Development and Validation of a Kit to Measure Drink Antioxidant Capacity Using a Novel Colorimeter
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Tested Drinks
3.2. DPPH Radical Scavenging Assay
3.3. Radical Scavenging Capacity (RSC) and Drink Potency Determination
3.4. Colorimeter Development
3.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Elnakish, M.T.; Hassanain, H.H.; Janssen, P.M.; Angelos, M.G.; Khan, M. Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: Important role of Rac/NADPH oxidase. J. Pathol. 2013, 231, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Abdali, D.; Samson, S.E.; Grover, A.K. How Effective Are Antioxidant Supplements in Obesity and Diabetes? Med. Princ. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Santos-Ocana, C.; Sanchez-Alcazar, J.A.; Fernandez-Ayala, D.J.M.; Asencio-Salcedo, C.; Rodriguez-Aguilera, J.C.; Navas, P. Mitochondrial responsibility in ageing process: innocent, suspect or guilty. Biogerontology 2015, 16, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernandez, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Sintara, M.; Chang, T. Multi-radical (ORAC MR5) antioxidant capacity of selected berries and effects of food processing. J. Berry Res. 2016, 6, 159–173. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Matthaiou, C.M.; Goutzourelas, N.; Stagos, D.; Sarafoglou, E.; Jamurtas, A.; Koulocheri, S.D.; Haroutounian, S.A.; Tsatsakis, A.M.; Kouretas, D. Pomegranate juice consumption increases GSH levels and reduces lipid and protein oxidation in human blood. Food Chem. Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available from the authors.
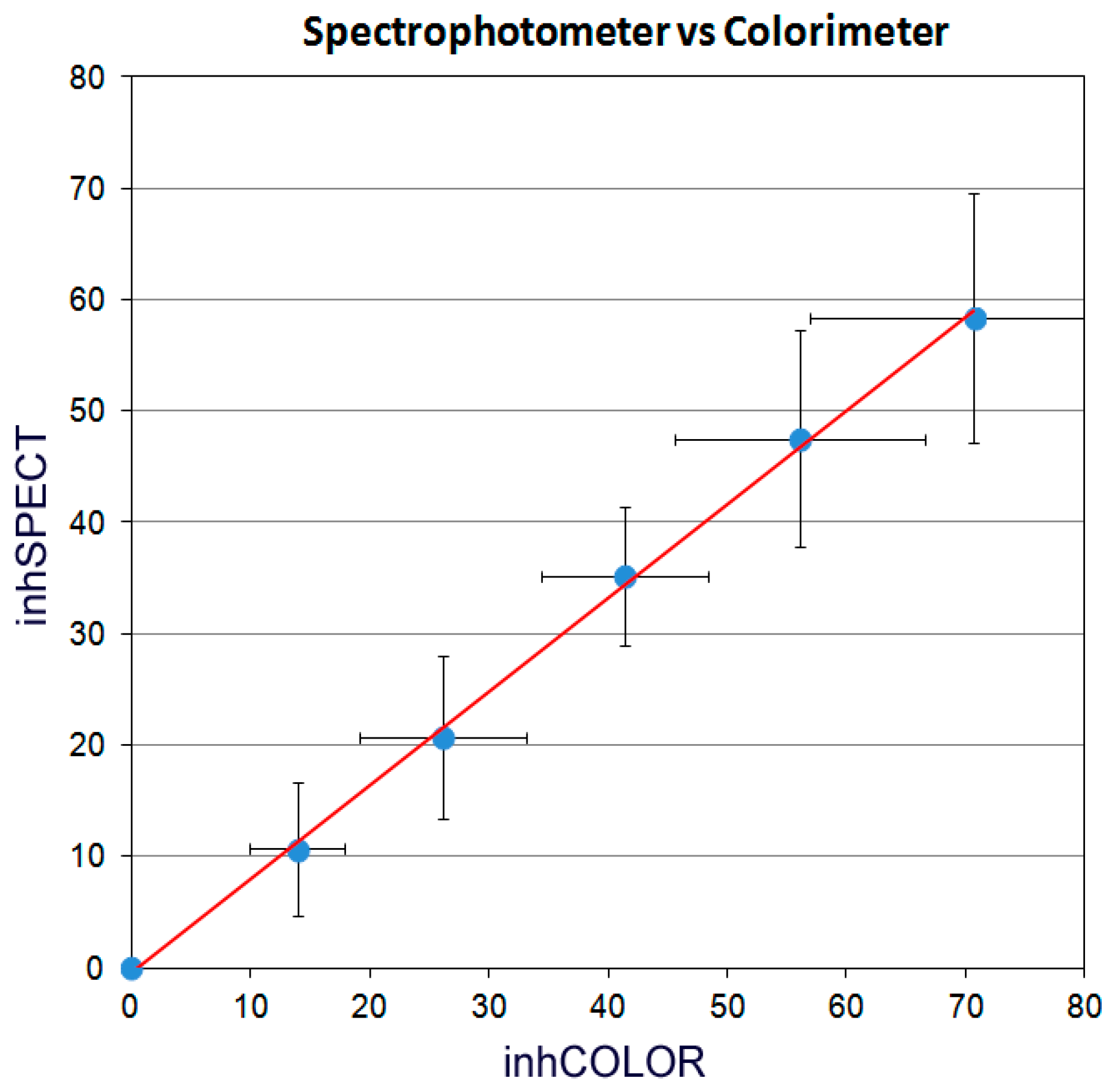
| Sample | inhSPECT | inhCOLOR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 μL | 0.10 μL | 0.20 μL | 0.30 μL | 0.40 μL | 0.50 μL | 0.00 μL | 0.10 μL | 0.20 μL | 0.30 μL | 0.40 μL | 0.50 μL | |
| 1 | 0.00 | 18.28 ± 0.52 | 28.43 ± 0.90 | 41.30 ± 1.35 | 59.46 ± 1.96 | 67.85 ± 2.14 | 0.00 | 14.92 ± 0.16 | 28.62 ± 0.70 | 47.23 ± 1.35 | 68.08 ± 2.01 | 86.75 ± 2.43 |
| 2 | 0.00 | 7.07 ± 0.16 | 18.58 ± 0.76 | 35.62 ± 1.54 | 48.58 ± 1.55 | 58.85 ± 1.86 | 0.00 | 11.43 ± 0.11 | 25.39 ± 0.71 | 40.61 ± 1.15 | 54.65 ± 1.56 | 71.64 ± 1.78 |
| 3 | 0.00 | 8.07 ± 0.24 | 26.62 ± 1.02 | 42.30 ± 1.22 | 55.38 ± 1.33 | 70.42 ± 1.99 | 0.00 | 13.29 ± 0.09 | 33.57 ± 0.95 | 51.28 ± 1.44 | 69.65 ± 2.15 | 92.59 ± 2.56 |
| 4 | 0.00 | 17.22 ± 0.51 | 32.08 ± 1.28 | 42.90 ± 1.15 | 55.44 ± 1.24 | 64.83 ± 2.12 | 0.00 | 19.77 ± 0.30 | 37.09 ± 1.01 | 51.53 ± 1.65 | 69.02 ± 2.08 | 83.24 ± 1.97 |
| 5 | 0.00 | 18.43 ± 0.35 | 29.13 ± 1.05 | 34.79 ± 1.00 | 50.32 ± 1.01 | 69.21 ± 2.20 | 0.00 | 19.28 ± 0.23 | 33.32 ± 0.78 | 40.32 ± 1.04 | 57.88 ± 1.76 | 76.63 ± 1.99 |
| 6 | 0.00 | 18.52 ± 0.43 | 30.61 ± 0.78 | 41.65 ± 1.76 | 53.12 ± 1.86 | 62.66 ± 1.66 | 0.00 | 19.90 ± 0.17 | 33.90 ± 0.55 | 50.44 ± 1.47 | 64.13 ± 1.43 | 75.21 ± 2.01 |
| 7 | 0.00 | 7.16 ± 0.12 | 16.58 ± 0.54 | 26.38 ± 0.95 | 32.68 ± 0.97 | 40.80 ± 0.98 | 0.00 | 7.29 ± 0.07 | 17.47 ± 0.16 | 28.00 ± 0.67 | 39.52 ± 1.02 | 50.17 ± 1.43 |
| 8 | 0.00 | 6.24 ± 0.09 | 18.91 ± 0.43 | 28.80 ± 0.78 | 30.23 ± 0.78 | 39.00 ± 0.77 | 0.00 | 12.06 ± 0.10 | 23.40 ± 0.46 | 32.67 ± 0.88 | 42.77 ± 1.13 | 51.81 ± 1.32 |
| 9 | 0.00 | 6.29 ± 0.11 | 16.86 ± 0.87 | 35.23 ± 0.96 | 45.69 ± 1.06 | 61.82 ± 2.03 | 0.00 | 15.93 ± 0.33 | 30.33 ± 1.01 | 44.31 ± 1.21 | 58.65 ± 1.43 | 78.37 ± 1.93 |
| 10 | 0.00 | 16.59 ± 0.39 | 17.36 ± 0.66 | 34.48 ± 0.66 | 43.05 ± 1.15 | 53.05 ± 1.55 | 0.00 | 17.24 ± 0.18 | 29.80 ± 0.88 | 42.84 ± 1.00 | 56.58 ± 1.72 | 69.10 ± 1.77 |
| 11 | 0.00 | 9.86 ± 0.20 | 17.14 ± 0.34 | 27.37 ± 0.74 | 36.56 ± 1.05 | 48.38 ± 1.13 | 0.00 | 15.78 ± 0.40 | 26.30 ± 0.76 | 38.96 ± 0.73 | 48.66 ± 1.13 | 63.75 ± 1.04 |
| 12 | 0.00 | 0.54 ± 0.01 | 14.70 ± 0.66 | 32.70 ± 0.76 | 44.03 ± 1.24 | 47.53 ± 1.22 | 0.00 | 6.26 ± 0.15 | 18.28 ± 0.45 | 34.22 ± 0.89 | 43.86 ± 1.10 | 49.90 ± 0.55 |
| 13 | 0.00 | 3.79 ± 0.09 | 9.79 ± 0.23 | 23.58 ± 0.69 | 36.29 ± 0.99 | 48.95 ± 1.65 | 0.00 | 9.06 ± 0.23 | 16.38 ± 0.35 | 32.05 ± 0.80 | 40.29 ± 1.01 | 51.51 ± 1.43 |
| 14 | 0.00 | 8.99 ± 0.12 | 15.93 ± 0.49 | 41.25 ± 1.23 | 60.33 ± 2.01 | 72.75 ± 2.23 | 0.00 | 12.23 ± 0.22 | 22.70 ± 0.57 | 42.55 ± 1.10 | 62.34 ± 1.49 | 77.31 ± 2.04 |
| 15 | 0.00 | 11.87 ± 0.25 | 22.59 ± 0.75 | 38.70 ± 1.04 | 55.98 ± 1.96 | 70.29 ± 2.10 | 0.00 | 13.35 ± 0.38 | 27.61 ± 0.33 | 41.37 ± 1.14 | 61.03 ± 1.86 | 74.01 ± 1.67 |
| 16 | 0.00 | 4.17 ± 0.07 | 8.04 ± 0.32 | 30.48 ± 0.77 | 41.20 ± 1.29 | 47.43 ± 1.21 | 0.00 | 14.08 ± 0.41 | 11.86 ± 0.30 | 38.27 ± 0.99 | 48.26 ± 1.35 | 63.16 ± 1.59 |
| 17 | 0.00 | 18.28 ± 0.67 | 28.43 ± 1.03 | 41.30 ± 0.55 | 59.46 ± 1.78 | 67.85 ± 1.97 | 0.00 | 14.92 ± 0.09 | 28.62 ± 0.18 | 47.23 ± 1.21 | 68.08 ± 1.56 | 86.75 ± 2.32 |
| Average | 0.00 | 10.67 | 20.69 | 35.22 | 47.52 | 58.33 | 0.00 | 13.93 | 26.15 | 41.40 | 56.09 | 70.70 |
| SD | 6.04 | 7.32 | 6.22 | 9.77 | 11.19 | 4.02 | 7.02 | 6.96 | 10.45 | 13.71 | ||
| Drink | Type | Antioxidant Activity (μmol DPPH/mL) | Antioxidant Activity Per Dose a |
|---|---|---|---|
| Pomegranate Juice | − | 19.30–71.40 | 3860–14280 |
| Juice with pomegranate and other fruits (grape, orange and carrot) | − | 11.90–21.70 | 2380–4340 |
| Juice without pomegranate | Orange | 1.12–4.40 | 224–880 |
| Lemon | 0.91–1.08 | 182–216 | |
| Mandarin | 0.82–1.28 | 164–256 | |
| Apple-Orange | 2.27–3.06 | 454–612 | |
| Apple-Orange-Carrot | 1.83–2.75 | 366–550 | |
| Cranberry-Raspberry-Blueberry | 4.36–4.87 | 872–974 | |
| Sour Cherry | 2.56–5.45 | 512–1090 | |
| Peach | 0.72–1.76 | 144–352 | |
| Blackcurrant | 7.11–8.37 | 1422–1674 | |
| Orange-Apple-Apricot | 2.48–3.64 | 496–728 | |
| Orange-Apple-Carrot | 2.34–4.35 | 468–870 | |
| 9 fruits | 3.30–6.01 | 660–1202 | |
| Coffee | Espresso Coffee | 60.30–116 | 1807–3489 |
| Instant Coffee | 28.30–43.10 | 7062–10775 | |
| Greek Coffee | 16.40–28.60 | 735–1285 | |
| Filtered Coffee | 5.25–6.35 | 1522–1841 | |
| Milk | Milk (full fat) | 0.33–0.49 | 66–98 |
| Wine | White Wine | 0.50–2.50 | 100–500 |
| Beer | Blonde Beer | 1.20 | 396 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priftis, A.; Stagos, D.; Tzioumakis, N.; Konstantinopoulos, K.; Patouna, A.; Papadopoulos, G.E.; Tsatsakis, A.; Kouretas, D. Development and Validation of a Kit to Measure Drink Antioxidant Capacity Using a Novel Colorimeter. Molecules 2016, 21, 1154. https://doi.org/10.3390/molecules21091154
Priftis A, Stagos D, Tzioumakis N, Konstantinopoulos K, Patouna A, Papadopoulos GE, Tsatsakis A, Kouretas D. Development and Validation of a Kit to Measure Drink Antioxidant Capacity Using a Novel Colorimeter. Molecules. 2016; 21(9):1154. https://doi.org/10.3390/molecules21091154
Chicago/Turabian StylePriftis, Alexandros, Dimitrios Stagos, Nikolaos Tzioumakis, Konstantinos Konstantinopoulos, Anastasia Patouna, Georgios E. Papadopoulos, Aristides Tsatsakis, and Dimitrios Kouretas. 2016. "Development and Validation of a Kit to Measure Drink Antioxidant Capacity Using a Novel Colorimeter" Molecules 21, no. 9: 1154. https://doi.org/10.3390/molecules21091154







