colchicine induced AD Model in Wistar rats | Piperine-loaded oral microemulsion: -associated with increased delivery to brain. -increased efficacy. -better therapeutic outcome. | [393] |
| in-vivo studies NMRI mice | Piperine-loadedchitosan-sodium tripolyphosphate nanoparticles: -associated with enhanced neuroprotection. -amelioration of the astrocytes activation. | [394] |
| Design | Treatment | Patients (n) | Key Effects | References |
|---|---|---|---|---|
| Clinical trial randomized double-blind placebo-controlled | Kava Extract (3 X 100 mg)/day Duration: 4 Weeks | n = 58 | -Hamilton-Anxiety-Scale (HAMA) Score significantly reduced in drug receiving group compared to placebo group. -No adverse effects. | [367] |
| Clinical trial randomized double-blind placebo-controlled | Kava Extract (3 X 110mg)/day Duration: 25 Weeks | n = 101 | -Significant superiority of kava extract in Hamilton-Anxiety-Scale (HAMA) Score over the placebo starting from week 8. -Rare and evenly distributed adverse effects. | [368] |
| Clinical trial randomized double-blind placebo-controlled | Kava Extract (280 mg)/day Duration: 4 Weeks | n = 13 | -Significantly improved baroreflex control of heart rare following treatment with placebo. -No effect on respiratory sinus arrhythmia. | [407] |
| Clinical trial randomized open study | Kava Extract (100 and 200 mg)/day Duration: 3 Months | n = 80 | -In kava treatment groups anxiety climacteric score declined at 1.and 3. Months. -Depression reduced at month 3. -In control group no significant result. | [413] |
| Clinical trial double-blind crossover trial placebo-controlled | Kava Extract 5 tablets/day Containing 3.2 g extract (delivering 50 mg kavalactones) Duration: 1 month | n = 60 | -Kava resulted in 11.4 points reduction on HAMA Score over placebo. -Antidepressant effect. -No serious adverse effect. -No clinical hepatotoxicity. | [416] |
| Clinical trial randomized double-blind placebo-controlled | Kava Extract1 or 2 tablet/day (120 or 240 mg) kavalactone Duration: 6 Weeks | n = 75 | -Significant increase in female’s sexual drive in Kava group compared to placebo (assessed by ASEX). -Significant reduction of anxiety in Kava group compared to placebo (assessed by HAMA). -No significant differences across groups for liver function test. -No differences in withdraw or addiction. | [417,418] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. https://doi.org/10.3390/molecules24071364
Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, Khan T, Sharifi-Rad J, Ozleyen A, Turkdonmez E, et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules. 2019; 24(7):1364. https://doi.org/10.3390/molecules24071364
Chicago/Turabian StyleSalehi, Bahare, Zainul Amiruddin Zakaria, Rabin Gyawali, Salam A. Ibrahim, Jovana Rajkovic, Zabta Khan Shinwari, Tariq Khan, Javad Sharifi-Rad, Adem Ozleyen, Elif Turkdonmez, and et al. 2019. "Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications" Molecules 24, no. 7: 1364. https://doi.org/10.3390/molecules24071364