1. Introduction
Solid oxide fuel cells (SOFCs) are power generation devices that directly convert fuels into electricity via electrochemical reactions through clean and highly efficient operations. To improve the reliability and stability of the cells, lowering the operating temperature to an intermediate-temperature range (below 800 °C) [
1,
2] is one of the main targets in the recent research efforts. These advantages of lower-temperature operation, including the ease of material selection, reduction in total cost and accelerating the start-up process, are generally recognized. At present, the operation of SOFCs at 650 °C to 800 °C is currently feasible for a SOFC using ultrathin yttria-doped zirconia [
3]. However, a lower operating temperature results in sluggish oxygen reduction reaction (ORR) at the cathode and apparently increases the resistance of the electrolyte/cathode, potentially degrading the performance of SOFCs [
4,
5].
High performance cathodes based on La-perovskite (LaMO
3) materials have been studied, and it was recently reported that cobalt-containing (La,Sr)CoO
3 perovskites exhibit the best activity of electro-catalytic ORR at intermediate temperatures (600 °C–800 °C). Since cobalt is a good catalyst for reduction oxygen molecules to ions and flexible redox behavior via valence transition [
4], the cobalt containing perovskites, e.g., La
0.6Sr
0.4Fe
0.8Co
0.2O
3−δ (LSFC6482), have a good electronic conductivity, However, they have very low ionic conductivity and the high coefficient of thermal expansion (CTE) of the LSFC cathode mismatches to that of zirconia-based electrolyte, which is also unfavorable to thermal cycling of the cells [
6,
7,
8,
9,
10]. Besides, an interfacial reaction might occur in various cathode/yttria-stabilized zirconia (YSZ) combinations. Amorphization of YSZ by the diffusion of the Mn from (La,Sr)MnO
3−δ cathode [
11], or the formation of a new Co-phase by (La,Sr)(Fe,Co)O
3−δ [
12] are reported in the literature.
A new cathode material based on (Bi,Sr)FeO
3 (BSF) was synthesized and reported with interesting conductivity [
13,
14,
15,
16,
17,
18,
19,
20,
21]. To enhance the conductivity and ORR capability, Co [
18,
21], Ca [
19] and Mn [
20] were doped in the BSF perovskite structure. Besides, a higher oxygen exchange rate of pore-free BSF was reported, even higher than that of (La,Sr)FeO
3−δ. The literature [
21] concludes that the BSF structure with large ionic sizes of Bi
3+ and Sr
2+ at A-site, and Fe
3+ in B-site are basically performed favorable oxygen ion conductivity and matched CTE to the Y-doped ZrO
2 electrolyte. The substitution of A and B sites in the perovskite structure also affect the oxygen stoichiometry. A controversial report on the low oxygen stoichiometry of (Bi,Sr)FeO
3−δ varying over the range of 0 < δ < 0.016 [
19] and δ = 0.23 [
15], is noted in the literature. Besides, the measurement method of the ionic conductivity, i.e., electrochemical polarization by electric impedance spectroscopy (EIS), was used in many papers and might give over-promising conductivity values (1.4 × 10
−2–2.6 × 10
−1 S·cm
−1) of the BSF at 650 °C. The ionic conductivity of the ferrites with respect to oxygen deficiency needs careful verification.
A series of Sr-doped LaMnO
3 cathode material likely enhances the electronic conductivity due to the charge exchange of Sr
La and Mn
n+ ions in the high-temperature conditions of the stoichiometry composition [
11,
22]. The possible reaction is shown as below:
This likely goes through the double-exchange of electrons between neighboring cations (M). In a more general form, the double-exchange conductive mechanism of moving electron between cations can also be shown as below when considering the presence of oxygen between the cations (M
+):
An additional possible conductive mechanism, small polaron, was proposed by Tuller and Nowick [
23], which was mainly controlled by the existence of sufficient oxygen vacancy concentration, ≥1%. The association of electron with point defects, e.g., oxygen vacancies, inducing rapid transport of electron between the vacancies is generally accepted due to a weak polarization interaction, and is used in the interpretation of oxide conductivity at high temperature [
24,
25].
This study uses a molecular-scale synthesis method (termed the “EDTA-citrate method”) to fabricate homogeneous (Bi
1−xSr
x)(Fe
1−yM
y)O
3−δ powders, and low temperature sintering to obtain various porous cathode materials. Therefore, we can understand the conductive mechanisms of the cathode materials. The electric conductivity, including ionic and electronic, will be measured by the two-probe and four-probe ionic DC method [
26]. The mixed-ionic-electric (MIE) property of the doped ferrites is tailored in this study to optimize the electrochemical properties of the cathodes and used to improve the performance of made fuel cells.
3. Discussion
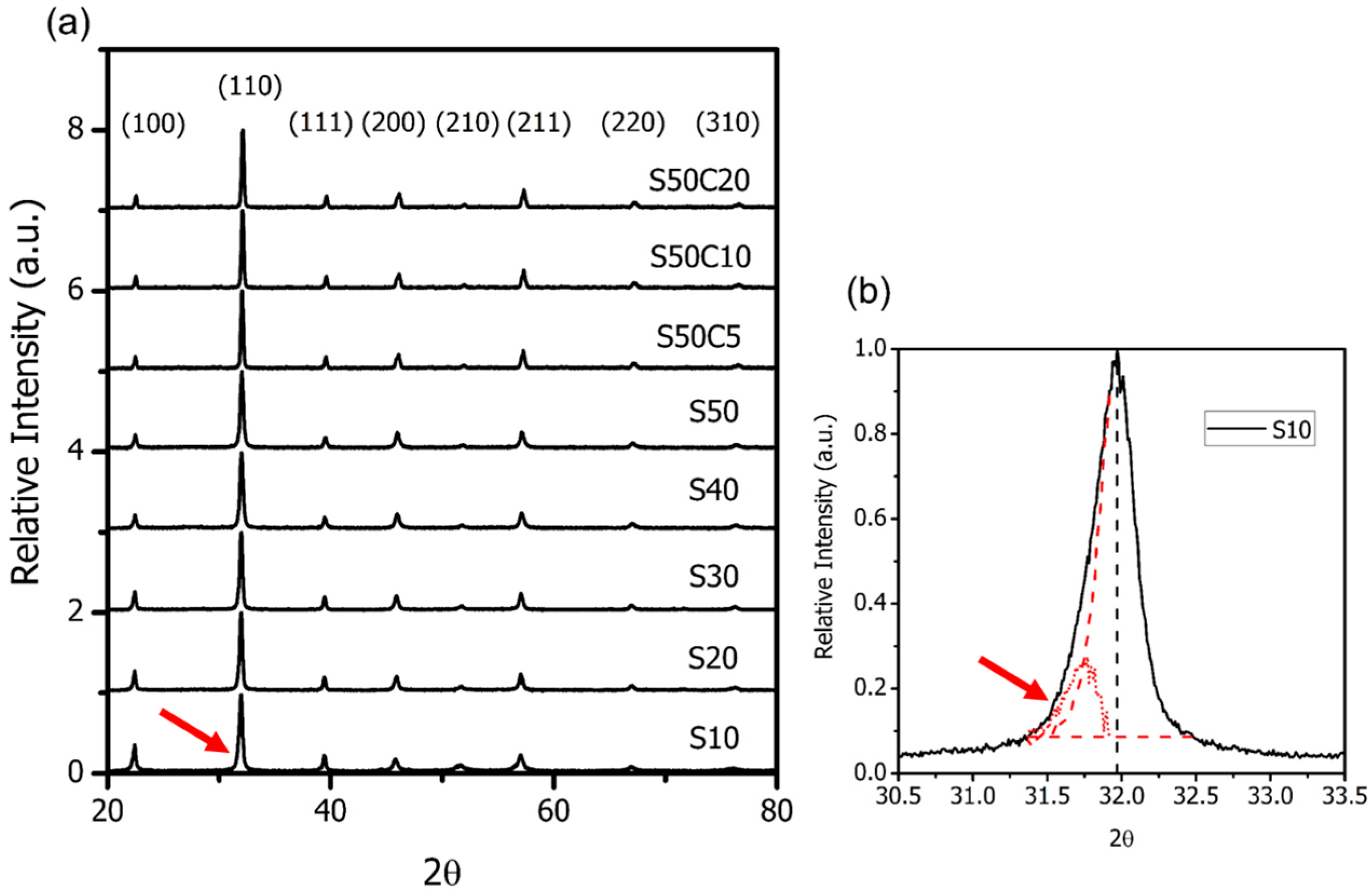
A substitution of trivalent cation (e.g., Bi
3+) on the A-site of perovskite by divalent cation (e.g., Sr
2+) induces two possible reactions in the materials. One is the increase in the valence state of B-site cations, and the other is the formation of oxygen vacancy. If the B-site cations are transition metals (M), the change in valence state creates M
n+/M
(n+1)+ couples, which act as the hopping sites for electrons via neighboring oxygen, as shown in Equation (2). A three-dimensional MO
6 octahedral network [
36] in the perovskite structure acts as the double exchange media and induces electronic conduction, causing valence exchange along the MO
6 octahedron, as shown in Equation (2) [
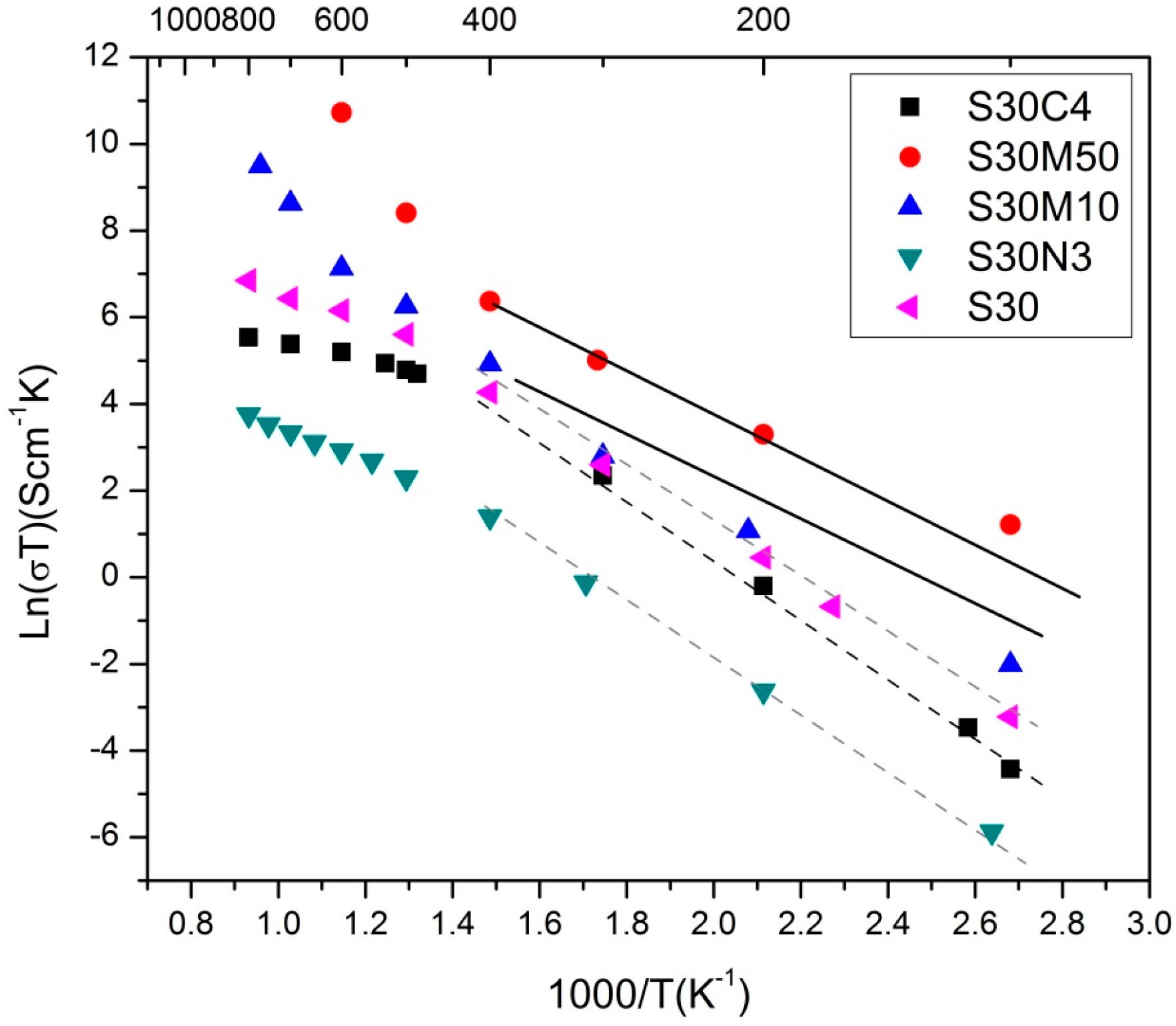
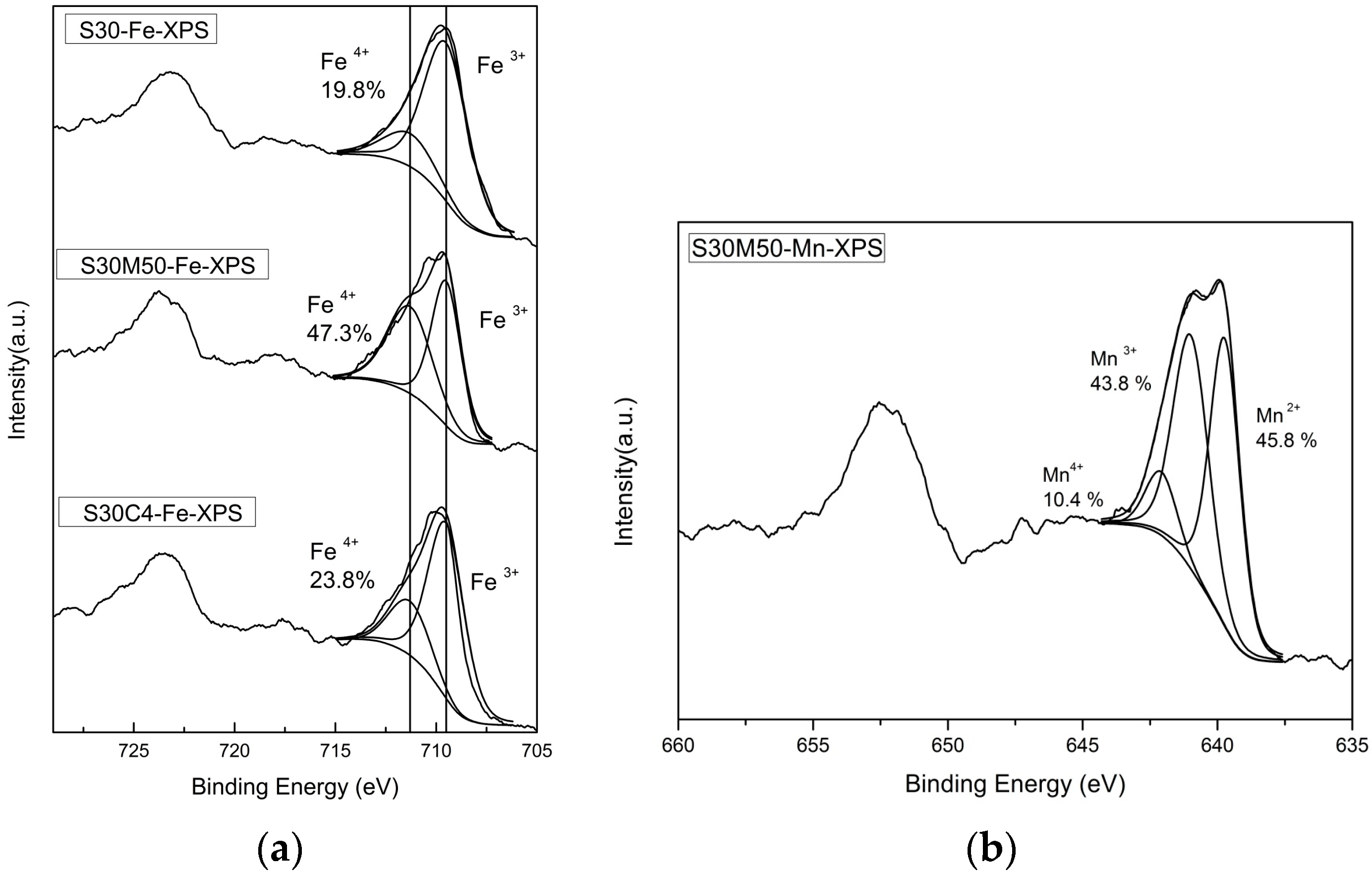
37]. The electrical conductivity of S30 (
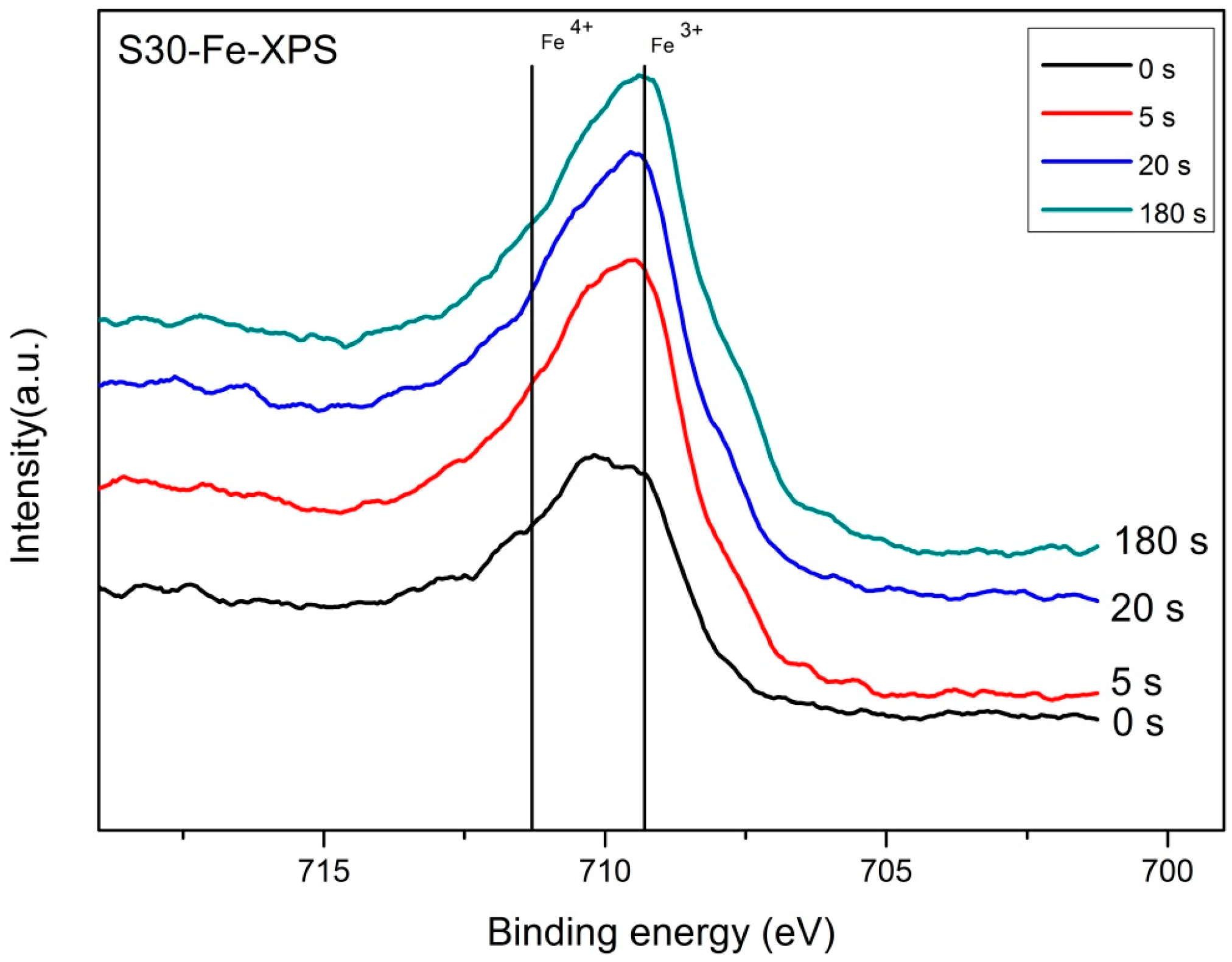
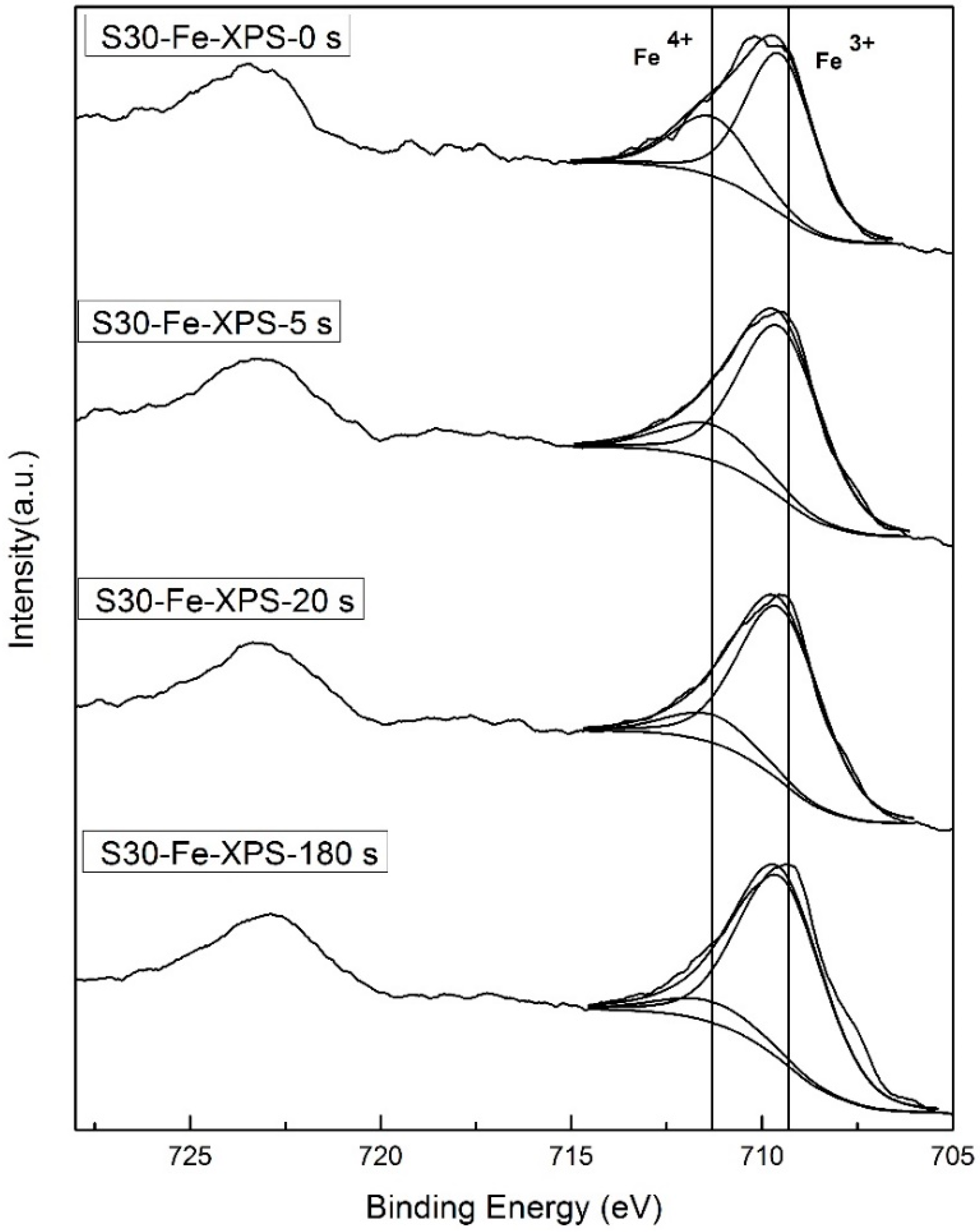
Figure 2a) and XPS results (
Figure 5 and
Figure 6) depicting the valence states of Fe (4
+ and 3
+) in nearly equal quantity at the B-sites are mostly favored as hopping sites (e.g., Fe
3+/Fe
4+ and Mn
3+/Mn
4+) and generates electron conduction at low temperatures.
The ionic conductivity of the perovskite/fluorite structure occurs in more complex way at higher temperature via the vacancy mechanism of oxygen ion diffusion. Two factors, the concentration of the oxygen vacancy and the mobility µο of the oxygen vacancy are effective and affect the oxygen ionic conductivity in the perovskite structure.
However, there are several factors influencing the ionic mobility, basically, to which the ions block transport of the oxygen in the perovskite structure.
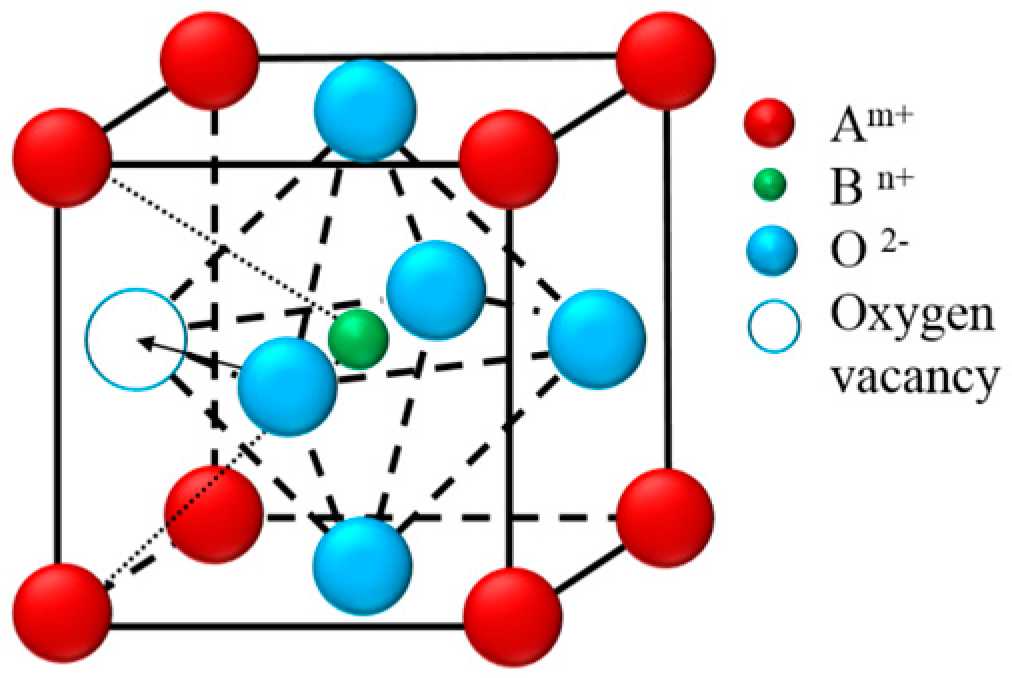
Several structural factors have been proposed to explain the relationship of ionic transportation behavior with the oxygen vacancies, etc. These factors can be explained by a schematic diagram in
Figure 9.
Goldschmidt et al. [
38] defined a tolerance factor,
S, to reveal the stability of the perovskite structure, as shown below:
where
rA and
rB are the mean ionic radii for A- and B-site cations, and
rO is the radius of an oxygen ion. For a stable perovskite structure, the tolerance factor
S is between 0.77 and 1.0. When
S = 1.0, a perfect cubic structure is achieved, and the B–O–B bond angle is 180°; when
S < 1.0, the lattice structure tends to change from cubic to rhombohedral and to orthorhombic.
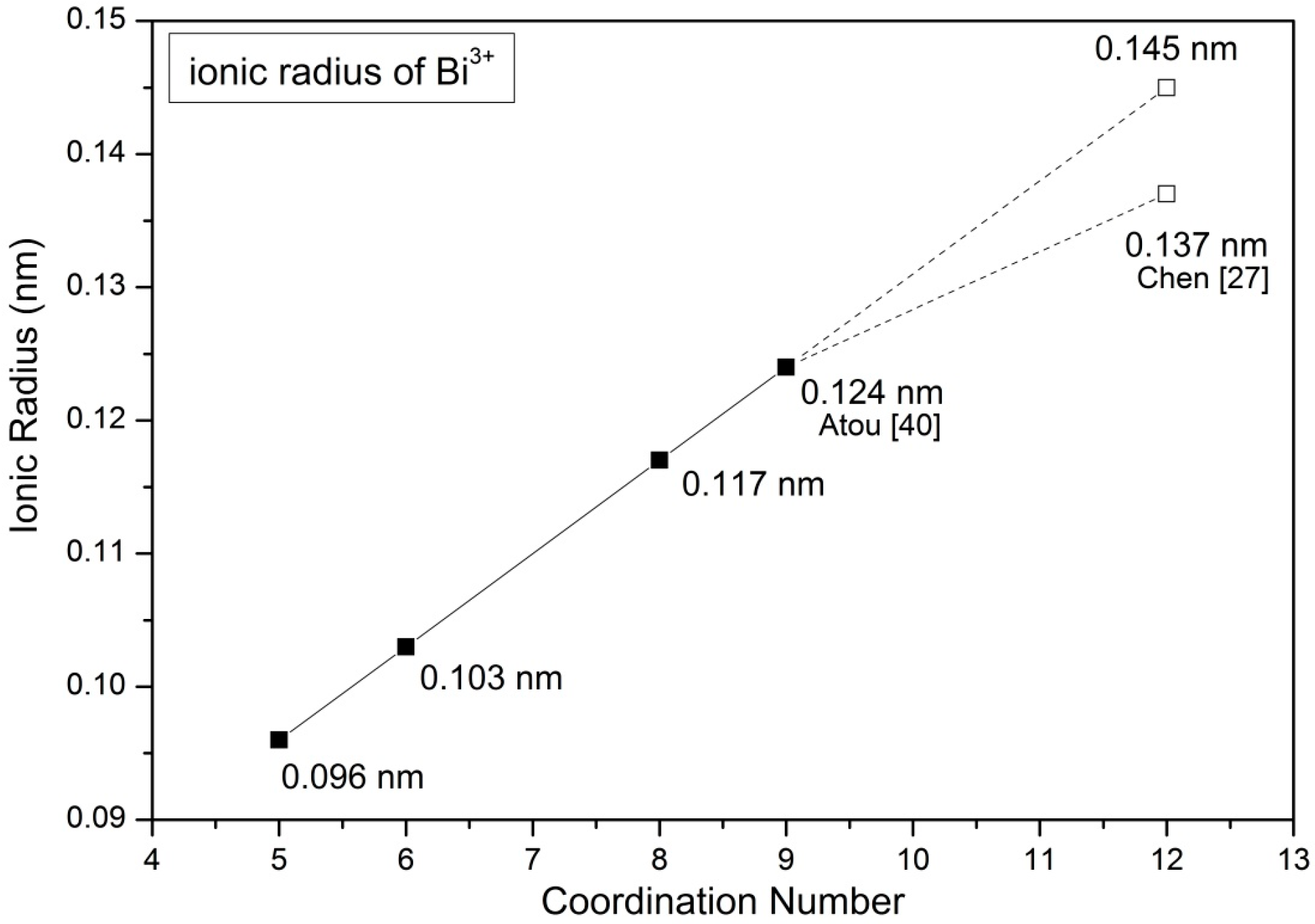
Table 5 shows the ionic radii of various cations of a specified coordination number (CN) referenced from Shannon [
39]. Since the ionic radius of Bi
3+ of CN = 12 is not reported in the literature, one extrapolated value was calculated from a linear relationship between the reported ionic radii and CNs, as shown in
Figure 10. An ionic size 1.45 Å of Bi
3+ is obtained, which is slightly greater than 1.37 Å calculated from X-ray data in our previous work [
27].
If the value 1.45 Å of Bi
3+ (CN = 12) was used to calculate the tolerance factor of LSCF6428, S30, S30N3 and S30M50, the results are shown in
Table 6. Since the
S values of all samples are less and close to 1.0, the result indicates all BSFM samples have a stable perovskite structure.
Other than the tolerance factor, Richter et al. [
36] also proposed another parameter, critical radius
rcr, to describe the open space of the triangle (
Figure 9) allowing oxygen ion diffusion in the perovskite structure, and it could be calculated by the following equation:
where
a0 is the pseudo-perovskite lattice parameter, and
a0 can be derived experimentally or as below [
41] if the structure is nearly cubic:
In other words, the
rcr describes the maximum size of the gap for a mobile oxygen ion to pass through the “saddle-point” between the triangle surround by two A-site cations and one B-site cation. The critical radii
rcr of four samples are calculated and shown in
Table 5. As all critical radii are close to 0.90 Å and far smaller than the oxygen ions radius (1.40 Å in six-fold coordination or 1.36 Å in three-fold coordination in the saddle-point), the oxygen ions need a strong thermal vibration of the lattice to assist the migration [
36].
Sammells et al. [
42] introduced another parameter, lattice free volume
Vf, which was defined as equal to the difference in the unit cell volume (
V =
and the total volume of the constituent ions. Moreover, Hayashi et al. [
43] modified the
Vf and proposed the other parameter, the specific free volume (
Vsf) to describe the same diffusion phenomena:
They used the parameters,
Vf and
Vsf, as the bases to compare the free space in the perovskite structure of different compositions. The larger the
Vsf, the lower the driving force and activation energy of the migration. Based on the calculated results shown in
Table 5, the difference between the two structural factors, the tolerance factor and critical radius are not significant, having less than 1% difference. However, a
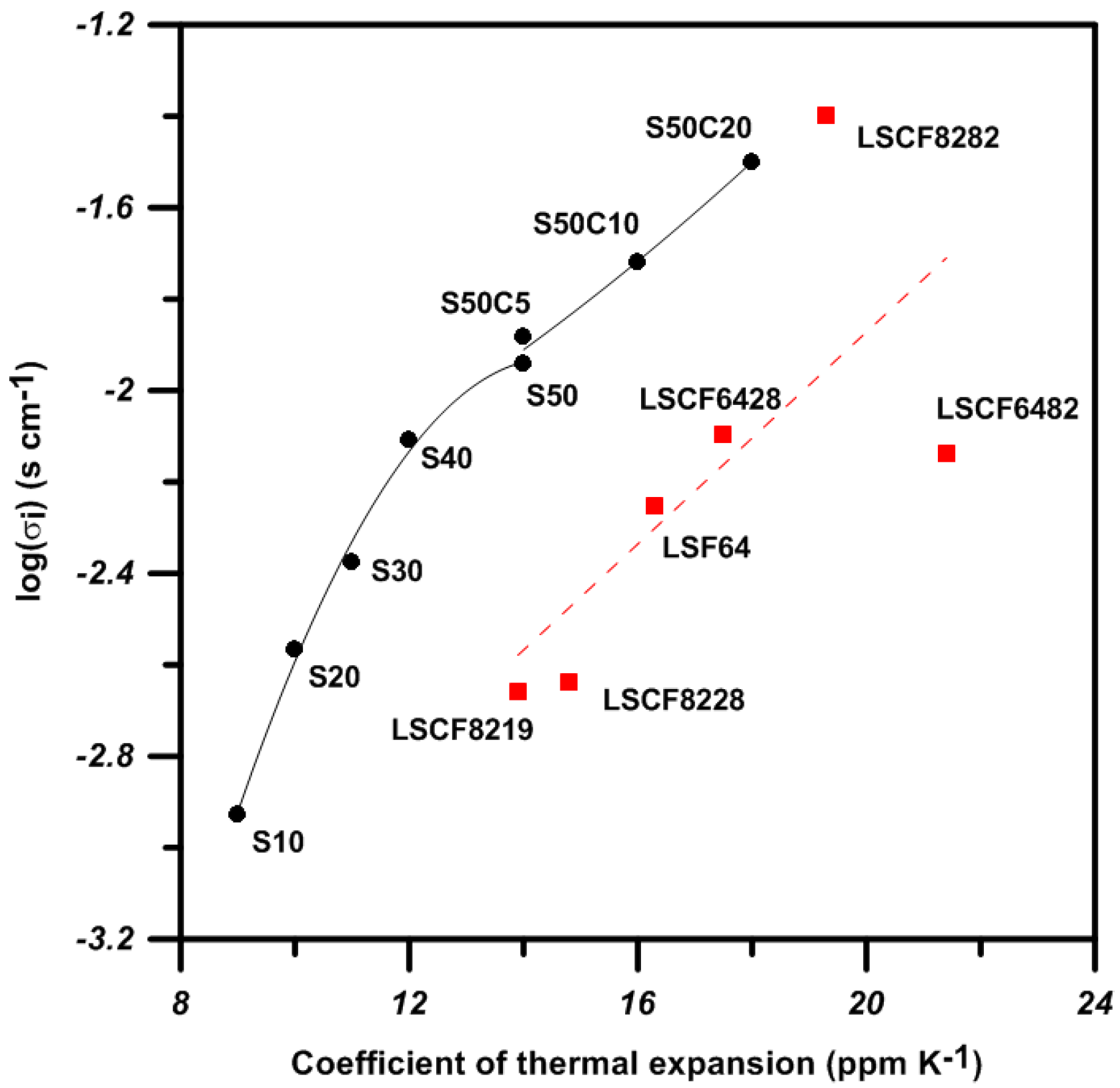
Vsf valued of S30 and S30M50 being 10% more than that of LSCF6428 provides more free space for mobile oxygen diffusing in the perovskite structure. Therefore, the structural advantage of S50C20 offering a lower activation energy (
Ea) of 1.2 eV and a higher mobility (µ
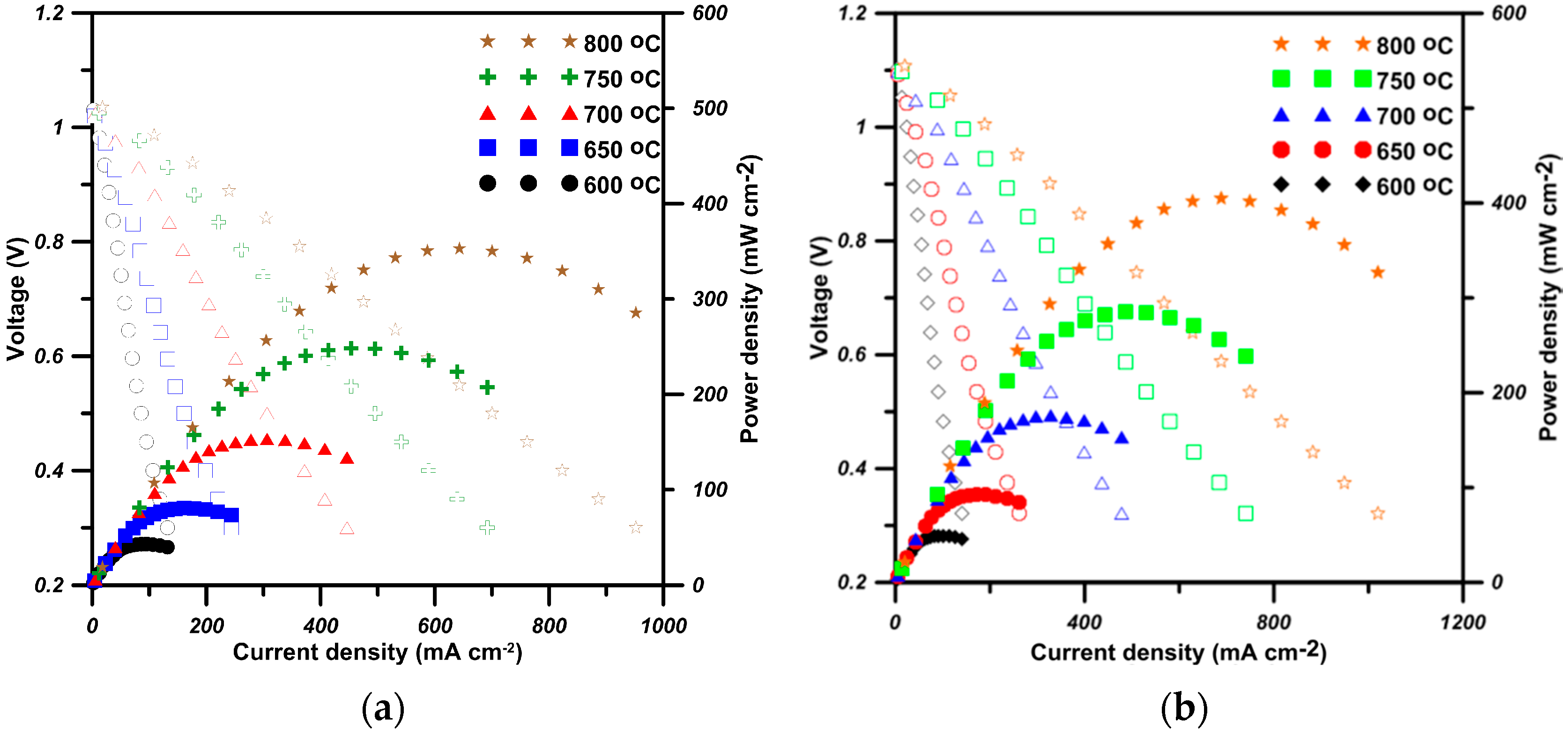
0) of oxygen migration are expected. The power density output of the cell with the S50C20 cathode is better than that with LSCM6328.
4. Materials and Experimental Procedures
4.1. Synthesis of Cathode Materials
Bi
0.7Sr
0.3Fe
1−xM
xO
3−δ powders (M = Co, Ni, Mn) were synthesized by the EDTA-citric method [
44]. Bi
2O
3 (Solar Applied Materials Technology Corp., Tainan City, Taiwan) was dissolved in a HNO
3 solution (65%, Sigma-Aldrich, St. Louis, MO, USA), and Sr(NO
3)
2 (Showa Chemical Industry Co., Ltd., Tokyo, Japan), Fe(NO
3)
3·9H
2O (Showa Chemical Industry Co., Ltd.), Ni(NO
3)
2·6H
2O (Showa Chemical Industry Co., Ltd.), MnN
2O
6·4H
2O (Alfa Aesar, Ward Hill, MA, USA) were separately dissolved in deionized (DI) water to obtain the solutions for a 0.1 M molar concentration. EDTA (ACROS, Geel, Belgium) was dissolved in the NH
4OH solution (J.T. Baker Chemical, Center Valley, PA, USA) as a buffer solution. The nitrite solutions were mixed together as the formulation BSFM specified, then added drop-by-drop into the buffer solution, following the addition of citric acid (Showa Chemical Industry Co., Ltd.) in a molar ratio of EDTA/cations/citric acid = 1:1:2. After the solution was mixed and stirred, NH
4OH was added to control pH to 7.0. Then the solution was heated on a hot plate at 200 °C until a gel solution was obtained. The dried gel was combusted at 200 °C in an oven and left overnight. The ash-like powder was obtained and calcined at 800 °C–900 °C to obtain a single-phase powder.
4.2. Preparation of Fuel Cell
SOFC was prepared by the following procedures. In brief, 8 mol % yttria stabilized zirconia (8YSZ, Tosoh Co., Tokyo, Japan), NiO (F grade, INCO Ltd., Toronto, ON, Canada), and mesophase carbon powder (MCMB, National Chung-Shan Institute of Science & Technology, Taoyuan, Taiwan) in a mass ratio of 45:45:10 were used to prepare three separated slurries in a solid loading of 28 vol %. The slurries contained de-ionized water as a liquid carrier and Darvan C (PMAA-N, Vanderbilt Co. Ltd., Norwalk, CT, USA) as dispersant. After ball-milling for 24 h, the slurries in a specified composition of the anode, were mixed for an additional 1 h, and then dried to powder form. The anode powder was pressed into the pellets of 20 mm in diameter and 1.0 mm in thickness. The pellets were calcined at 1000 °C for 2 h in air before spin-coating of the 8YSZ layer.
The slurry for spin-coating of 8YSZ electrolyte layer was prepared by mixing YSZ powder (TZ-8Y, Tosoh, Tokyo, Japan) with ethyl cellulose (E0266, TCI, Tokyo, Japan) and terpineol (regent grade, ACROS, NJ, USA) in a mass ratio of 30:2:68. The mixture was ball-milled for 24 h until the slurry was stable and homogeneous. Spin-coating of the YSZ slurry on the anode was conducted in three steps: (1) a few drops of the slurry were coated onto a spinning anode pellet in a turning rate increasing from 0 to 6000 rpm; (2) the spinning speed was kept at 6000 rpm for 60 s; and (3) after coating, the film and the substrate were co-calcined at 500 °C for 10 min. In some cases, the coating was repeated up to three times. A thick coating, made by three cycles, for example, was capable of forming a ca. 15 µm thickness 8YSZ electrolyte layer after sintering. Finally, the coated pellets (half cells) were sintered at 1400 °C for 4 h. A layer of cathode with the pastes of LSFC6482 was screen-printed on a half cell. The S50C20 cathode in an area of 1.00 cm diameter, and a thickness of ca. 10 µm was made. After drying and binder-burn-out of the printed cathode layer, the cell was sintered at 1000 °C for 2 h at a heating rate of 5 °C·min−1.
4.3. Characterization
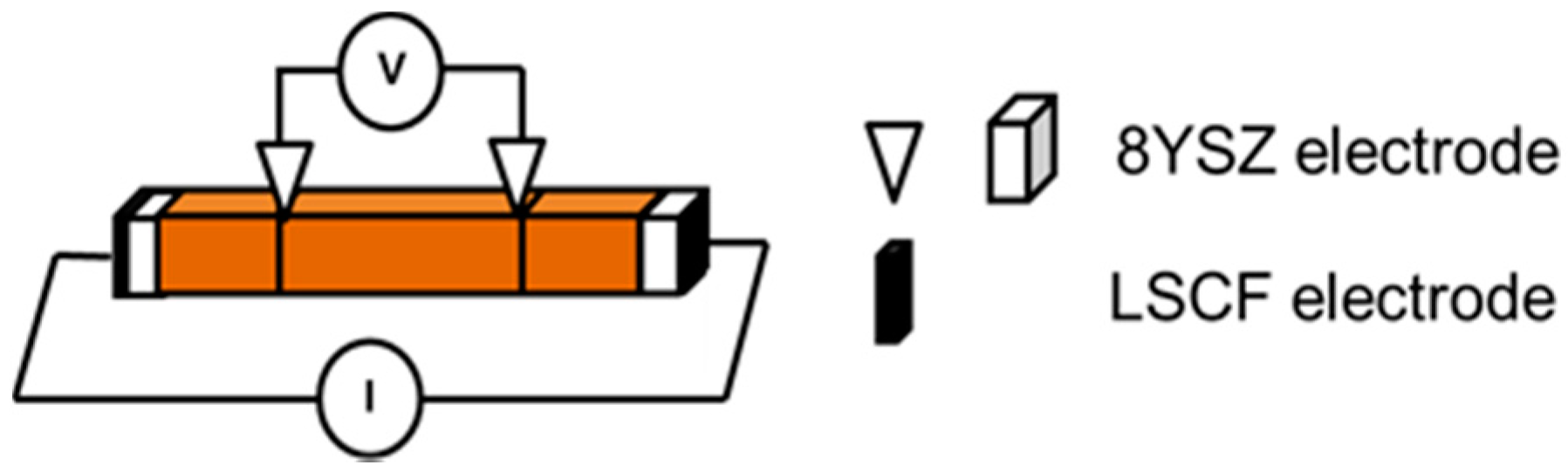
The crystal structure and phases identification of the calcined powder were determined by X-ray diffractometry (XRD, TTRAX 3, Rigaku Ltd., Tokyo, Japan). The total electrical conductivity of the sintered sample in a disk shape and the ionic conductivity of the rectangular samples were measured from 150 °C to 800 °C by a two-probe or four-probe DC technique (
Figure 11), respectively [
26]. The valence states of Fe and Mn in Bi
0.7Sr
0.3Fe
1−xM
xO
3−δ were investigated by X-ray photoelectron spectroscopy (XPS) (Thermo Scientific, Theta Probe, Waltham, MA, USA). Spectra were recorded before and after a step of bombardment cleaning using Ar
+ ion at 3 keV for a selected period (0 s–180 s). Thermo-gravimetric analysis (TGA) was conducted for estimating the oxygen non-stoichiometry of the ferrite samples using a TA Q600 instrument (SDT Q600, TA Waters LLC, New Castle, DE, USA). The samples underwent through careful drying and sintering steps before the XPS and TGA tests.
5. Conclusions
Single-phase doped (Bi,Sr)FeO
3−δ materials were synthesized and displayed interesting mixed electronic and ionic conductivities at temperatures up to 800 °C. The electric conductivity of S
xC
y (
x = 0–30,
y = 0–5) shows two-stage temperature dependence, but the others (
x = 40–50,
y = 10–20) show only one-stage dependence. However, for the ionic conductivity of S
x and S
xC
y samples, the dependence follows only one
Ea 1.24 ± 0.06 eV to the entire temperature region 300–800 °C, which is lower than that (1.32–1.34 eV) of LSCF cathodes [
11].
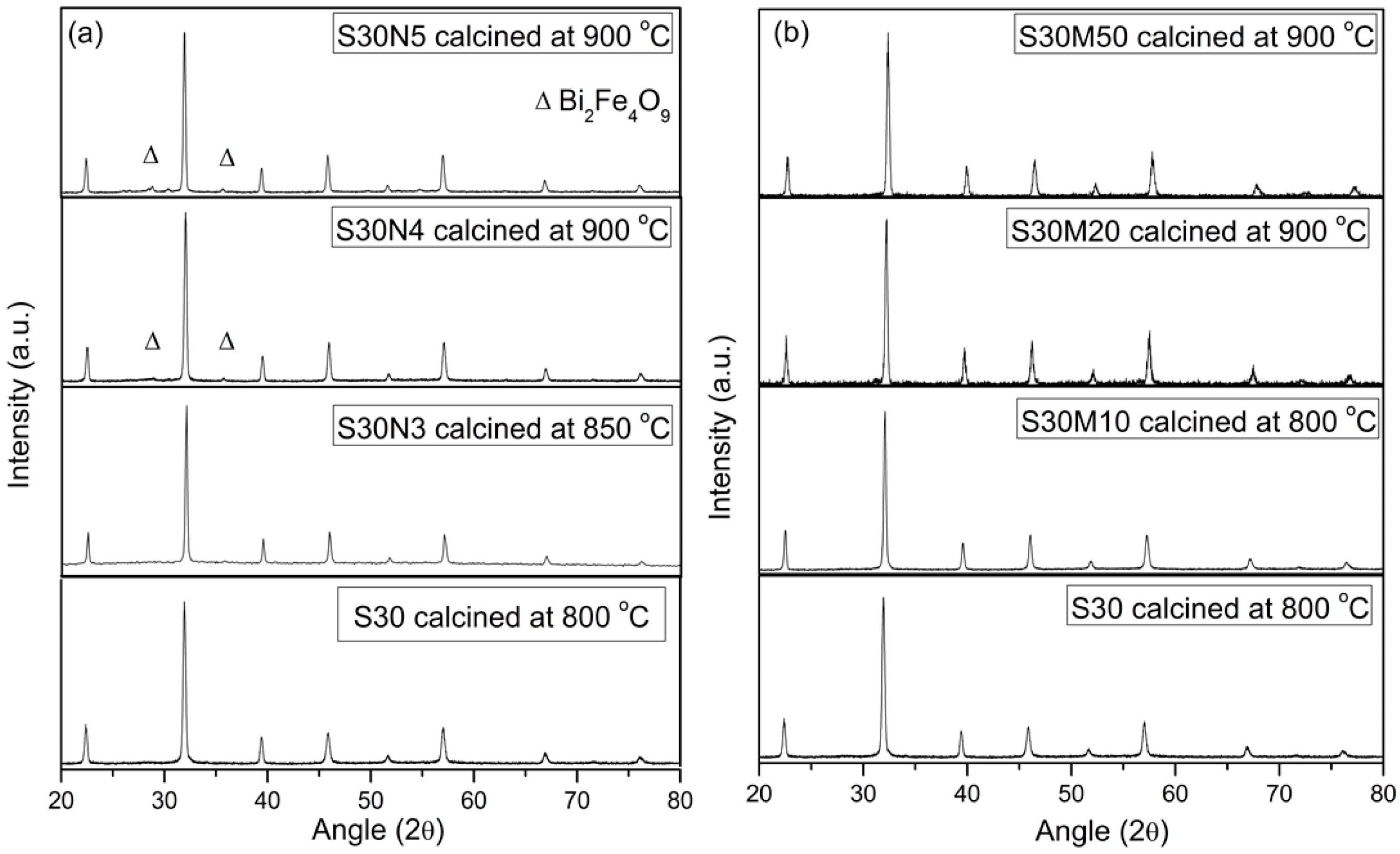
The solubility limit of Ni in S30 is 0.03, but there is no limit for Mn up to 0.50 to keep the single perovskite phase. Three samples, doped S30, S30N3 and S30M50, show CTE values ranging between 12.8 × 10−6 and 13.7 × 10−6 K−1, which are close to that of zirconia- or ceria-based electrolyte.
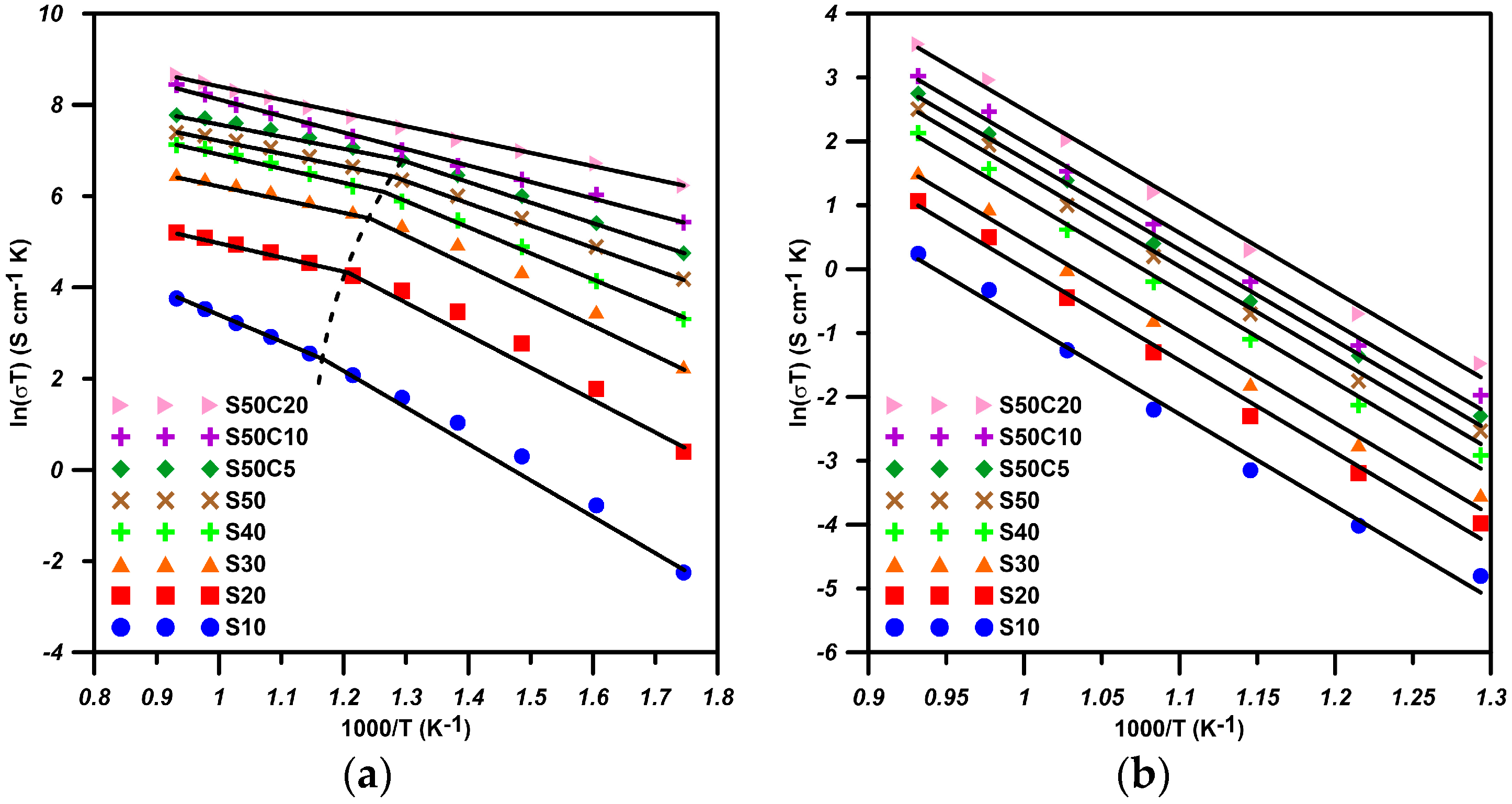
The results of the valence state of Fe by XPS show the fraction of Fe3+ and Fe4+ are in a ratio of 0.47 vs. 0.53 in S30M50 at room temperature. Similar XPS analysis on the Mn shows divalent and trivalent Mn ions appear in nearly equal quantity (0.46 vs. 0.44). Both B-site ions in equal quantity are favored for the double-exchange conduction of electrons along the Fe(Mn)O6 octahedral chains in the perovskite structure at lower temperature. At temperatures greater than 400 °C, the oxygen vacancies in BSF samples increase by more than a few percentages, and result in not only an interruption of the double-exchange mechanism, but also enhancement of a small-polaron conduction by the help in neighboring oxygen vacancies.
One of the structural factors, specific free volume (Vsf) for BSFM materials, offers 10% more free space than LSCF6428 and may result in a better diffusive mobility of oxygen/vacancy in BSF materials at higher temperature.
All doped BSFs show similar two-stage temperature dependence of conductivity: when lower than the transition temperature (Ttr), all samples show similar double-exchange conductive mechanism with an activation energy of 0.54–0.59 eV·mol−1 or 0.37–0.44 eV·mol−1. At temperatures higher than Ttr, the conductive mechanism relies on a few percent concentration of oxygen vacancies, offering the exchange media to electrons to hop between small polarons (). This allows the cells with Co-doped S50C20 to reach a maximum power density of 406 mW·cm−2, with 15% being better than the cell with LSCF6428 testing at 800 °C.