Phosphoproteomics of Retinoblastoma: A Pilot Study Identifies Aberrant Kinases
Abstract
:1. Introduction
2. Results
2.1. Phosphoproteome of Human Retina
2.2. Differentially Phosphorylated Proteins in Retinoblastoma
2.3. Categorization of Differentially Phosphorylated Proteins Based on Gene Ontology (GO) Annotation
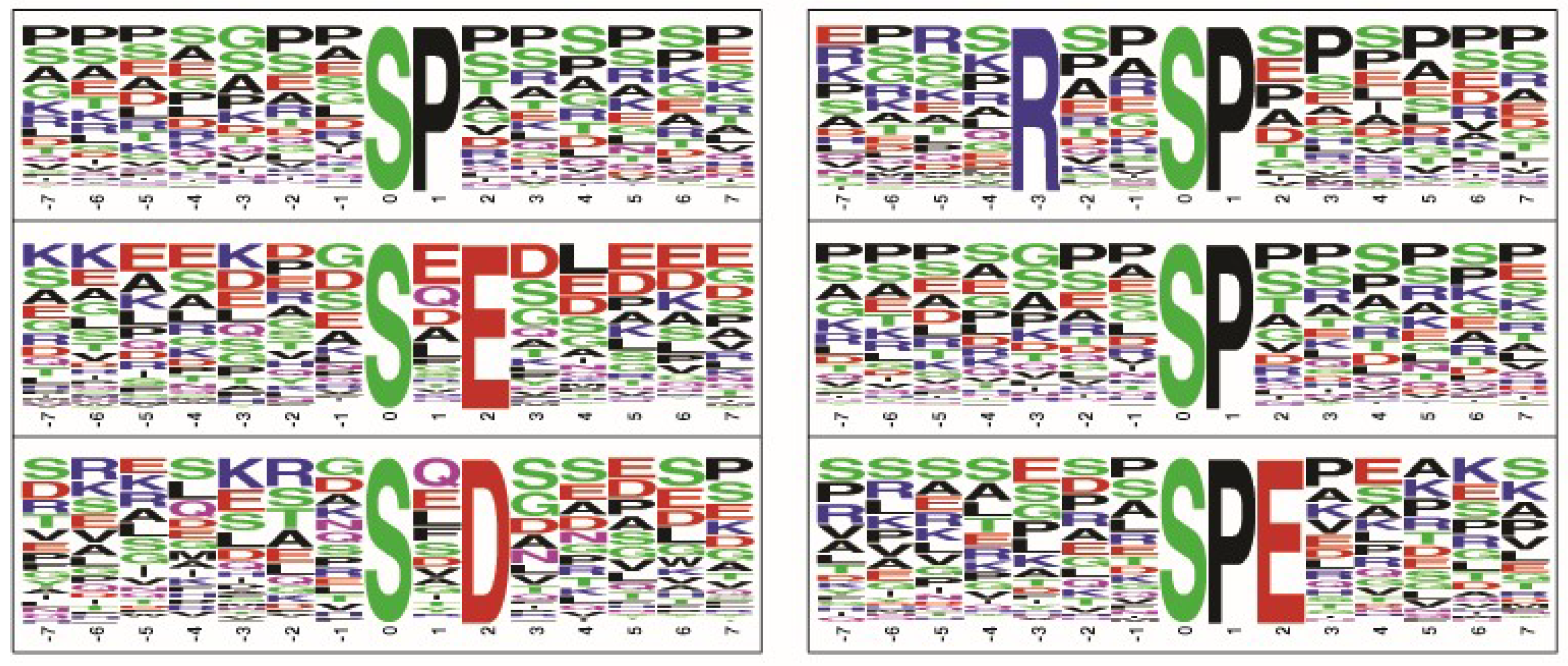
2.4. Phosphorylated Motifs Identified in Retinoblastoma
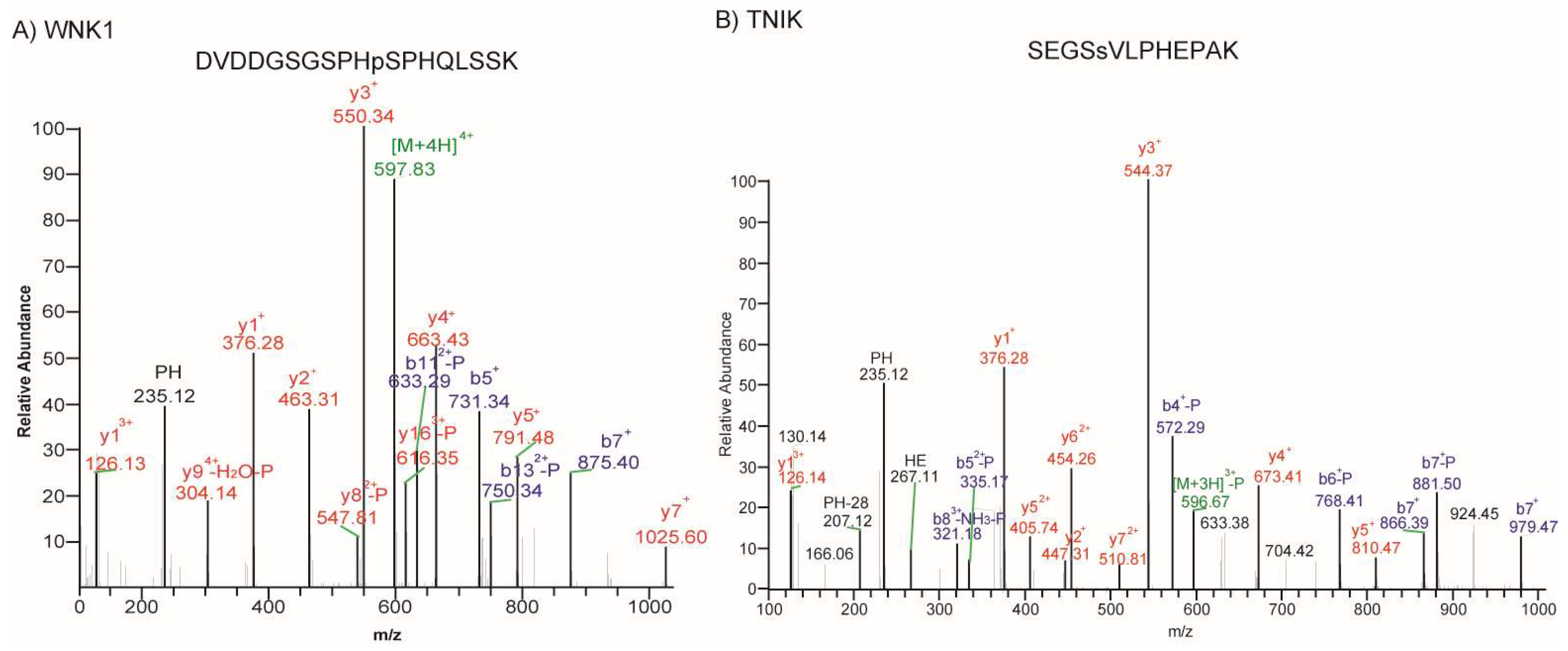
2.5. Phosphorylated Kinases Identified in Retinoblastoma
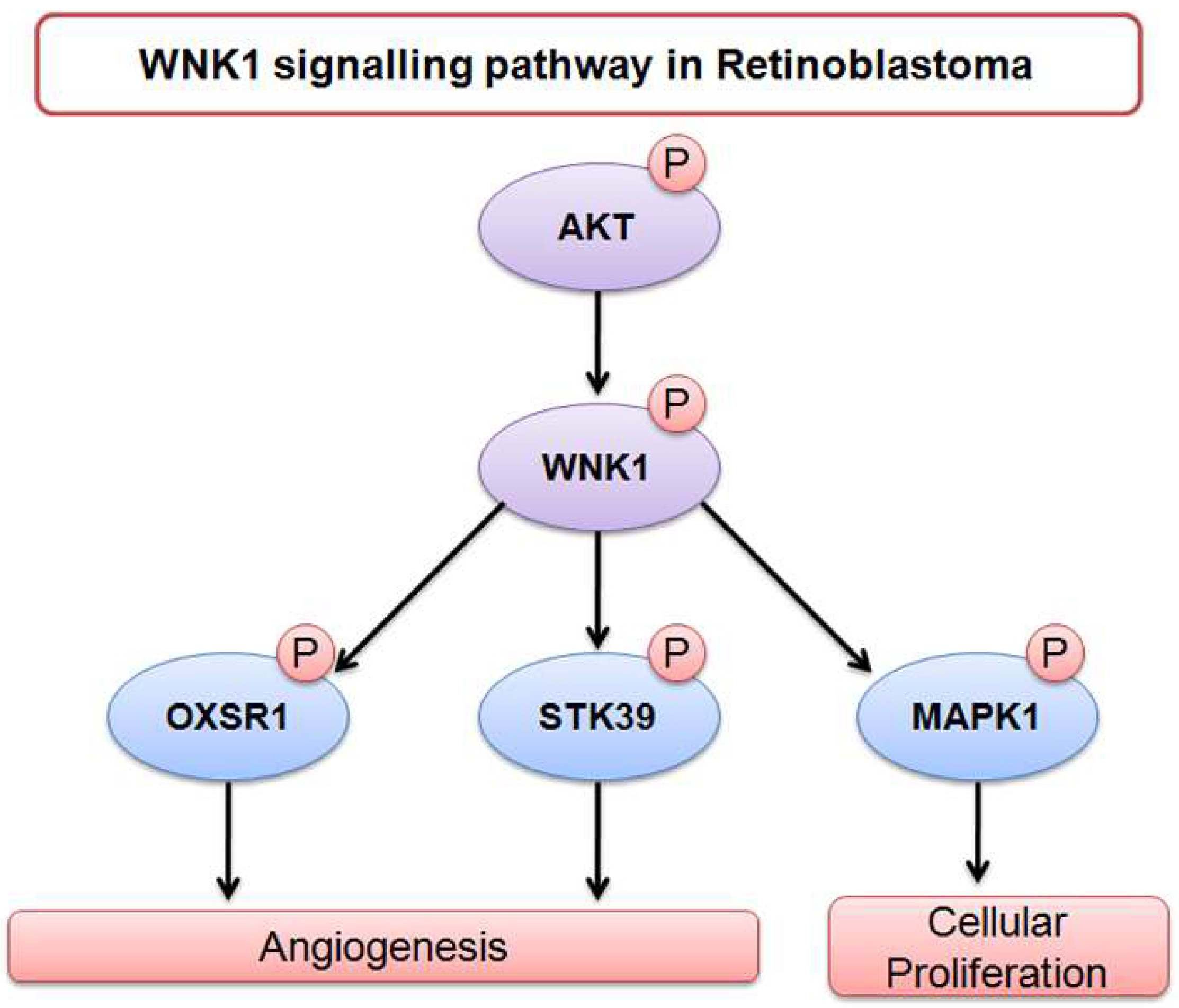
3. Discussion
4. Materials and Methods
4.1. Tumour Samples and Lysate Preparation
4.2. Trypsin Digestion and Tandem Mass Tag (TMT) Labelling
4.3. Basic pH Reversed-Phase Liquid Chromatography (bRPLC) and TiO2-Based Phosphopeptide Enrichment
4.4. LC MS/MS Analysis of the Enriched Phosphopeptides
4.5. Data Analysis
4.6. Gene Ontology Analysis
4.7. Motif Enrichment Analysis
4.8. Kinase–Substrate Network Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hubbard, M.J.; Cohen, P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci. 1993, 18, 172–177. [Google Scholar] [CrossRef]
- Cohen, P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Haab, B.B. Antibody arrays in cancer research. Mol. Cell. Proteomics 2005, 4, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Ong, S.E.; Gronborg, M.; Steen, H.; Jensen, O.N.; Pandey, A. Analysis of protein phosphorylation using mass spectrometry: Deciphering the phosphoproteome. Trends Biotechnol. 2002, 20, 261–268. [Google Scholar] [CrossRef]
- Krutzik, P.O.; Nolan, G.P. Intracellular phospho-protein staining techniques for flow cytometry: Monitoring single cell signaling events. Cytom. Part A J. Int. Soc. Anal. Cytol. 2003, 55, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nita-Lazar, A. Quantitative analysis of phosphorylation-based protein signaling networks in the immune system by mass spectrometry. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 368–376. [Google Scholar] [CrossRef] [PubMed]
- White, F.M.; Wolf-Yadlin, A. Methods for the Analysis of Protein Phosphorylation-Mediated Cellular Signaling Networks. Annu. Rev. Anal. Chem. 2016, 9, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Rigbolt, K.T.; Blagoev, B. Quantitative phosphoproteomics to characterize signaling networks. Semin. Cell Dev. Biol. 2012, 23, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kosako, H.; Nagano, K. Quantitative phosphoproteomics strategies for understanding protein kinase-mediated signal transduction pathways. Expert Rev. Proteom. 2011, 8, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashman, K.; Villar, E.L. Phosphoproteomics and cancer research. Clin. Transl. Oncol. 2009, 11, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.T.; Liu, N.M.; Vu, J.Q.; Patel, D.; Cohn, W.; Capri, J.; Ziegler, M.; Patel, N.; Tramontano, A.; Williams, R.; et al. Phospho-Network Analysis Identifies and Quantifies Hepatitis C Virus (HCV)-induced Hepatocellular Carcinoma (HCC) Proteins Regulating Viral-mediated Tumor Growth. Cancer Genom. Proteom. 2016, 13, 339–357. [Google Scholar]
- Narumi, R.; Murakami, T.; Kuga, T.; Adachi, J.; Shiromizu, T.; Muraoka, S.; Kume, H.; Kodera, Y.; Matsumoto, M.; Nakayama, K.; et al. A strategy for large-scale phosphoproteomics and SRM-based validation of human breast cancer tissue samples. J. Proteome Res. 2012, 11, 5311–5322. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Cho, W.C. Phosphoproteomics and lung cancer research. Int. J. Mol. Sci. 2012, 13, 12287–12314. [Google Scholar] [CrossRef] [PubMed]
- Lescarbeau, R.S.; Lei, L.; Bakken, K.K.; Sims, P.A.; Sarkaria, J.N.; Canoll, P.; White, F.M. Quantitative Phosphoproteomics Reveals Wee1 Kinase as a Therapeutic Target in a Model of Proneural Glioblastoma. Mol. Cancer Ther. 2016, 15, 1332–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theriault, B.L.; Dimaras, H.; Gallie, B.L.; Corson, T.W. The genomic landscape of retinoblastoma: A review. Clin. Exp. Ophthalmol. 2014, 42, 33–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, G.I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006, 24, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, J.K.; Hornicek, F.J.; Kan, Q.; Duan, Z. The emerging roles and therapeutic potential of cyclin-dependent kinase 11 (CDK11) in human cancer. Oncotarget 2016, 7, 40846–40859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, D.P.; Foulkes, D.M.; Eyers, P.A. Pseudokinases: Update on their functions and evaluation as new drug targets. Future Med. Chem. 2017, 9, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, E.D.; Zhang, L.; Wu, J.; Daly, R.J. The kinome ‘at large’ in cancer. Nat. Rev. Cancer 2016, 16, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, L.; Zhang, S. Comprehensive Modeling and Discovery of Mebendazole as a Novel TRAF2- and NCK-interacting Kinase Inhibitor. Sci. Rep. 2016, 6, 33534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowles, D.W.; Jimeno, A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin. Investig. Drugs 2011, 20, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–9017. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.E.; Gardner, T.W. Functions of insulin and insulin receptor signaling in retina: Possible implications for diabetic retinopathy. Prog. Retinal Eye Res. 2003, 22, 545–5462. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Xie, C.; Lu, H.; Nomura, A.; Hanse, E.A.; Forster, C.L.; Parker, J.B.; Linden, M.A.; Karasch, C.; Hallstrom, T.C. Co-deleting Pten with Rb in retinal progenitor cells in mice results in fully penetrant bilateral retinoblastomas. Mol. Cancer 2015, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, C.; Gratias, S.; Stephan, H.; Schuler, A.; Schramm, A.; Klein-Hitpass, L.; Rieder, H.; Schneider, S.; Kappes, F.; Eggert, A.; et al. Gains and overexpression identify DEK and E2F3 as targets of chromosome 6p gains in retinoblastoma. Oncogene 2005, 24, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Kappes, F.; Damoc, C.; Knippers, R.; Przybylski, M.; Pinna, L.A.; Gruss, C. Phosphorylation by protein kinase CK2 changes the DNA binding properties of the human chromatin protein DEK. Mol. Cell. Biol. 2004, 24, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Allen, H.V.; Jones, E.E.; Habash, K.B.; Matsuo, H.; Wells, S.I. Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol. Cell. Biol. 2006, 26, 7506–7519. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Katsov, A.; Hu, L.; Petros, A.; Fesik, S.W.; Yaffe, M.B.; Greenberg, M.E. 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell 2000, 6, 41–51. [Google Scholar] [CrossRef]
- Sakamaki, J.; Daitoku, H.; Ueno, K.; Hagiwara, A.; Yamagata, K.; Fukamizu, A. Arginine methylation of BCL-2 antagonist of cell death (BAD) counteracts its phosphorylation and inactivation by Akt. Proc. Natl. Acad. Sci. USA 2011, 108, 6085–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Zhang, Y.; Shacter, E. Rac1 inhibits apoptosis in human lymphoma cells by stimulating Bad phosphorylation on Ser-75. Mol. Cell. Biol. 2004, 24, 6205–6214. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, H.; Vaidyanathan, H.; Knapinska, A.; Ramos, J.W. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem. J. 2005, 390, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.; Jordan, P. Emerging roles for WNK kinases in cancer. Cell. Mol. Life Sci. 2010, 67, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.G.; Tsai, S.M.; Tu, H.C.; Chen, W.C.; Kou, F.J.; Lu, J.W.; Wang, H.D.; Huang, C.L.; Yuh, C.H. Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling. PLoS ONE 2014, 9, e106129. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, L.; Yu, R.K.; Zeng, G. Down-regulation of WNK1 protein kinase in neural progenitor cells suppresses cell proliferation and migration. J. Neurochem. 2006, 99, 1114–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dbouk, H.A.; Weil, L.M.; Perera, G.K.; Dellinger, M.T.; Pearson, G.; Brekken, R.A.; Cobb, M.H. Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK. Proc. Natl. Acad. Sci. USA 2014, 111, 15999–16004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.A.; Shen, M.; Huang, B.C.; Lasaga, J.; Payan, D.G.; Luo, Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J. Biol. Chem. 1999, 274, 30729–30737. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Uno, Y.; Ohbayashi, N.; Ohata, H.; Mimata, A.; Kukimoto-Niino, M.; Moriyama, H.; Kashimoto, S.; Inoue, T.; Goto, N.; et al. TNIK inhibition abrogates colorectal cancer stemness. Nat. Commun. 2016, 7, 12586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizcaino, J.A.; Csordas, A.; del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.F.; Schwartz, D. Biological sequence motif discovery using motif-x. Curr. Protoc. Bioinform. 2011, 13, 15–24. [Google Scholar]
- Horn, H.; Schoof, E.M.; Kim, J.; Robin, X.; Miller, M.L.; Diella, F.; Palma, A.; Cesareni, G.; Jensen, L.J.; Linding, R. KinomeXplorer: An integrated platform for kinome biology studies. Nat. Methods 2014, 11, 603–604. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Pathways | Genes | Count | p-Value |
|---|---|---|---|
| Spliceosome | NCBP1, TRA2B, PPIL1, U2AF2, TRA2A, HSPA1A, SART1, SF3B2, CTNNBL1, HNRNPA3, SF3B1, DDX46, HNRNPK, DDX23, RBM8A, PCBP1, USP39, DHX16, SNRNP70, ACIN1, HNRNPC, PRPF40B, RBM25, PRPF40A, DDX42, PRPF31, PRPF3, SNW1, SF3A1, HNRNPA1, HNRNPU, SNRNP200, SLU7, THOC2, PRPF38B, RBM17, THOC1, PRPF38A | 38 | 4.47 × 10−14 |
| Tight junction | PARD3, CASK, CLDN11, PTEN, TJAP1, MYL9, CTTN, MLLT4, AKT2, PRKCA, SYMPK, EPB41, MPDZ, PRKCI, MYH9, PRKCE, CTNNA1, CTNNA2, PRKCB, EPB41L2, EPB41L3, TJP1, EPB41L1, MYH14, TJP3, TJP2, SPTAN1, MYH10 | 28 | 8.77 × 10−7 |
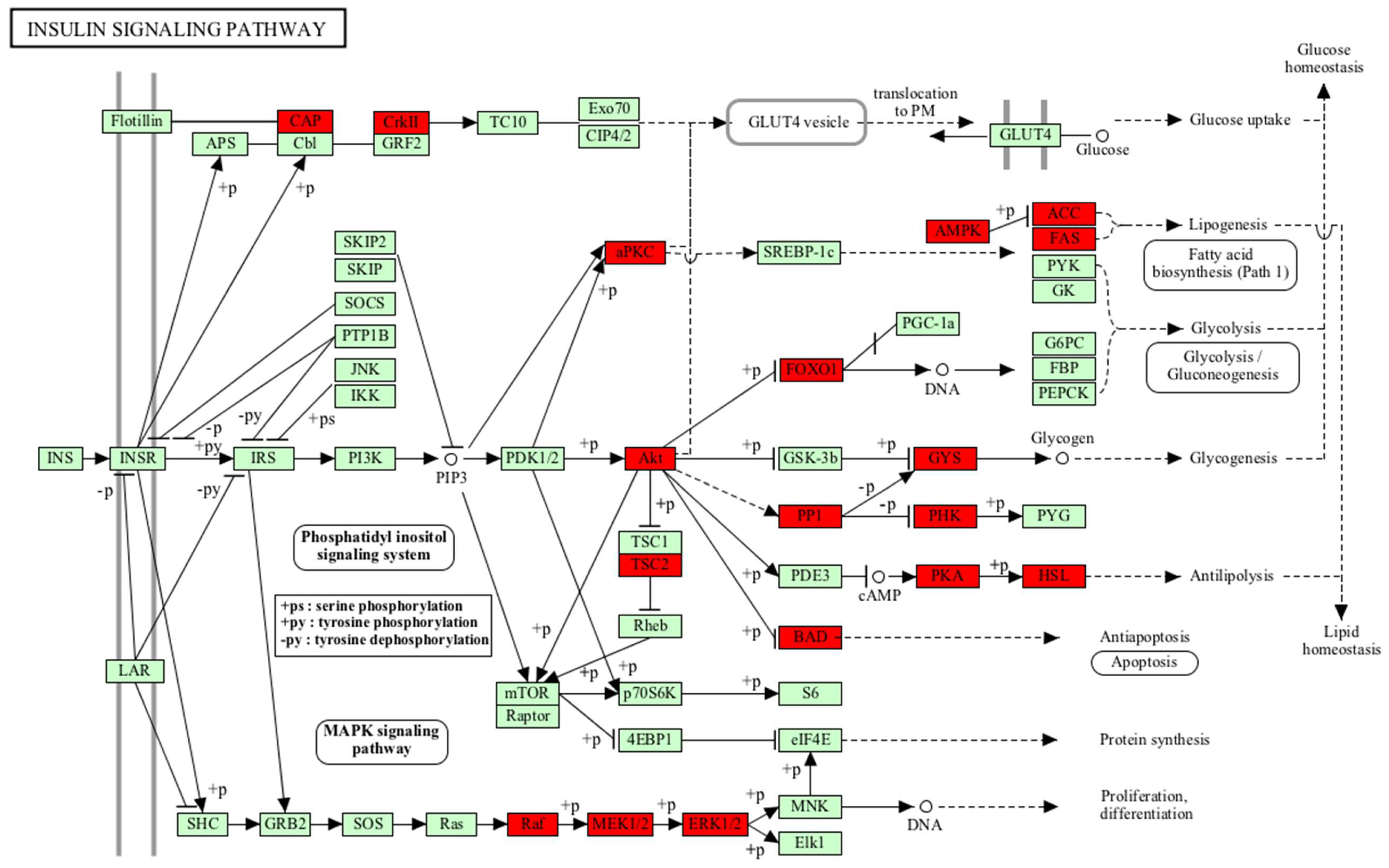
| Insulin signalling pathway | PHKB, FOXO1, PPP1R3D, PRKAR2B, PRKAR2A, SORBS1, GYS1, FASN, PRKACA, PRKAA1, PRKACB, AKT2, MAP2K1, BRAF, ACACA, PRKCI, PRKAB1, RAF1, BAD, MAPK1, CRKL, PRKAR1B, TSC2, PRKAR1A, MAPK3, CRK, LIPE | 27 | 3.44 × 10−6 |
| Fc gamma R-mediated phagocytosis | PRKCA, DNM3, DNM1L, MAP2K1, SPHK2, LYN, MARCKSL1, ASAP2, ASAP1, RAF1, PRKCE, AMPH, PRKCB, MAPK1, CRKL, MAPK3, CFL1, MARCKS, PAK1, CRK, AKT2 | 21 | 1.20 × 10−5 |
| ErbB signalling pathway | PRKCA, EGFR, MAP2K1, BRAF, CAMK2G, RAF1, BAD, PRKCB, MAPK1, CRKL, PAK2, PAK4, NCK1, MAPK3, CAMK2D, PAK1, CRK, ABL2, AKT2 | 19 | 4.20 × 10−5 |
| # | Gene Symbol | Kinase | Ser/Thr/Tyr Kinase | Primary Localization | Available Drugs |
|---|---|---|---|---|---|
| 1 | CDK1 | Cyclin-dependent kinase 1 | Ser/Thr protein kinase | Cytoplasm | Flavopiridol, dinaciclib, PD0332991 [18] |
| 2 | CDK11B | Cyclin-dependent kinase 11B | Ser/Thr protein kinase | Cytoplasm; Nucleus | Proposed target for cancer treatment [19] |
| 3 | WNK1 | Lysine deficient protein kinase 1 | Ser/Thr protein kinase | Cytoplasm | Proposed target for cancer treatment [20,21] |
| 4 | TNIK | TRAF2 and NCK interacting kinase | Ser/Thr protein kinase | Cytoplasm | Mebendazole [22] |
| 5 | BAZ1B | Bromodomain adjacent to zinc finger domain 1B | Tyrosine kinase | Nucleus; Cytoplasm | Belongs to the bromodomain- and extra terminal domain (BET) family of proteins. Probably targeted by BET inhibitors |
| 6 | PI4K2A | Phosphatidylinositol 4-kinase type 2 alpha | Golgi apparatus | Small-molecule inhibitors are available for phosphatidylinositol 3-kinase [23] | |
| 7 | AAK1 | AP2 associated kinase 1 | Ser/Thr protein kinase | Cytoskeleton | - |
| 8 | BRD4 | Bromodomain containing 4 | Ser/Thr protein kinase | Nucleus; Cytoplasm | BET inhibitors—JQ1, OTX015, GSK 525762, TEN-010 [24] |
| 9 | CASK | Calcium/calmodulin-dependent serine threonine kinase | Ser/Thr protein kinase | Plasma membrane | - |
| 10 | DLG3 | Discs large MAGUK scaffold protein 3 | Plasma membrane | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvan, L.D.N.; Danda, R.; Madugundu, A.K.; Puttamallesh, V.N.; Sathe, G.J.; Krishnan, U.M.; Khetan, V.; Rishi, P.; Prasad, T.S.K.; Pandey, A.; et al. Phosphoproteomics of Retinoblastoma: A Pilot Study Identifies Aberrant Kinases. Molecules 2018, 23, 1454. https://doi.org/10.3390/molecules23061454
Selvan LDN, Danda R, Madugundu AK, Puttamallesh VN, Sathe GJ, Krishnan UM, Khetan V, Rishi P, Prasad TSK, Pandey A, et al. Phosphoproteomics of Retinoblastoma: A Pilot Study Identifies Aberrant Kinases. Molecules. 2018; 23(6):1454. https://doi.org/10.3390/molecules23061454
Chicago/Turabian StyleSelvan, Lakshmi Dhevi Nagarajha, Ravikanth Danda, Anil K. Madugundu, Vinuth N. Puttamallesh, Gajanan J. Sathe, Uma Maheswari Krishnan, Vikas Khetan, Pukhraj Rishi, Thottethodi Subrahmanya Keshava Prasad, Akhilesh Pandey, and et al. 2018. "Phosphoproteomics of Retinoblastoma: A Pilot Study Identifies Aberrant Kinases" Molecules 23, no. 6: 1454. https://doi.org/10.3390/molecules23061454






