HSP70 Acetylation Prevents Combined mTORC1/2 Inhibitor and Curcumin Treatment-Induced Apoptosis
Abstract
:1. Introduction
2. Results
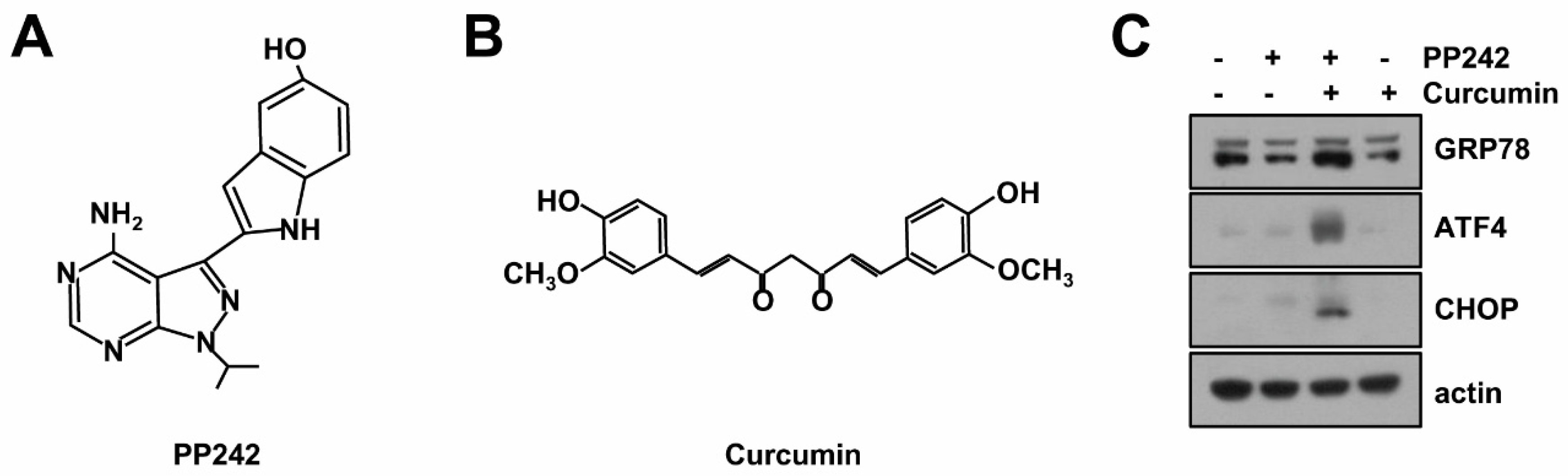
2.1. Combined mTORC1/2 Inhibitor and Curcumin Treatment Induces Endoplasmic Reticulum (ER) Stress
2.2. Combined Treatment Increases Cytosolic Ca2+ Levels
2.3. Combined PP242 and Curcumin-Induced ER Stress Does Not Affect Cell Death
2.4. HSP70 Acetylation Inhibits PP242 Plus Curcumin-Induced Apoptosis
3. Discussion
4. Material and Methods
4.1. Cell Culture and Materials
4.2. Western Blot Analysis and Flow Cytometry Analysis
4.3. Intracellular Ca2+ Detection and Measurement of LMP
4.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CHX | Cycloheximide |
| ROS | Reactive Oxygen Species |
| ER | Endoplasmic Reticulum |
| UPR | Unfolded Protein Response |
| CHOP | CCAAT-Enhancer-Binding Protein Homologous Protein |
| ATF4 | Aactivating Transcription Factor 4 |
| PARP | Poly (ADP-ribose) Polymerase |
References
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Z.; Chen, M.; Chen, X.; Liang, W. The anti-osteosarcoma cell activity by a mTORC1/2 dual inhibitor RES-529. Biochem. Biophys. Res. Commun. 2018, 497, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, B.; Zhang, Y.; Li, K.; Hao, K.; Jiang, L.; Wang, Y.; Mou, X.; Xu, X.; Wang, Z. The anti-hepatocellular carcinoma cell activity by a novel mTOR kinase inhibitor CZ415. Biochem. Biophys. Res. Commun. 2017, 487, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Benavides, A.; Shi, Y.; Yang, Y.; Frost, P.; Gera, J.; Lichtenstein, A. The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J. Biol. Chem. 2012, 287, 21796–21805. [Google Scholar] [PubMed]
- Soares, H.P.; Ni, Y.; Kisfalvi, K.; Sinnett-Smith, J.; Rozengurt, E. Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS ONE 2013, 8, e57289. [Google Scholar] [CrossRef] [PubMed]
- Blaser, B.; Waselle, L.; Dormond-Meuwly, A.; Dufour, M.; Roulin, D.; Demartines, N.; Dormond, O. Antitumor activities of ATP-competitive inhibitors of mTOR in colon cancer cells. BMC Cancer 2012, 12, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Woo, S.M.; Lee, H.S.; Kim, S.H.; Min, K.J.; Kwon, T.K. mTORC1/2 inhibitor and curcumin induce apoptosis through lysosomal membrane permeabilization-mediated autophagy. Oncogene 2018, 37, 5205–5220. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.R. Curcumin and dietary polyphenol research: Beyond drug discovery. Acta Pharmacol. Sin. 2018, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, H.; Yoon, H. ER Stress-Mediated Signaling: Action Potential and Ca2+ as Key Players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E. The HSP70 family and cancer. Carcinogenesis 2013, 34, 1181–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankiewicz, A.R.; Lachapelle, G.; Foo, C.P.; Radicioni, S.M.; Mosser, D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005, 280, 38729–38739. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Srinivasula, S.M.; Balkir, L.; Robbins, P.D.; Alnemri, E.S. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2000, 2, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Nylandsted, J.; Gyrd-Hansen, M.; Danielewicz, A.; Fehrenbacher, N.; Lademann, U.; Hoyer-Hansen, M.; Weber, E.; Multhoff, G.; Rohde, M.; Jaattela, M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 2004, 200, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Lee, H.; Jung, B.H. Metabolite identification and pharmacokinetic profiling of PP242, an ATP-competitive inhibitor of mTOR using ultra high-performance liquid chromatography and mass spectrometry. J. Chromatogr. B 2018, 1072, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V.; Reddy, B.S. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.; Zhao, S.; Xiang, R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016, 6, 27486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Park, J.H.; Lee, E.J.; Vo, T.T.; Choi, H.; Kim, J.Y.; Jang, J.K.; Wee, H.J.; Lee, H.S.; Jang, S.H.; et al. ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation. Nat. Commun. 2016, 7, 12882–12894. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Zotano, A.; Mayer, I.A.; Arteaga, C.L. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016, 35, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The unfolded protein response as a target for anticancer therapeutics. Crit. Rev. Oncol. Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Min, K.J.; Woo, S.M.; Choe, M.; Choi, K.S.; Lee, Y.K.; Yoon, G.; Kwon, T.K. Inhibition of Cathepsin S Induces Mitochondrial ROS That Sensitizes TRAIL-Mediated Apoptosis through p53-Mediated Downregulation of Bcl-2 and c-FLIP. Antioxid. Redox Signal. 2017, 27, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohfeld, J.; Cyr, D.M.; Patterson, C. From the cradle to the grave: Molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001, 2, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Ruckova, E.; Halada, P.; Coates, P.J.; Hrstka, R.; Lane, D.P.; Vojtesek, B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene 2013, 32, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Seo, J.H.; Park, J.H.; Lee, H.S.; Kim, K.W. Hsp70 acetylation prevents caspase-dependent/independent apoptosis and autophagic cell death in cancer cells. Int. J. Oncol. 2017, 51, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kwon, Y.J.; Chun, Y.J. CYP1B1 Activates Wnt/β-Catenin Signaling through Suppression of Herc5-Mediated ISGylation for Protein Degradation on β-Catenin in HeLa Cells. Toxicol. Res. 2017, 33, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.; Shin, D.Y. Repression of the F-box protein Skp2 is essential for actin damage-induced tetraploid G1 arrest. BMB Rep. 2017, 50, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.U.; Min, K.J.; Woo, S.M.; Kwon, T.K. Z-FL-COCHO, a cathepsin S inhibitor, enhances oxaliplatin-mediated apoptosis through the induction of endoplasmic reticulum stress. Exp. Mol. Med. 2018, 50, 107–117. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.U.; Min, K.-j.; Woo, S.M.; Seo, J.H.; Kwon, T.K. HSP70 Acetylation Prevents Combined mTORC1/2 Inhibitor and Curcumin Treatment-Induced Apoptosis. Molecules 2018, 23, 2755. https://doi.org/10.3390/molecules23112755
Seo SU, Min K-j, Woo SM, Seo JH, Kwon TK. HSP70 Acetylation Prevents Combined mTORC1/2 Inhibitor and Curcumin Treatment-Induced Apoptosis. Molecules. 2018; 23(11):2755. https://doi.org/10.3390/molecules23112755
Chicago/Turabian StyleSeo, Seung Un, Kyoung-jin Min, Seon Min Woo, Ji Hae Seo, and Taeg Kyu Kwon. 2018. "HSP70 Acetylation Prevents Combined mTORC1/2 Inhibitor and Curcumin Treatment-Induced Apoptosis" Molecules 23, no. 11: 2755. https://doi.org/10.3390/molecules23112755





