Profiling and Structural Characterization of High Neu5Gc or Sulfate-Containing O-glycans from Hyla Rabbit Intestinal Mucin
Abstract
:1. Introduction
2. Results
2.1. Purification and Chemical Analysis of Hyla Rabbit Intestinal Mucins
2.2. Structural Analysis of O-glycan by LC-MS/MS
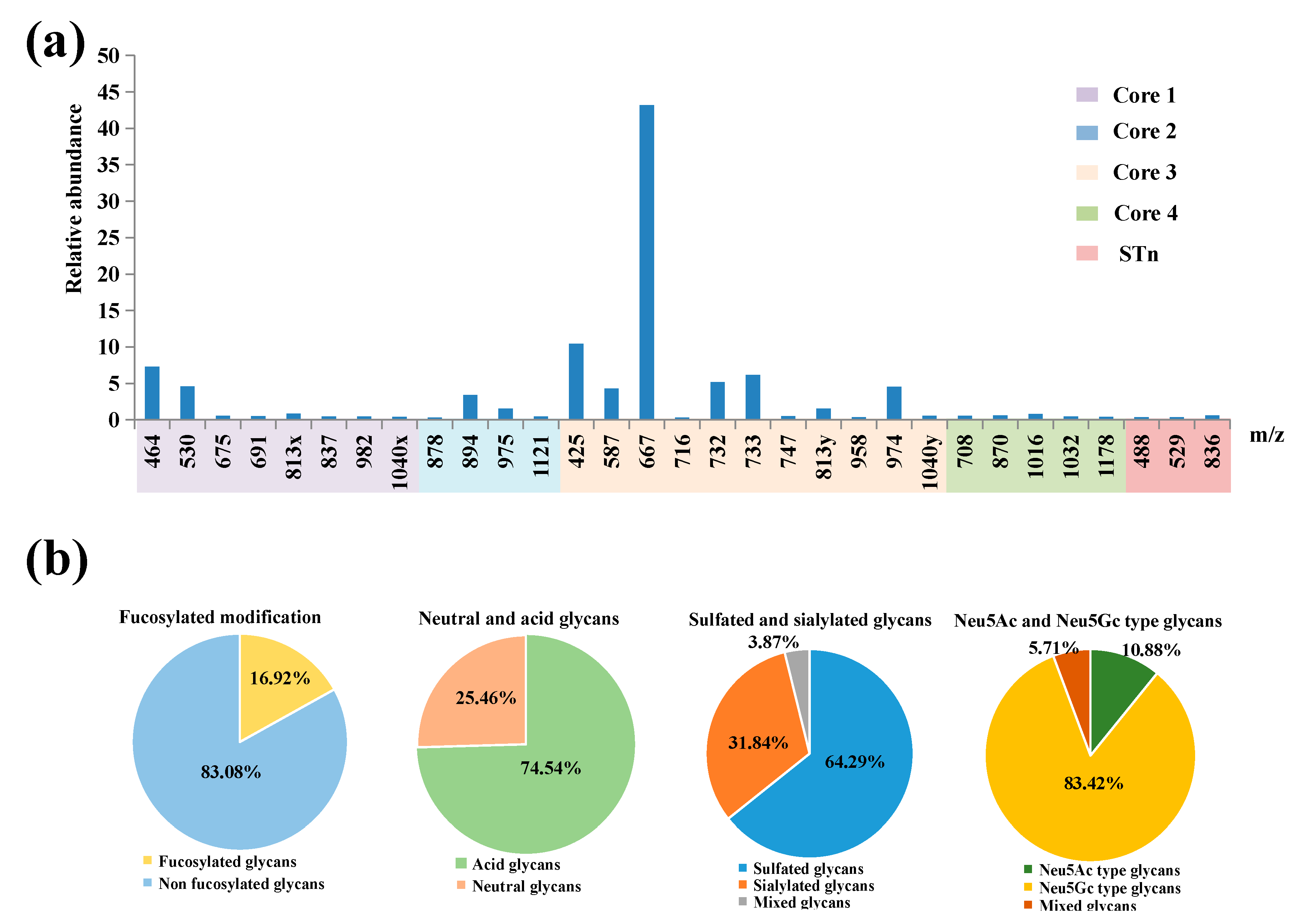
2.2.1. LC−MS/MS Revealed Overall Structural Characteristics
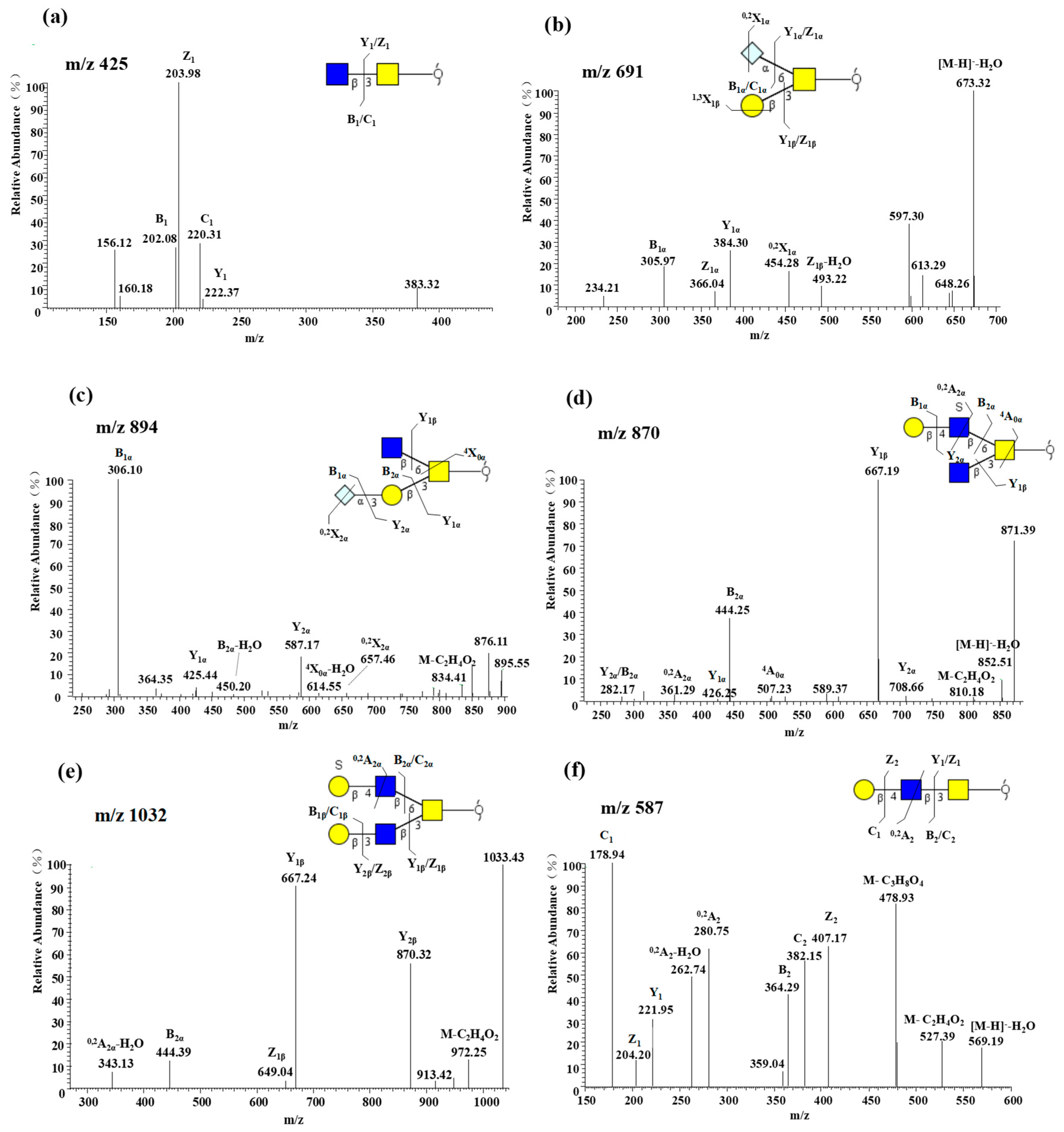
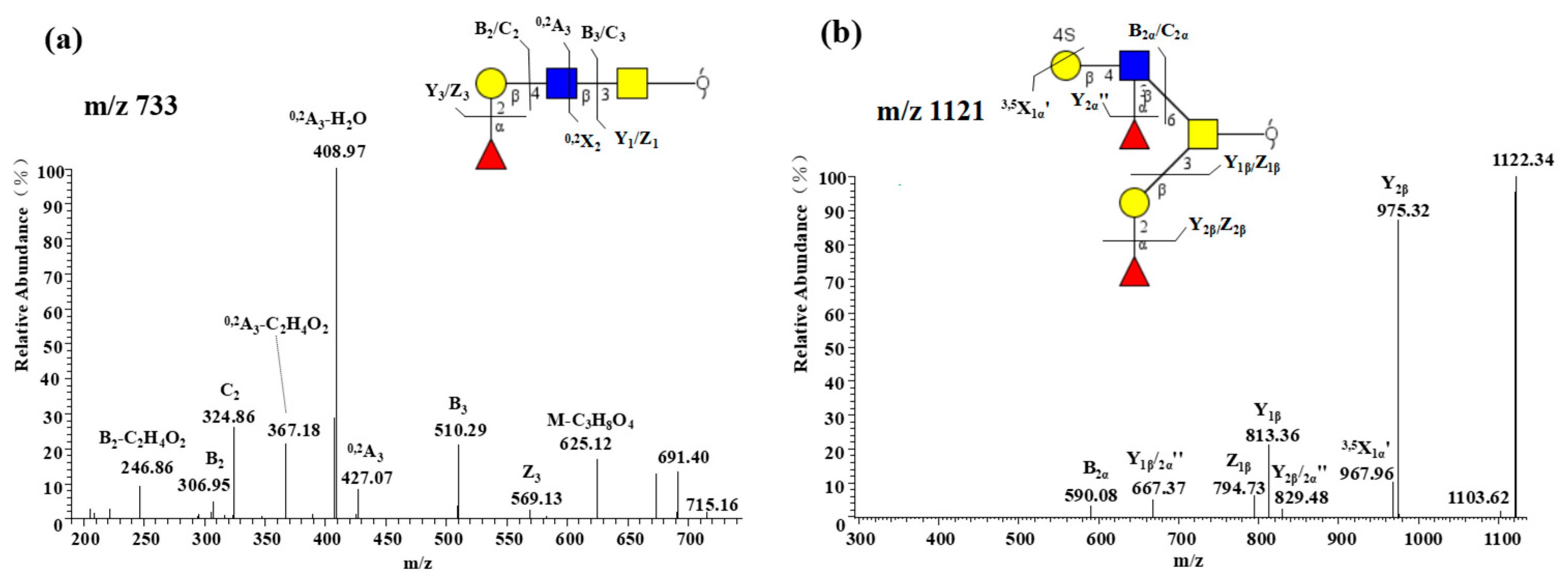
2.2.2. Core 3 Extended with Type II LacNAc Is the Main O-glycan Type
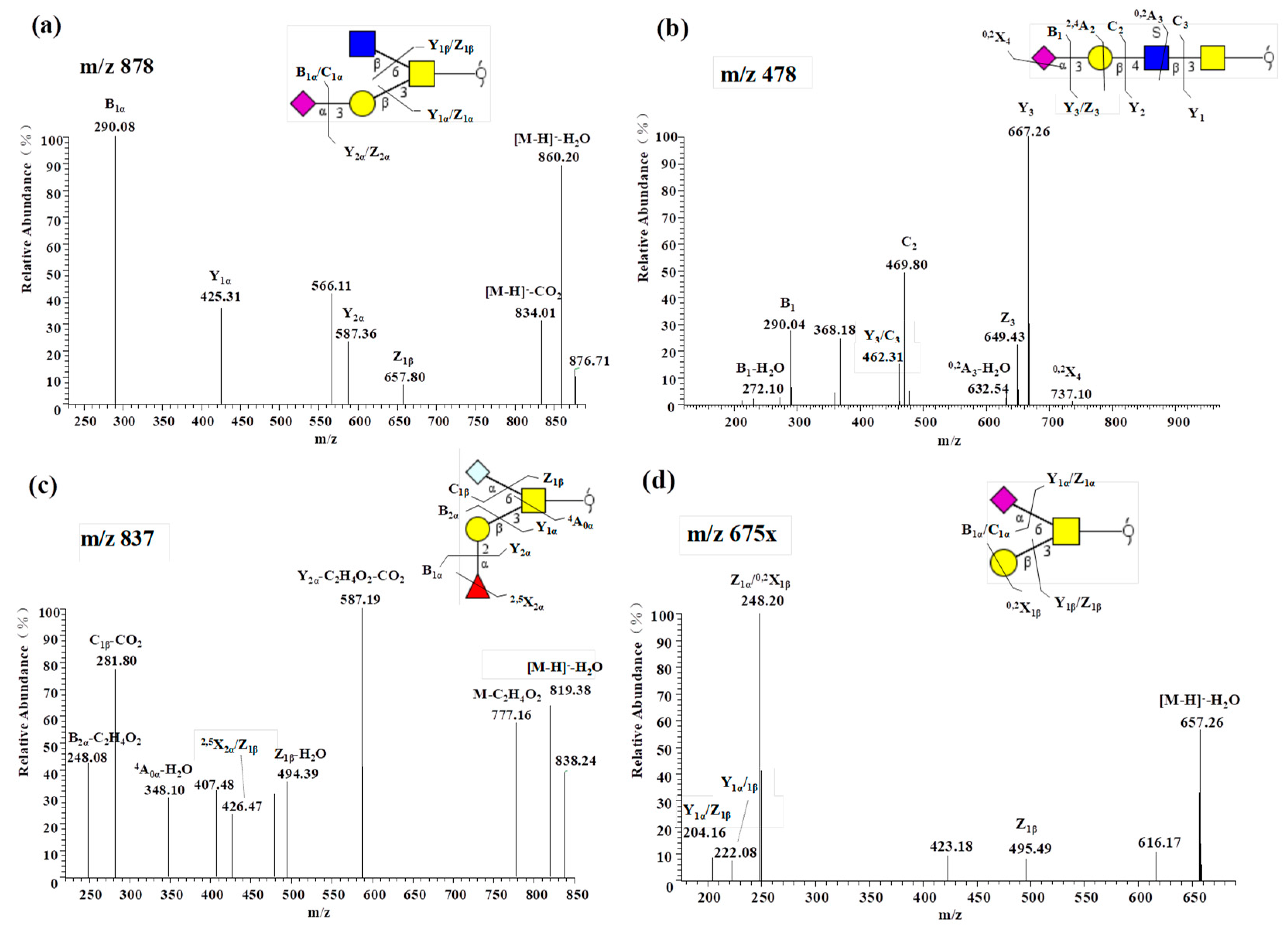
2.2.3. Notable Feature of High Neu5Gc-containing O-glycans
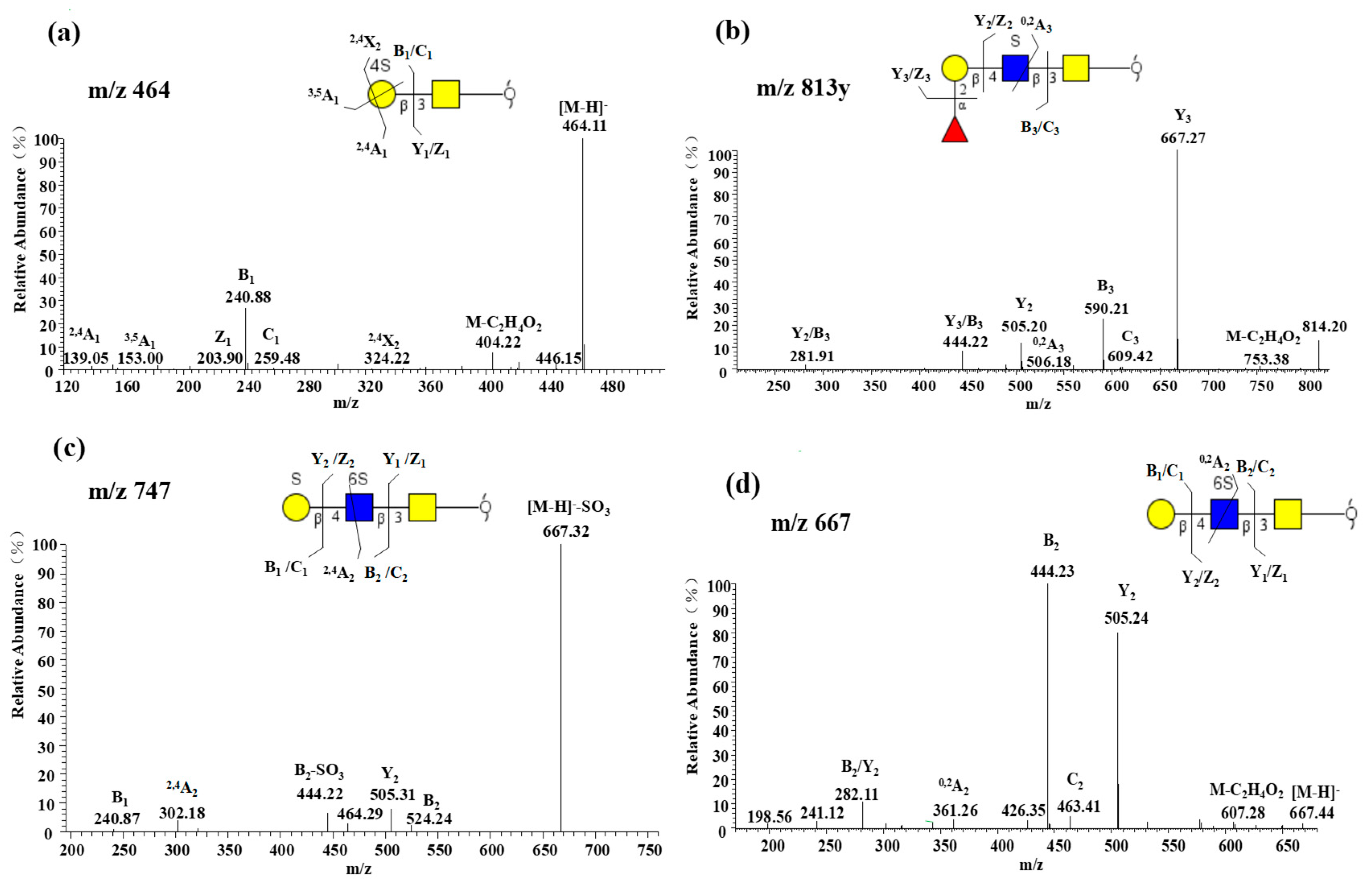
2.2.4. Characterization of Highly Sulfated O-glycans
2.2.5. Presence of α1-2 Fucose-containing O-glycans
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Purification and Chemical Compositions of Rabbit Intestinal Mucin-Type Glycopeptides
4.3. O-Linked Glycans Released from RIF6
4.4. Analysis of O-glycans Released from RIF6 by Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
4.5. O-glycan Structural Annotation and Assignments
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mccracken, V.J.; Lorenz, R.G. The gastrointestinal ecosystem: A precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 2001, 3, 1–11. [Google Scholar] [CrossRef]
- Gum, J.R., Jr.; Hicks, J.W.; Gillespie, A.; Carlson, E.J.; Kömüves, L.; Karnik, S.; Hong, J.C.; Epstein, C.J.; Kim, Y.S. Goblet cell-specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G666–G676. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; Mcguckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183. [Google Scholar] [CrossRef]
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1999, 1473, 67–95. [Google Scholar] [CrossRef]
- An, H.J.; Ninonuevo, M.; Aguilan, J.; Liu, H.; Lebrilla, C.B.; Alvarenga, L.S.; Mannis, M.J. Glycomics analyses of tear fluid for the diagnostic detection of ocular rosacea. J. Proteome Res. 2005, 4, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Furr, A.E.; Ranganathan, S.; Finn, O.J. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr. Dev. Pathol. 2010, 13, 24–31. [Google Scholar] [CrossRef]
- Kirkham, S.; Kolsum, U.; Rousseau, K.; Singh, D.; Vestbo, J.; Thornton, D.J. MUC5B Is the Major Mucin in the Gel Phase of Sputum in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care 2008, 178, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Royle, L.; Matthews, E.; Corfield, A.; Berry, M.; Rudd, P.M.; Dwek, R.A.; Carrington, S.D. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj. J. 2008, 25, 763. [Google Scholar] [CrossRef] [PubMed]
- Seipert, R.R.; Barboza, M.; Niñonuevo, M.R.; Locascio, R.G.; Mills, D.A.; Freeman, S.L.; German, J.B.; Lebrilla, C.B. Analysis and quantitation of fructooligosaccharides using matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2008, 80, 159–165. [Google Scholar] [CrossRef]
- Xia, B.; Royall, J.A.; Damera, G.; Sachdev, G.P.; Cummings, R.D. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology 2005, 15, 747–775. [Google Scholar] [CrossRef]
- Smith, R.F.; Stern, B.H.; Smith, A.A. Mucin immunohistochemistry in the diagnosis and mapping of extramammary Paget’s disease. J. Cell. Mol. Med. 2008, 12, 1605–1610. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- Pacheco, A.R.; Curtis, M.M.; Ritchie, J.M.; Munera, D.; Waldor, M.K.; Moreira, C.G.; Sperandio, V. Fucose sensing regulates bacterial intestinal colonization. Nature 2012, 492, 113–117. [Google Scholar] [CrossRef]
- Carabaño, R.; Badiola, I.; Chamorro, S.; García, J.; García-Ruiz, A.I.; García-Rebollar, P.; Gómez-Conde, M.S.; Gutiérrez, I.; Nicodemus, N.; Villamide, M.J.; et al. New trends in rabbit feeding: Influence of nutrition on intestinal health. A Review. Span. J. Agric. Res. 2008, 6, 15. [Google Scholar] [CrossRef]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Schulz, B.L.; Packer, N.H. Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MSn. J. Am. Soc. Mass Spectr. 2004, 15, 659–672. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Karlsson, H.; Hansson, G.C. Sulphated mucin oligosaccharides from porcine small intestine analysed by four-sector tandem mass spectrometry. J. Mass Spectrom. 1996, 31, 560–572. [Google Scholar] [CrossRef]
- Everest-Dass, A.V.; Abrahams, J.L.; Kolarich, D.; Packer, N.H.; Campbell, M.P. Structural Feature Ions for Distinguishing N- and O-Linked Glycan Isomers by LC-ESI-IT MS/MS. J. Am. Soc. Mass Spectr. 2013, 24, 895–906. [Google Scholar] [CrossRef]
- Robbe, C.; Capon, C.; Coddeville, B.; Michalski, J.C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004, 384, 307–316. [Google Scholar] [CrossRef]
- Robbe, C.; Capon, C.; Coddeville, B.; Michalski, J. Diagnostic ions for the rapid analysis by nano-electrospray ionization quadrupole time-of-flight mass spectrometry of O-glycans from human mucins. Rapid Commun. Mass Spectrom. 2004, 18, 412–420. [Google Scholar] [CrossRef]
- Thomsson, K.A.; Karlsson, H.; Hansson, G.C. Sequencing of Sulfated Oligosaccharides from Mucins by Liquid Chromatography and Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2000, 72, 4543–4549. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Robbe, C.; Michalski, J.; Capon, C. Structural determination of O-glycans by tandem mass spectrometry. In Glycobiology Protocols; Springer: Berlin, Germany, 2006; pp. 109–123. [Google Scholar]
- An, G.; Wei, B.; Xia, B.; Mcdaniel, J.M.; Ju, T.; Cummings, R.D.; Braun, J.; Xia, L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 2007, 204, 1417–1429. [Google Scholar] [CrossRef]
- Struwe, W.B.; Gough, R.; Gallagher, M.E.; Kenny, D.T.; Carrington, S.D.; Karlsson, N.G.; Rudd, P.M. Identification of O-glycan structures from chicken intestinal mucins provides insight into Campylobactor jejuni pathogenicity. Mol. Cell. Proteom. 2015, 14, 1464–1477. [Google Scholar] [CrossRef]
- Venkatakrishnan, V.; Quintana-Hayashi, M.P.; Mahu, M.; Haesebrouck, F.; Pasmans, F.; Lindén, S.K. Brachyspira hyodysenteriae Infection Regulates Mucin Glycosylation Synthesis Inducing an Increased Expression of Core-2 O-Glycans in Porcine Colon. J. Proteome Res. 2017, 16, 1728–1742. [Google Scholar] [CrossRef]
- Holmén Larsson, J.M.; Thomsson, K.A.; Rodríguez-Piñeiro, A.M.; Karlsson, H.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G357–G363. [Google Scholar] [CrossRef]
- Jin, C.; Padra, J.T.; Sundell, K.; Sundh, H.; Karlsson, N.G.; Lindén, S.K. Atlantic Salmon Carries a Range of Novel O-Glycan Structures Differentially Localized on Skin and Intestinal Mucins. J. Proteome Res. 2015, 14, 3239–3251. [Google Scholar] [CrossRef]
- Robinson, L.S.; Lewis, W.G.; Lewis, A.L. The sialate O-acetylesterase EstA from gut Bacteroidetes species enables sialidase-mediated cross-species foraging of 9-O-acetylated sialoglycans. J. Biol. Chem. 2017, 292, 11861–11872. [Google Scholar] [CrossRef]
- Varki, A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am. J. Phys. Anthropol. 2001, 116, 54–69. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef]
- Ito, T.; Suzuki, Y.; Suzuki, T.; Takada, A.; Horimoto, T.; Wells, K.; Kida, H.; Otsuki, K.; Kiso, M.; Ishida, H. Recognition of N-glycolylneuraminic acid linked to galactose by the α2, 3 linkage is associated with intestinal replication of influenza A virus in ducks. J. Virol. 2000, 74, 9300–9305. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Olson, F.J.; Jovall, P.A.; Andersch, Y.; Enerback, L.; Hansson, G.C. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem. J. 2000, 350 Pt 3, 805–814. [Google Scholar] [CrossRef]
- Malykh, Y.N.; Schauer, R.; Shaw, L. N-Glycolylneuraminic acid in human tumours. Biochimie 2001, 83, 623–634. [Google Scholar] [CrossRef]
- Schauer, R.; Srinivasan, G.V.; Coddeville, B.; Zanetta, J.P.; Guérardel, Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr. Res. 2009, 344, 1494–1500. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Yu, H.; Cao, H.; Chokhawala, H.; Karp, F.; Varki, N.; Chen, X.; Varki, A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology 2008, 18, 818–830. [Google Scholar] [CrossRef]
- Mawhinney, T.P.; Adelstein, E.; Gayer, D.A.; Landrum, D.C.; Barbero, G.J. Structural analysis of monosulfated side-chain oligosaccharides isolated from human tracheobronchial mucous glycoproteins. Carbohydr. Res. 1992, 223, 187–207. [Google Scholar] [CrossRef]
- Thomsson, K.A.; Holmén-Larsson, J.M.; ångström, J.; Johansson, M.E.; Xia, L.; Hansson, G.C. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology 2012, 22, 1128–1139. [Google Scholar] [CrossRef]
- Brockhausen, I. Sulphotransferases acting on mucin-type oligosaccharides. Biochem. Soc. Trans. 2003, 318–325. [Google Scholar] [CrossRef]
- Tobisawa, Y.; Imai, Y.; Fukuda, M.; Kawashima, H. Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice. J. Biol. Chem. 2010, 285, 6750–6760. [Google Scholar] [CrossRef]
- Uchimura, K.; El-Fasakhany, F.M.; Hori, M.; Hemmerich, S.; Blink, S.E.; Kansas, G.S.; Kanamori, A.; Kumamoto, K.; Kannagi, R.; Muramatsu, T. Specificities of N-Acetylglucosamine-6-O-sulfotransferases in Relation to L-selectin Ligand Synthesis and Tumor-associated Enzyme Expression. J. Biol. Chem. 2002, 277, 3979–3984. [Google Scholar] [CrossRef]
- Berry, M.; Harris, A.; Lumb, R.; Powell, K. Commensal ocular bacteria degrade mucins. Br. J. Ophthalmol. 2002, 86, 1412–1416. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.; van de Bovenkamp, J.H.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-Pathogen Interactions in Campylobacter Infections: The Host Perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef]
- Naughton, J.A.; Mariño, K.; Dolan, B.; Reid, C.; Gough, R.; Gallagher, M.E.; Kilcoyne, M.; Gerlach, J.Q.; Joshi, L.; Rudd, P.; et al. Divergent Mechanisms of Interaction of Helicobacter pylori and Campylobacter jejuni with Mucus and Mucins. Infect. Immun. 2013, 81, 2838–2850. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Yin, L.A.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Xue, C.; Dong, P.; Sheng, W.; Yu, G.; Chai, W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj. J. 2008, 25, 481–492. [Google Scholar] [CrossRef]
- Stanton, P.G.; Shen, Z.; Kecorius, E.A.; Burgon, P.G.; Robertson, D.M.; Hearn, M.T.W. Application of a sensitive HPLC-based fluorometric assay to determine the sialic acid content of human gonadotropin isoforms. J. Biochem. Biophys. Methods 1995, 30, 37–48. [Google Scholar] [CrossRef]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |
| Core 1 | |||||
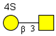 |  |  |  |  | |
| m/z 464 | m/z 530 | m/z 675x | m/z 675y | m/z 691 | |
 | 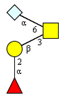 | 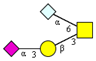 |  | ||
| m/z 813x | m/z 837 | m/z 982 | m/z 1040 x | ||
| Core 2 | |||||
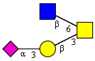 | 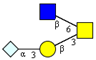 | 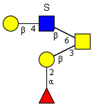 | 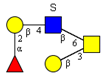 | 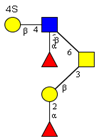 | |
| m/z 878 | m/z 894 | m/z 975x | m/z 975y | m/z 1121 | |
| Core 3 | |||||
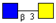 |  |  |  | 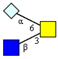 | 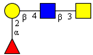 |
| m/z 425 | m/z 587 | m/z 667 (333) | m/z 716 | m/z 732 | m/z 733 |
 |  |  | 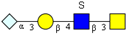 |  | |
| m/z 747 (373) | m/z 813y | m/z 958 (478) | m/z 974 | m/z 1040 y (519) | |
| Core 4 | |||||
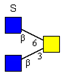 | 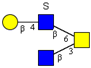 | 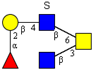 | 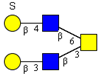 | 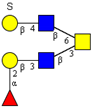 | |
| m/z 708 | m/z 870 | m/z 1016 | m/z 1032 | m/z 1178 | |
| Other special structures | |||||
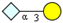 | 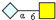 |  | |||
| m/z 488 (peeling) | m/z 529 (STn*) | m/z 836 (STn*) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Li, G.; Wang, C.; Wang, Y.; Li, Q.; Hao, J.; Yu, G. Profiling and Structural Characterization of High Neu5Gc or Sulfate-Containing O-glycans from Hyla Rabbit Intestinal Mucin. Molecules 2019, 24, 1365. https://doi.org/10.3390/molecules24071365
Fu Q, Li G, Wang C, Wang Y, Li Q, Hao J, Yu G. Profiling and Structural Characterization of High Neu5Gc or Sulfate-Containing O-glycans from Hyla Rabbit Intestinal Mucin. Molecules. 2019; 24(7):1365. https://doi.org/10.3390/molecules24071365
Chicago/Turabian StyleFu, Qianyun, Guoyun Li, Chen Wang, Ya Wang, Qinying Li, Jiejie Hao, and Guangli Yu. 2019. "Profiling and Structural Characterization of High Neu5Gc or Sulfate-Containing O-glycans from Hyla Rabbit Intestinal Mucin" Molecules 24, no. 7: 1365. https://doi.org/10.3390/molecules24071365






