Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. Maximum Pore Size (Bubble Point Method)
2.3.3. Contact Angle Analysis
2.3.4. Membrane Permeability
2.3.5. Porosity
2.3.6. Filtration Resistance Analysis
3. Results and Discussion
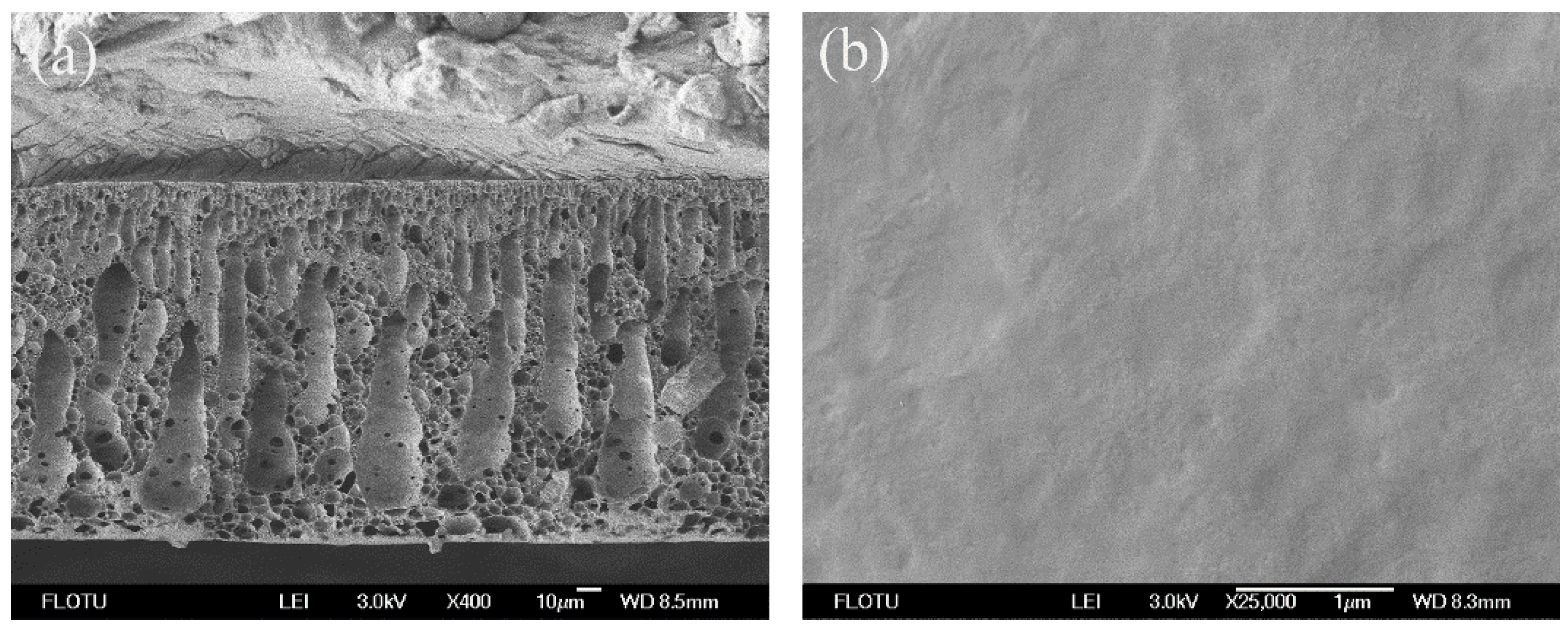
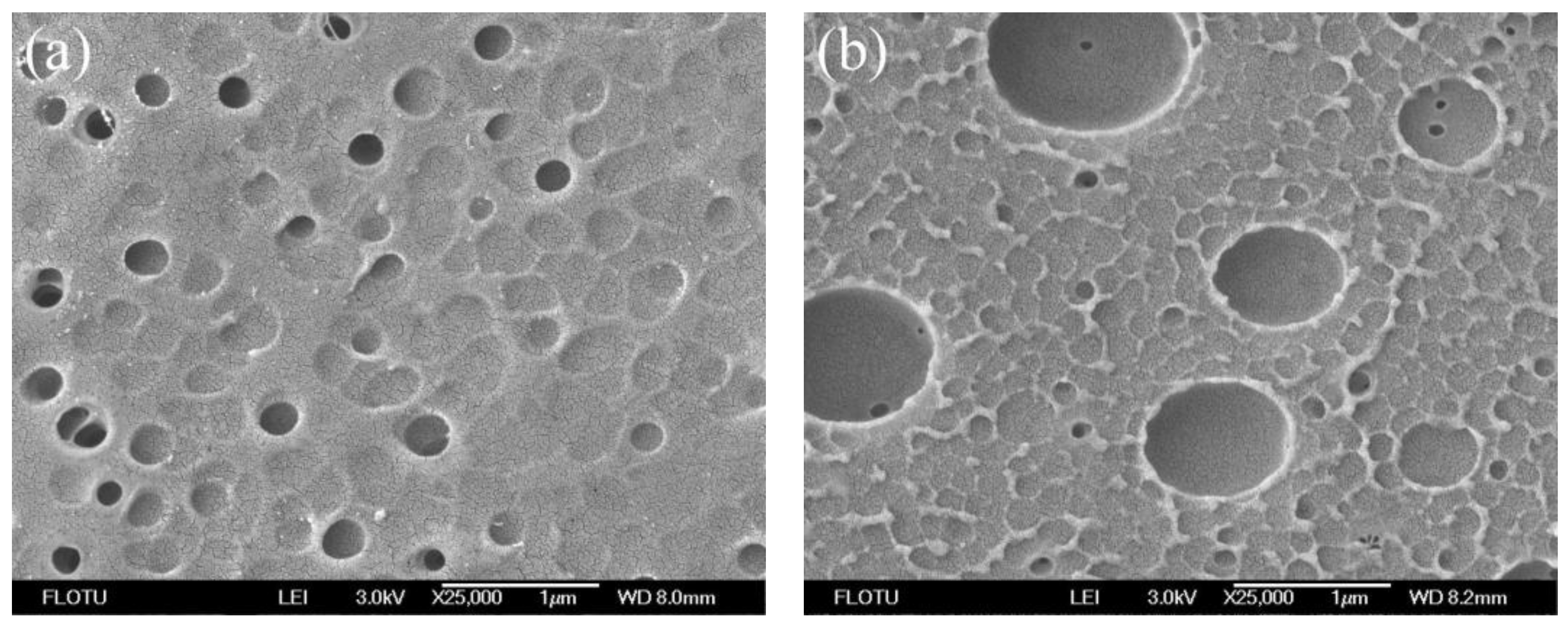
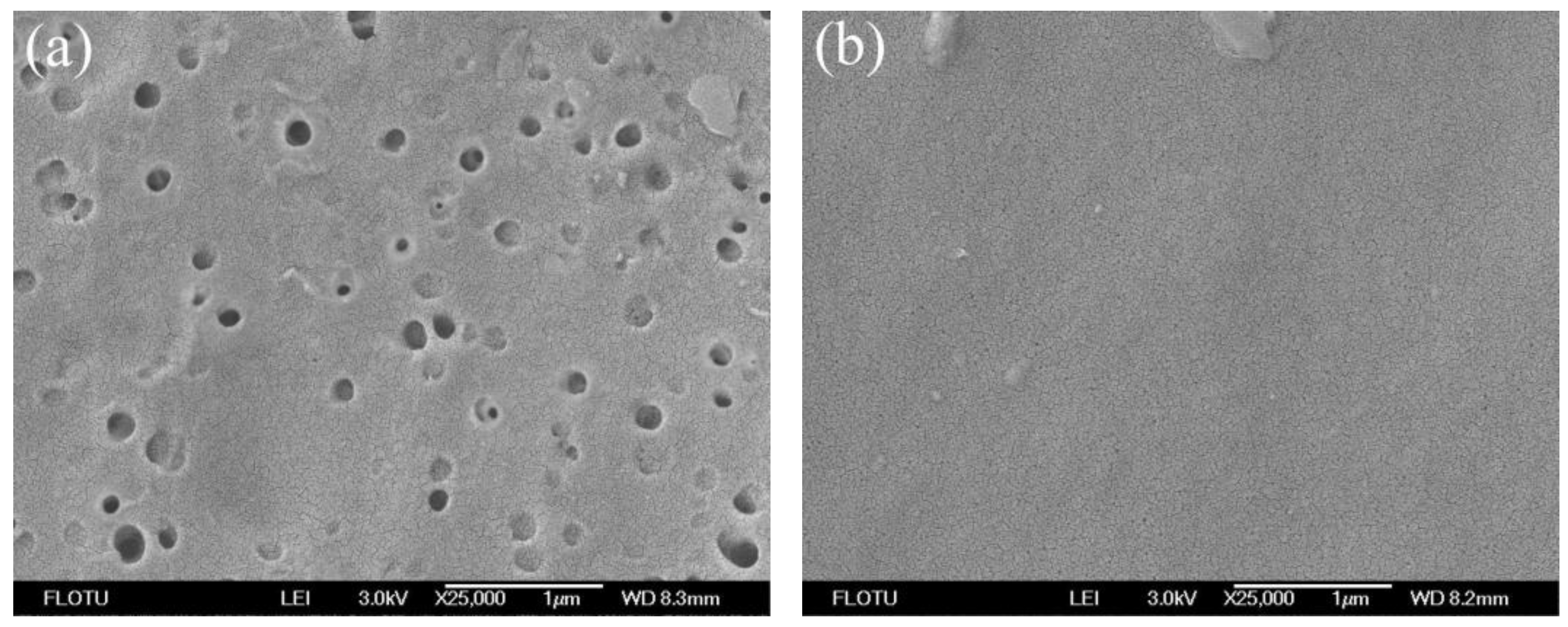
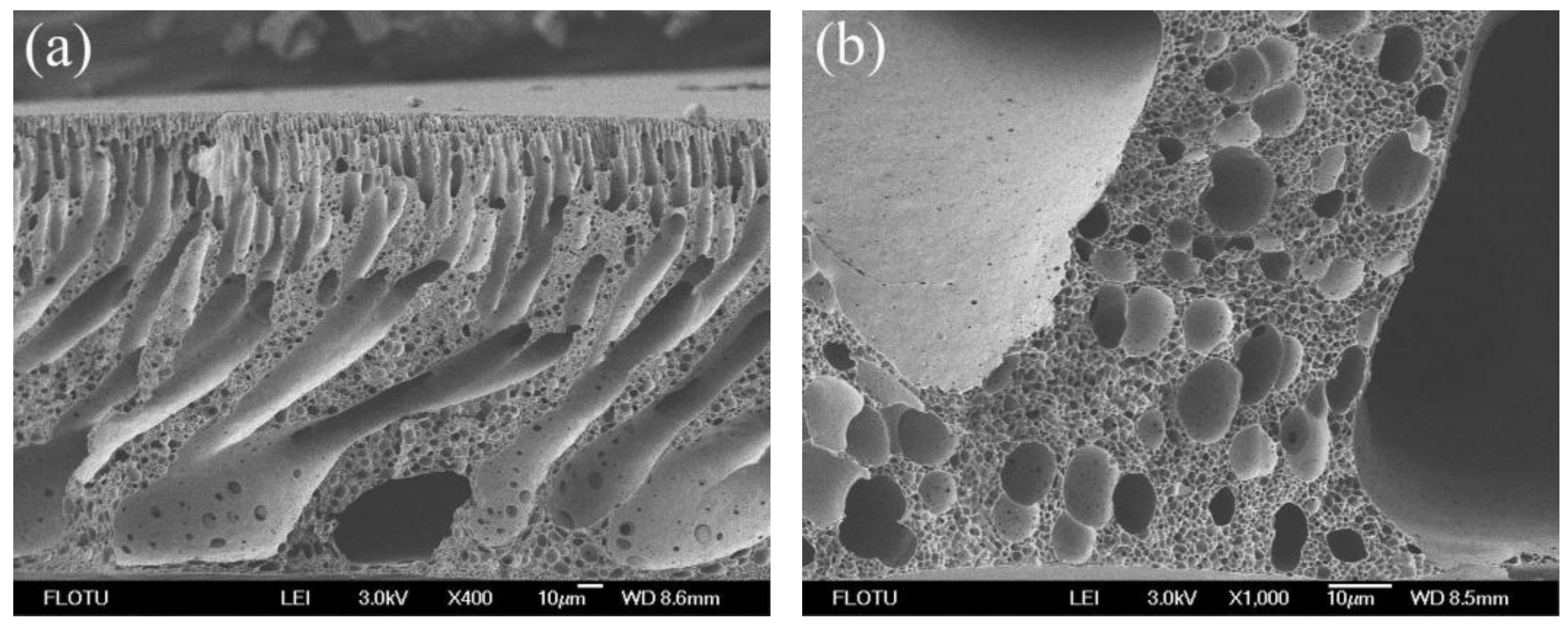
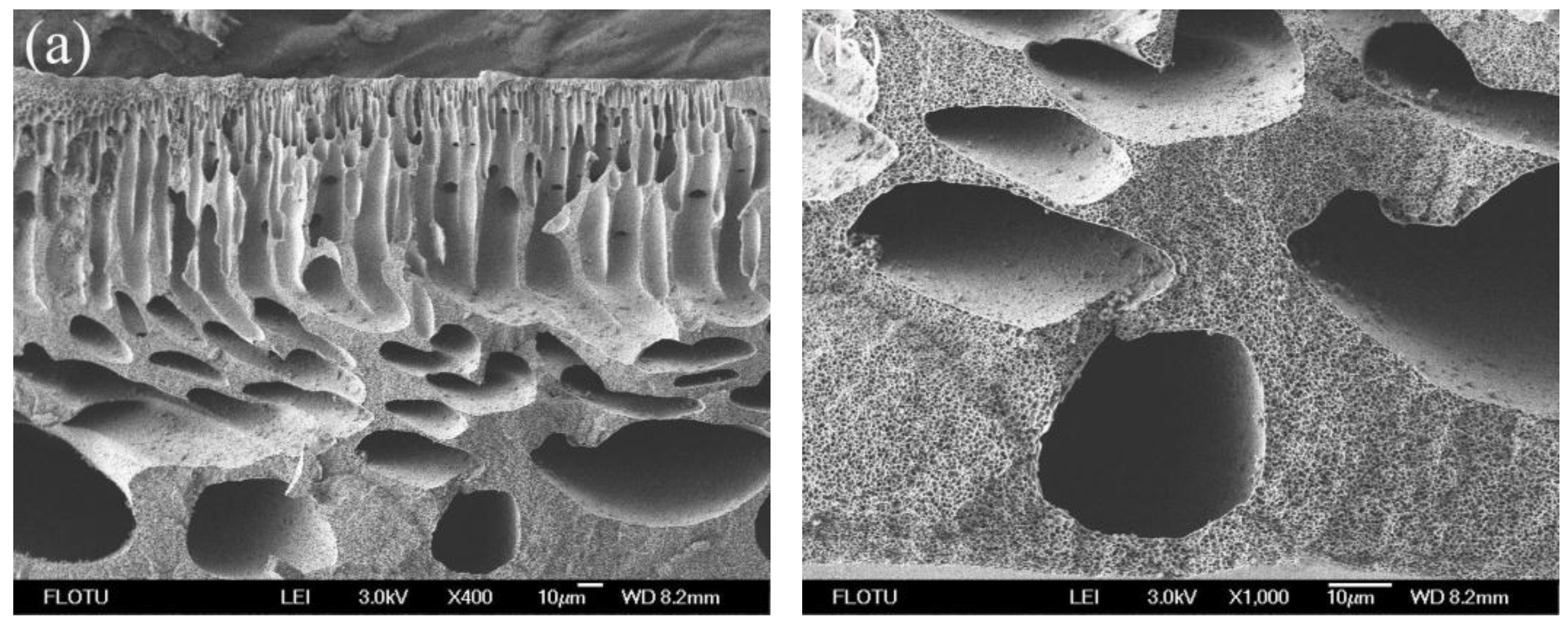
3.1. Morphological Studies
3.2. Structural Parameters and Properties of the Membranes
3.3. Analysis of Filtration Resistance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aghigh, A.; Alizadeh, V.; Wong, H.; Islam, M.S.; Amin, N.; Zaman, M. Recent advances in utilization of graphene for filtration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar]
- Qasim, M.; Darwish, N.A.; Sarp, S.; Hilal, N. Water desalination by forward (direct) osmosis phenomenon: A comprehensive review. Desalination 2015, 374, 47–69. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- He, Z.; Miller, D.J.; Kasemset, S.; Wang, L.; Paul, D.R.; Freeman, B.D. Fouling propensity of a poly (vinylidene fluoride) microfiltration membrane to several model oil/water emulsions. J. Membr. Sci. 2016, 514. [Google Scholar] [CrossRef]
- Chen, F.; Peldszus, S.; Peiris, R.H.; Ruhl, A.S.; Mehrez, R.; Jekel, M.; Legge, R.L.; Huck, P.M. Pilot-scale investigation of drinking water ultrafiltration membrane fouling rates using advanced data analysis techniques. Water. Res. 2014, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yu, W.; Liu, H.; Qu, J. Effect of low dosage of coagulant on the ultrafiltration membrane performance in feedwater treatment. Water. Res. 2014, 51, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hu, C.; Wang, X.; Xie, Y.; Jefferson, W.A.; Liu, H.; Qu, J. Effect of aluminum speciation on ultrafiltration membrane fouling by low dose aluminum coagulation with bovine serum albumin (BSA). J. Membr. Sci. 2015, 492, 88–94. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Mi, N.; Gao, Z.; Liu, T.; Lv, Y.; Wang, X.; Meng, X.; Yang, Y. Enhancement and mitigation mechanisms of protein fouling of ultrafiltration membranes under different ionic strengths. Environ. Sci. Technol. 2015, 49, 6574–6580. [Google Scholar] [CrossRef] [PubMed]
- Combe, C.; Molis, E.; Lucas, P.; Riley, R.; Clark, M. The effect of CA membrane properties on adsorptive fouling by humic acid. J. Membr. Sci. 1999, 154, 73–87. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N. Effect of membrane pore size and solution chemistry on the ultrafiltration of humic substances solutions. J. Membr. Sci. 2005, 255, 49–56. [Google Scholar] [CrossRef]
- Zhao, W.; He, C.; Wang, H.; Su, B.; Sun, S.; Zhao, C. Improved antifouling property of polyethersulfone hollow fiber membranes using additive of poly(ethylene glycol) methyl ether-b-poly(styrene) copolymers. Ind. Eng. Chem. Res. 2011, 50, 3295–3303. [Google Scholar] [CrossRef]
- Peeva, P.D.; Knoche, T.; Pieper, T.; Ulbricht, M. Performance of thin-layer hydrogel polyethersulfone composite membranes during dead-end ultrafiltration of various protein solutions. Ind. Eng. Chem. Res. 2012, 51, 7231–7241. [Google Scholar] [CrossRef]
- Hwang, L.L.; Tseng, H.H.; Chen, J.C. Fabrication of polyphenylsulfone/polyetherimide blend membranes for ultrafiltration applications: The effects of blending ratio on membrane properties and humic acid removal performance. J. Membr. Sci. 2011, 384, 72–81. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Praneeth, K.; James, T.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar]
- Karlsson, L.E.; Jannasch, P. Polysulfone ionomers for proton-conducting fuel cell membranes: 2. Sulfophenylated polysulfones and polyphenylsulfones. Electrochim. Acta 2005, 50, 1939–1946. [Google Scholar] [CrossRef]
- Decker, B.; Hartmann-Thompson, C.; Carver, P.I.; Keinath, S.E.; Santurri, P.R. Multilayer sulfonated polyhedral oligosilsesquioxane (S-POSS)-sulfonated polyphenylsulfone (S-PPSU) composite proton exchange membranes. Chem. Mater. 2009, 22, 942–948. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Merrington, A.; Carver, P.I.; Keeley, D.L.; Rousseau, J.L.; Hucul, D.; Bruza, K.J.; Thomas, L.S.; Keinath, S.E.; Nowak, R.M. Proton-conducting polyhedral oligosilsesquioxane nanoadditives for sulfonated polyphenylsulfone hydrogen fuel cell proton exchange membranes. J. Appl. Polym. Sci. 2008, 110, 958–974. [Google Scholar] [CrossRef]
- Jannasch, P. Fuel cell membrane materials by chemical grafting of aromatic main-chain polymers. Fuel Cells 2005, 5, 248–260. [Google Scholar] [CrossRef]
- Di Vona, M.L.; D’Epifanio, A.; Marani, D.; Trombetta, M.; Traversa, E.; Licoccia, S. SPEEK/PPSU-based organic-inorganic membranes: Proton conducting electrolytes in anhydrous and wet environments. J. Membr. Sci. 2006, 279, 186–191. [Google Scholar] [CrossRef]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Preparation and characterization of PPSU/PBNPI blend membrane for hydrogen separation. Int. J. Hydrogen. Energy 2008, 33, 4178–4182. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Jansen, J.C.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; van der Bruggen, B. Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Membr. Sci. 2011, 379, 60–68. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.C.; Tocci, E.; Bazzarelli, F.; Bernardo, P.; Luis, P.; Degrève, J.; Drioli, E.; van der Bruggen, B. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Membr. Sci. 2011, 384, 89–96. [Google Scholar] [CrossRef]
- Jansen, J.C.; Darvishmanesh, S.; Tasselli, F.; Bazzarelli, F.; Bernardo, P.; Tocci, E.; Friess, K.; Randova, A.; Drioli, E.; van der Bruggen, B. Influence of the blend composition on the properties and separation performance of novel solvent resistant polyphenylsulfone/polyimide nanofiltration membranes. J. Membr. Sci. 2013, 447, 107–118. [Google Scholar] [CrossRef]
- Jullok, N.; Darvishmanesh, S.; Luis, P.; van der Bruggen, B. The potential of pervaporation for separation of acetic acid and water mixtures using polyphenylsulfone membranes. Chem. Eng. J. 2011, 175, 306–315. [Google Scholar] [CrossRef]
- Tang, Y.; Widjojo, N.; Shi, G.M.; Chung, T.S.; Weber, M.; Maletzko, C. Development of flat-sheet membranes for C1–C4 alcohols dehydration via pervaporation from sulfonated polyphenylsulfone (sPPSU). J. Membr. Sci. 2012, 415, 686–695. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.L.; Wu, C.R. Effect of annealing conditions on crystallization behavior and mechanical properties of NIPS poly(vinylidene fluoride) hollow fiber membranes. J. Appl. Polym. Sci. 2013, 129, 1417–1425. [Google Scholar] [CrossRef]
- Keurentjes, J.T.F.; Harbrecht, J.G.; Brinkman, D.; Hanemaaijer, J.H.; Stuart, M.A.C.; Riet, K.V.T. Hydrophobicity measurements of microfiltration and ultrafiltration membranes. J. Membr. Sci. 1989, 47, 333–344. [Google Scholar] [CrossRef]
- Yun, Y.; Le-Clech, P.; Dong, G.; Sun, D.; Wang, Y.; Qin, P.; Chen, Z.; Li, J.; Chen, C. Formation kinetics and characterization of polyphthalazinone ether ketone hollow fiber ultrafiltration membranes. J. Membr. Sci. 2012, 389, 416–423. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (PES)-silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Lee, S.Y.; Lee, J.M.; Woo, K.T.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Poly(vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Song, Z.; Zhi, W.; Xin, W.; Zhao, B.; Wang, J.; Yang, S.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane using PANiEB as both pore forming agent and hydrophilic modifier. J. Membr. Sci. 2011, 385, 251–262. [Google Scholar]
- Rai, P.; Rai, C.; Majumdar, G.; DasGupta, S.; De, S. Resistance in series model for ultrafiltration of mosambi (Citrus sinensis (L.) Osbeck) juice in a stirred continuous mode. J. Membr. Sci. 2006, 283, 116–122. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, Netherland, 1996; Volume 203, p. 263. [Google Scholar]
- Strathmann, H.; Kock, K.; Amar, P.; Baker, R.W. The formation mechanism of asymmetric membranes. Desalination 1975, 16, 179–203. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Qin, P.; Chen, C.; Han, B.; Takuji, S.; Li, J.; Sun, B. Preparation of poly(phthalazinone ether sulfone ketone) asymmetric ultrafiltration membrane: II. The gelation process. J. Membr. Sci. 2006, 268, 181–188. [Google Scholar] [CrossRef]
- Yun, Y.; Tian, Y.; Shi, G.; Li, J.; Chen, C. Preparation, morphologies and properties for flat sheet PPESK ultrafiltration membranes. J. Membr. Sci. 2006, 270, 146–153. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.T.; Tay, J.H. Effect of additives on the fabrication of poly(vinylidene fluoride-co-hexafluropropylene)(PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2008, 315, 195–204. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation and performance of polysulfone-sulfonated poly(ether ether ketone) blend ultrafiltration membranes. Part I. Appl. Surf. Sci. 2007, 253, 8705–8712. [Google Scholar] [CrossRef]
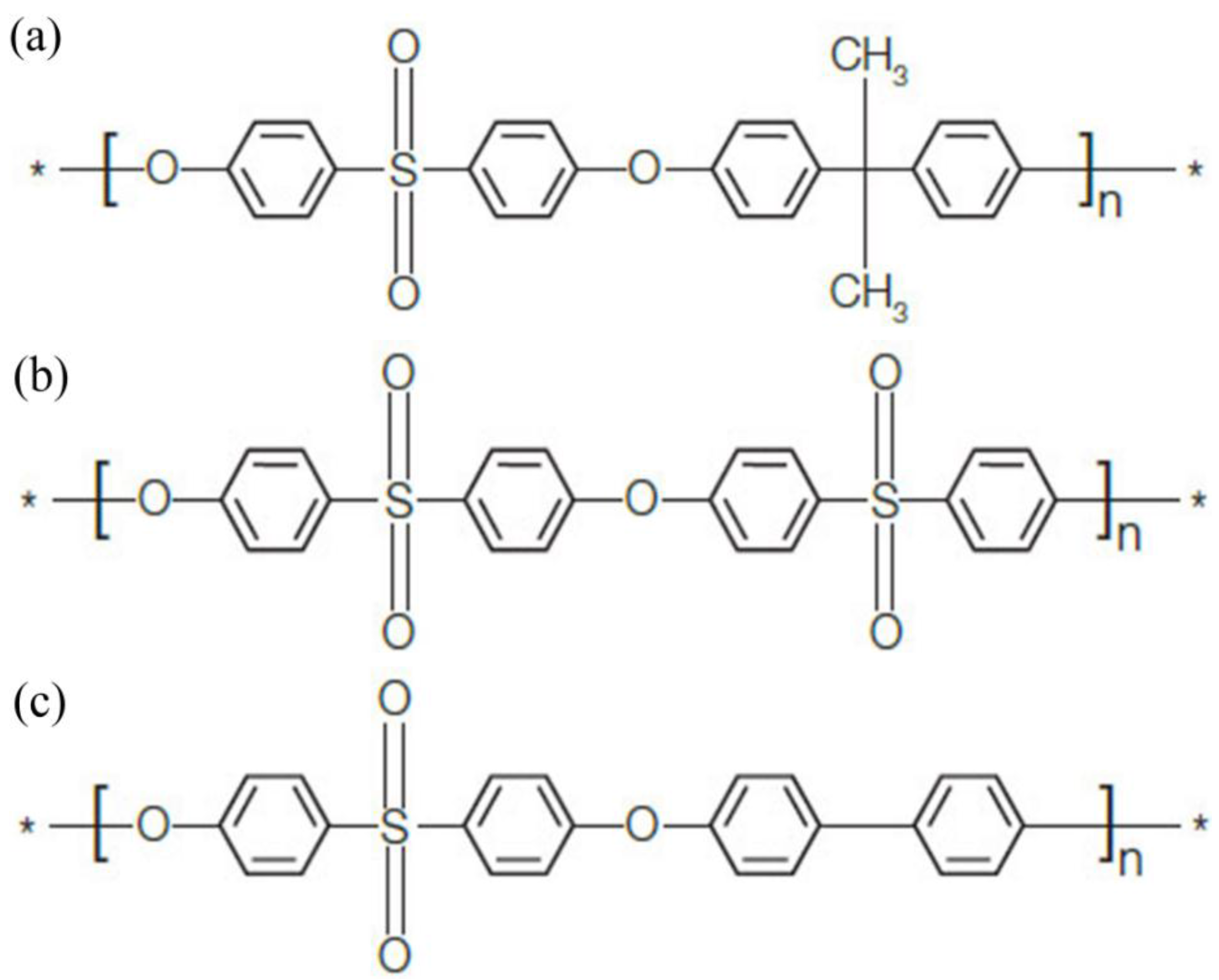



| Preparation Conditions | Parameters |
|---|---|
| Solution temperature (°C) | 70 |
| Coagulation bath | pure water |
| Coagulant temperature (°C) | 30 |
| Ambient temperature (°C) | 25 |
| Air humidity (%) | 40–50 |
| Membrane Samples | Casting Solution Compositions |
|---|---|
| PPSU1 | PPSU:NMP = 15:85 |
| PPSU2 | PPSU:PVP:NMP = 15:10:75 |
| PPSU3 | PPSU:PVP:Tween-80:PG:NMP = 15:6:3:1:75 |
| PPSU4 | PPSU:PVP:Tween-80:PG:NMP = 15:9:4.5:1.5:70 |
| PPSU5 | PPSU:PEG:NMP = 15:10:75 |
| PPSU6 | PPSU:PEG:Tween-80:PG:NMP = 15:6:3:1:75 |
| Samples | Maximum Pore Size (μm) | Porosity (%) | Contact Angle (°) | Jo (L/m2·h) | Jt (L/m2·h) | Ji (L/m2·h) | BSA Rejection (%) |
|---|---|---|---|---|---|---|---|
| PPSU2 | 0.31 ± 0.02 | 51.2 ± 3.1 | 65.1 ± 1.0 | 80.4 ± 3.3 | 31.3 ± 1.6 | 48.3 ± 1.0 | 53.2 ± 1.5 |
| PPSU3 | 0.21 ± 0.01 | 71.5 ± 2.1 | 63.0 ± 1.2 | 148.1 ± 2.3 | 52.3 ± 1.1 | 90.6 ± 0.9 | 81.5 ± 1.7 |
| PPSU4 | 0.26 ± 0.03 | 80.6 ± 4.5 | 54.4 ± 2.0 | 183.4 ± 1.4 | 63.6 ± 1.4 | 117.1 ± 1.9 | 70.1 ± 3.3 |
| PPSU5 | 0.29 ± 0.02 | 50.2 ± 3.4 | 67.3 ± 1.5 | 74.3 ± 2.4 | 29.3 ± 1.9 | 45.7 ± 1.3 | 62.3 ± 2.4 |
| PPSU6 | 0.18 ± 0.01 | 67.9 ± 2.4 | 60.5 ± 2.3 | 129.5 ± 1.7 | 43.9 ± 1.5 | 75.4 ± 0.8 | 89.5 ± 1.7 |
| Samples | Rm × 1011 (m−1) | Rp × 1011 (m−1) | Rc × 1011 (m−1) | Rt × 1011 (m−1) |
|---|---|---|---|---|
| PPSU2 | 2.00 ± 0.31 | 1.34 ± 0.10 | 0.84 ± 0.15 | 4.18 ± 0.76 |
| PPSU3 | 1.08 ± 0.23 | 0.70 ± 0.19 | 0.72 ± 0.14 | 2.50 ± 0.53 |
| PPSU4 | 0.88 ± 0.13 | 0.50 ± 0.12 | 0.68 ± 0.11 | 2.06 ± 0.41 |
| PPSU5 | 2.17 ± 0.27 | 1.36 ± 0.34 | 0.94 ± 0.19 | 4.47 ± 0.63 |
| PPSU6 | 1.24 ± 0.15 | 0.90 ± 0.22 | 0.84 ± 0.17 | 2.98 ± 0.35 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties. Membranes 2016, 6, 35. https://doi.org/10.3390/membranes6020035
Liu J, Zhong Z, Ma R, Zhang W, Li J. Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties. Membranes. 2016; 6(2):35. https://doi.org/10.3390/membranes6020035
Chicago/Turabian StyleLiu, Jie, Zhencheng Zhong, Rui Ma, Weichen Zhang, and Jiding Li. 2016. "Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties" Membranes 6, no. 2: 35. https://doi.org/10.3390/membranes6020035





