Synergistic Effects of Resveratrol and Pyrimethanil against Botrytis cinerea on Grape
Abstract
:1. Introduction
2. Results
2.1. Sensitivity of B. cinerea Isolates to Pyrimethanil and Cyprodinil
2.2. Determination of MICs and Interaction Analysis
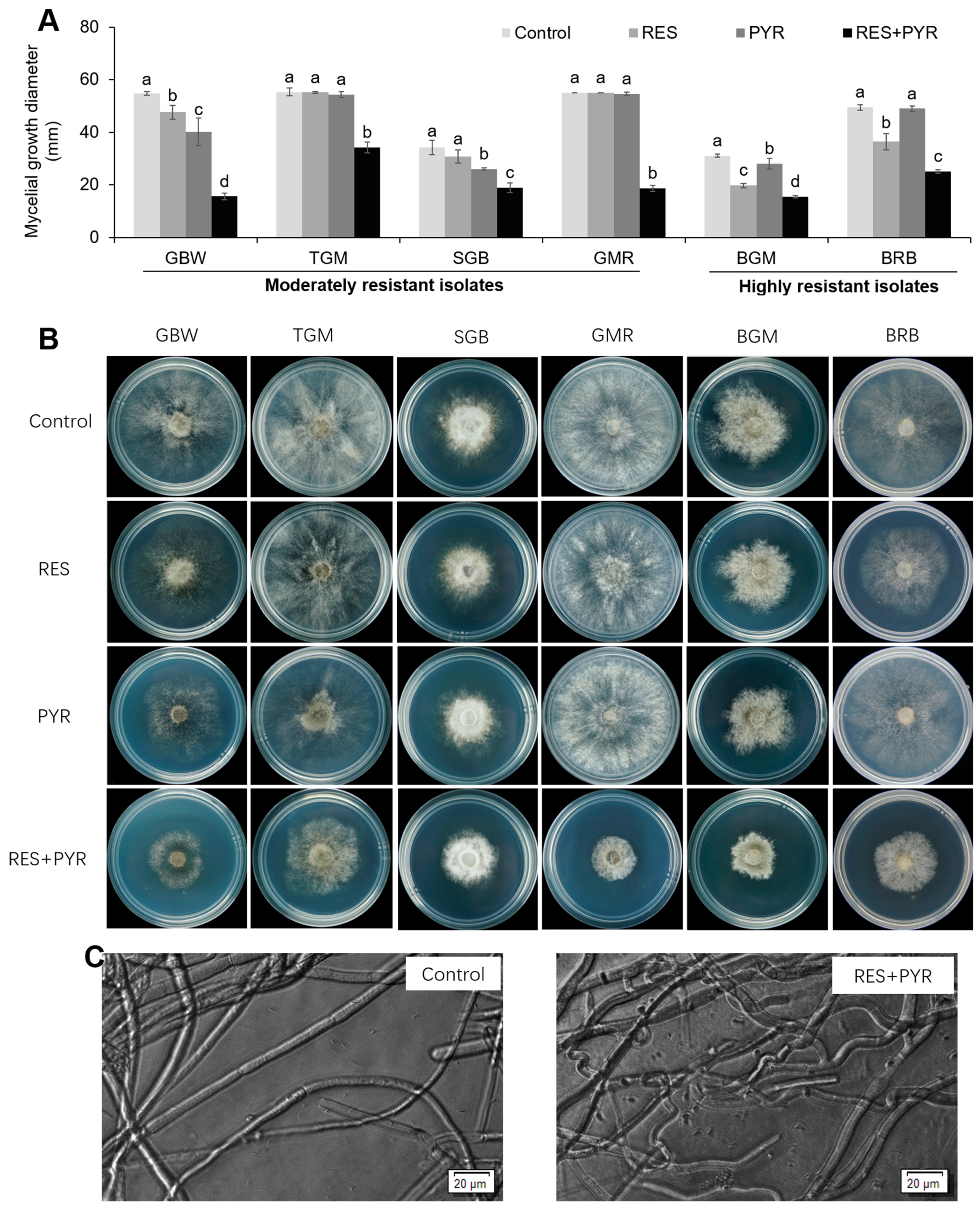
2.3. Synergistic Effect of Resveratrol and Pyrimethanil on Mycelial Growth
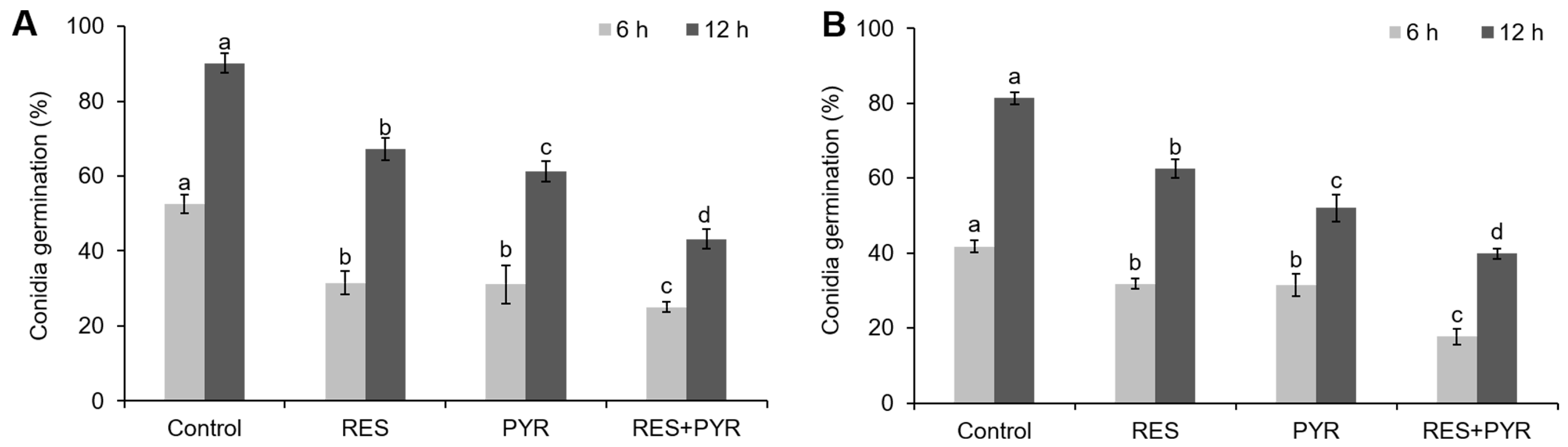
2.4. Synergistic Effect of Resveratrol and Pyrimethanil on Conidia Germination
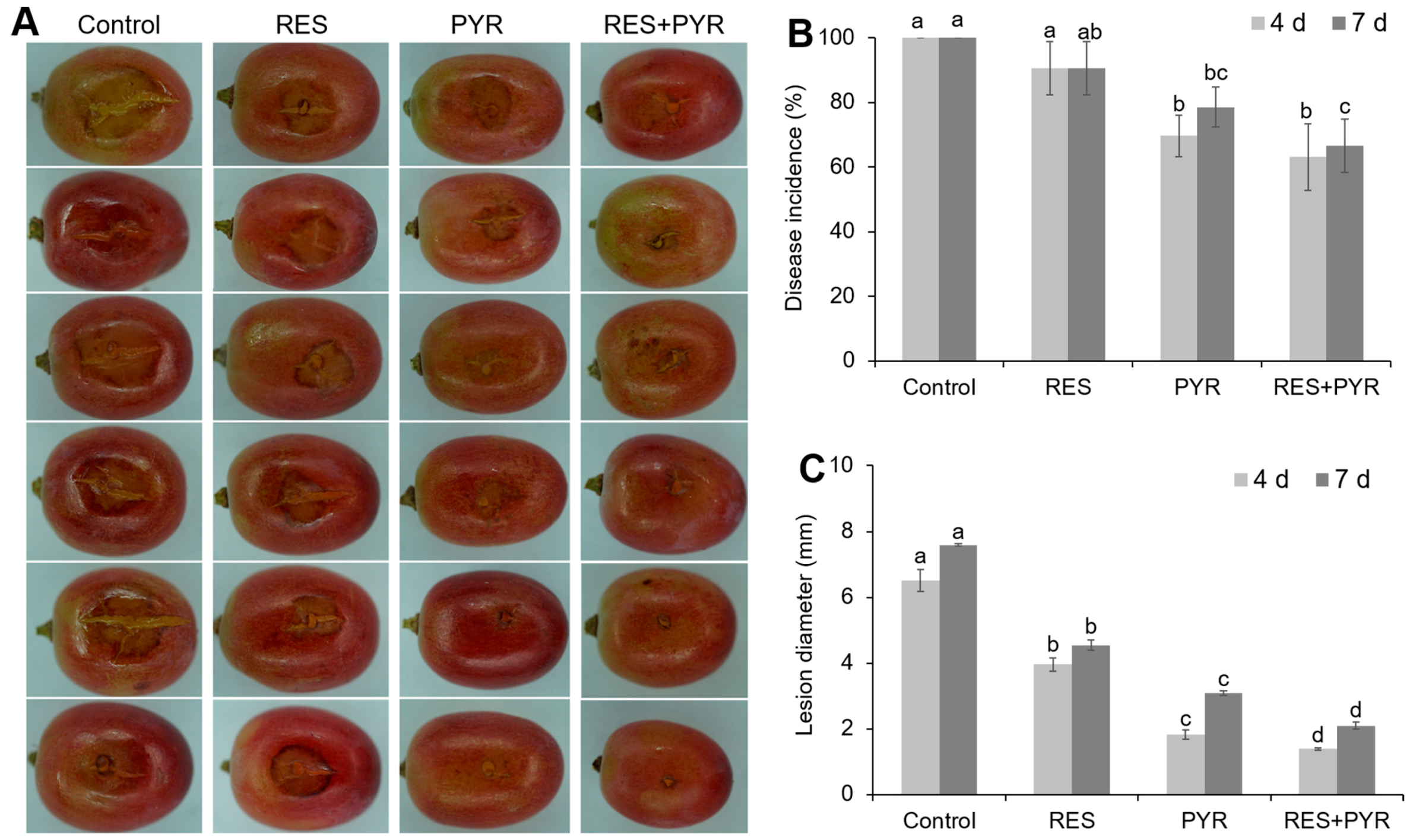
2.5. Synergistic Effect of Resveratrol and Pyrimethanil on Table Grape Gray Mold Disease Control
3. Discussion
4. Materials and Methods
4.1. Pathogen and Chemical Agents
4.2. Determination of the Fungicide Sensitivity of Tested Isolates
4.3. Classification of Resistance Level
4.4. Determination of Minimal Inhibitory Concentration (MIC)
4.5. Determination of MICs in the Drug Combination
4.6. Analysis of Drugs Interaction
4.7. Mycelial Growth Assay
4.8. Conidia Germination Assay
4.9. Pathogenicity Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides. Plant Dis. 2007, 91, 407–413. [Google Scholar] [CrossRef]
- Mosbach, A.; Edel, D.; Farmer, A.D.; Widdison, S.; Barchietto, T. Anilinopyrimidine resistance in Botrytis cinerea is linked to mitochondrial function. Front. Microbiol. 2017, 8, 2361. [Google Scholar] [CrossRef] [PubMed]
- McQuilken, M.P.; Thomson, J. Evaluation of anilinopyrimidine and other fungicides for control of grey mould (Botrytis cinerea) in container-grown Calluna vulgaris. Pest Manag. Sci. 2008, 64, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kim, Y.K.; Huang, L.; Xiao, C.L. Resistance to thiabendazole and baseline sensitivity to fludioxonil and pyrimethanil in Botrytis cinerea populations from apple and pear in Washington State. Postharvest Biol. Technol. 2010, 56, 12–18. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Psomopoulos, F.; Malandrakis, E.E.; Ganopoulos, I.; Madesis, P.; Vellios, E.K.; Drogoudi, P. Comparative genomics of Botrytis cinerea strains with differential multi-drug resistance. Front. Plant Sci. 2016, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.; Merillon, J. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crop. Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J. Phytopathol. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Pinto, E.P.; Perin, E.C.; Schott, I.B.; Da Silva Rodrigues, R.; Lucchetta, L.; Manfroi, V.; Rombaldi, C.V. The effect of postharvest application of UV-C radiation on the phenolic compounds of conventional and organic grapes (Vitis labrusca cv. ‘Concord’). Postharvest Biol. Technol. 2016, 120, 84–91. [Google Scholar] [CrossRef]
- Jeandet, P.; Breuil, A.C.; Adrian, M.; Weston, L.A.; Debord, S.; Meunier, P.; Maume, G.; Bessis, R. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Anal. Chem. 1997, 69, 5172–5177. [Google Scholar] [CrossRef]
- Breuil, A.C.; Jeandet, P.; Adrian, M.; Bessis, R. Changes in the phytoalexin content of various Vitis spp. in response to Ultraviolet C elicitation. J. Agric. Food Chem. 1999, 47, 4456–4461. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.N.; Carezzano, M.E.; Oliva, M.M.; Demo, M.S.; Pizzolitto, R.P.; Zunino, M.P.; Zygadlo, J.A.; Dambolena, J.S. In vitro activity of natural phenolic compounds against fluconazole-resistant Candida species: A quantitative structure-activity relationship analysis. J. Appl. Microbiol. 2014, 116, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in human health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, X.; Yan, L.J. Antioxidant activity, antitumor effect, and antiaging property of proanthocyanidins extracted from Kunlun Chrysanthemum flowers. Oxid. Med. Cell. Longev. 2015, 2015, 983484. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of Melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Bisson, J.; Waffo-Teguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.; Merillon, J.; Cluzet, S. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food Chem. 2012, 60, 11859–11868. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Deng, Y.; Han, T.; Jiang, L.; Xi, P.; Wang, Q.; Jiang, Z.; Gao, L. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Vestergaard, M.; Paulander, W.; Marvig, R.L.; Clasen, J.; Jochumsen, N. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2016, 47, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anju, S.; Kumar, N.S.; Krishnakumar, B.; Kumar, B.S.D. Synergistic combination of violacein and azoles that leads to enhanced killing of major human pathogenic dermatophytic fungi Trichophyton rubrum. Front. Cell. Infect. Microbiol. 2015, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int. J. Antimicrob. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tseng, C.; Wang, P.; Lu, P.; Weng, Y.; Yen, F.; Fang, J. Pterostilbene, a methoxylated resveratrol derivative, efficiently eradicates planktonic, biofilm, and intracellular MRSA by topical application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Qi, Z.; Zhao, P.; Cheng, G.; Gu, Z.; Wang, Y. Primary study on resistance of Botrytis cinerea to pyrimethanil in Tomato. J. Shenyang Agric. Univ. 2002, 33, 345–347. [Google Scholar]
- Fibach, E.; Prus, E.; Bianchi, N.; Zuccato, C.; Breveglieri, G.; Salvatori, F.; Finotti, A.; Lipucci, D.P.M.; Brognara, E.; Lampronti, I.; et al. Resveratrol: Antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and beta-thalassemia patients. Int. J. Mol. Med. 2012, 29, 974–982. [Google Scholar] [PubMed]
- Anya, A.; Rachel, S.S.; Naomi, S.S.; Adi, Y.B.; Sara, L.W.; Marina, K.H. Combination of rapamycin and resveratrol for treatment of bladder cancer. J. Cell. Physiol. 2017, 232, 436–446. [Google Scholar]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S. Comprehensive review on the antimicrobial potency of the plant polyphenol resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Seu, Y.B.; Lee, D.G. Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. Albicans. J. Microbiol. Biotechnol. 2007, 17, 1324. [Google Scholar] [PubMed]
- Caruso, F.; Mendoza, L.; Castro, P.; Cotoras, M.; Aguirre, M.; Matsuhiro, B.; Isaacs, M.; Rossi, M.; Viglianti, A.; Antonioletti, R. Antifungal activity of resveratrol against Botrytis cinerea is improved using 2-furyl derivatives. PLoS ONE 2011, 6, e2542110. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Garcia Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.S.D.; Gross, L.A.; Neto, E.; Lessey, B.A.; Savaris, R.F. The use of resveratrol as an adjuvant treatment of pain in endometriosis: A randomized clinical trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Alayev, A.; Berger, S.M.; Kramer, M.Y.; Schwartz, N.S.; Holz, M.K. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J. Cell. Biochem. 2015, 116, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Koo, B.S.; Park, S.Y.; Kim, Y.M. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyankanga, R.; Njogu, M.; Muthomi, J.; Olanya, M. Efficacy of fungicide combinations, phosphoric acid and plant extract from stinging nettle on potato late blight management and tuber yield. Arch. Phytopathol. Plant Prot. 2012, 45, 1449–1463. [Google Scholar] [CrossRef]
- Gado, E. Management of Cercospora leaf spot disease of sugar beet plants by some fungicides and plant extracts. Egypt. J. Phytopathol. 2007, 35, 1–10. [Google Scholar]
- Abdu-Allah, G.A.M.; Abo-Elyousr, K.A.M. Effect of certain plant extracts and fungicides against powdery mildew disease of Grapevines in Upper Egypt. Arch. Phytopathol. Plant Prot. 2017, 50, 957–969. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Chen, Y.; Li, H.; Chen, C.; Zhou, M. Multiple resistance of Botrytis cinerea from vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China. Plant Dis. 2010, 94, 551–556. [Google Scholar] [CrossRef]
- Liu, S.; Che, Z.; Chen, G. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Fernandez-Ortuno, D.; Chen, F.; Schnabel, G. Resistance to cyprodinil and lack of fludioxonil resistance in Botrytis cinerea isolates from strawberry in North and South Carolina. Plant Dis. 2013, 97, 81–85. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Wang, D.; Yang, Y.; Sun, W. Synergistic antifungal effect of fluconazole combined with licofelone against resistant Candida albicans. Front. Microbiol. 2017, 8, 2101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds resveratrol and pyrimethanil are available from the authors. |

| Isolates | Pyrimethanil | Cyprodinil | ||
|---|---|---|---|---|
| Phenotype | EC50 (mg/L) | Phenotype | EC50 (mg/L) | |
| GBW | MR | 33.8 | R | 66.5 |
| TGM | MR | 39.9 | MR | 10.4 |
| SGB | MR | 22.8 | MR | 15.1 |
| GMR | MR | 11.9 | S | 1.3 |
| BGM | HR | 70.7 | R | 22.1 |
| BRB | HR | 88.0 | MR | 11.1 |
| Isolates | MICs (mg/L) | FICs | ||||||
|---|---|---|---|---|---|---|---|---|
| Alone | In Combination | |||||||
| MICA | MICB | CA | CB | FICA | FICB | FICI | IN | |
| GBW MR | 40 | 0.625 | 2.5 | 0.15625 | 0.0625 | 0.25 | 0.3125 | SYN |
| TGM MR | 80 | 1.25 | 5 | 0.078125 | 0.0625 | 0.0625 | 0.125 | SYN |
| SGB MR | 40 | 0.625 | 2.5 | 0.3125 | 0.0625 | 0.5 | 0.5625 | IND |
| GMR MR | 20 | 1.25 | 1.25 | 0.078125 | 0.0625 | 0.0625 | 0.125 | SYN |
| BGM HR | 5 | 2.5 | 1.25 | 0.15625 | 0.25 | 0.0625 | 0.3125 | SYN |
| BRB HR | 20 | 2.5 | 1.25 | 0.3125 | 0.0625 | 0.125 | 0.1875 | SYN |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yu, G.; Xi, P.; Kong, X.; Wang, Q.; Gao, L.; Jiang, Z. Synergistic Effects of Resveratrol and Pyrimethanil against Botrytis cinerea on Grape. Molecules 2018, 23, 1455. https://doi.org/10.3390/molecules23061455
Xu D, Yu G, Xi P, Kong X, Wang Q, Gao L, Jiang Z. Synergistic Effects of Resveratrol and Pyrimethanil against Botrytis cinerea on Grape. Molecules. 2018; 23(6):1455. https://doi.org/10.3390/molecules23061455
Chicago/Turabian StyleXu, Dandan, Ge Yu, Pinggen Xi, Xiangyu Kong, Qi Wang, Lingwang Gao, and Zide Jiang. 2018. "Synergistic Effects of Resveratrol and Pyrimethanil against Botrytis cinerea on Grape" Molecules 23, no. 6: 1455. https://doi.org/10.3390/molecules23061455




