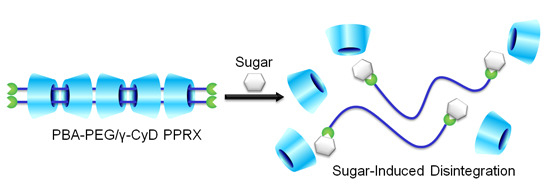
Sugar-Responsive Pseudopolyrotaxane Composed of Phenylboronic Acid-Modified Polyethylene Glycol and γ-Cyclodextrin
Abstract
:1. Introduction
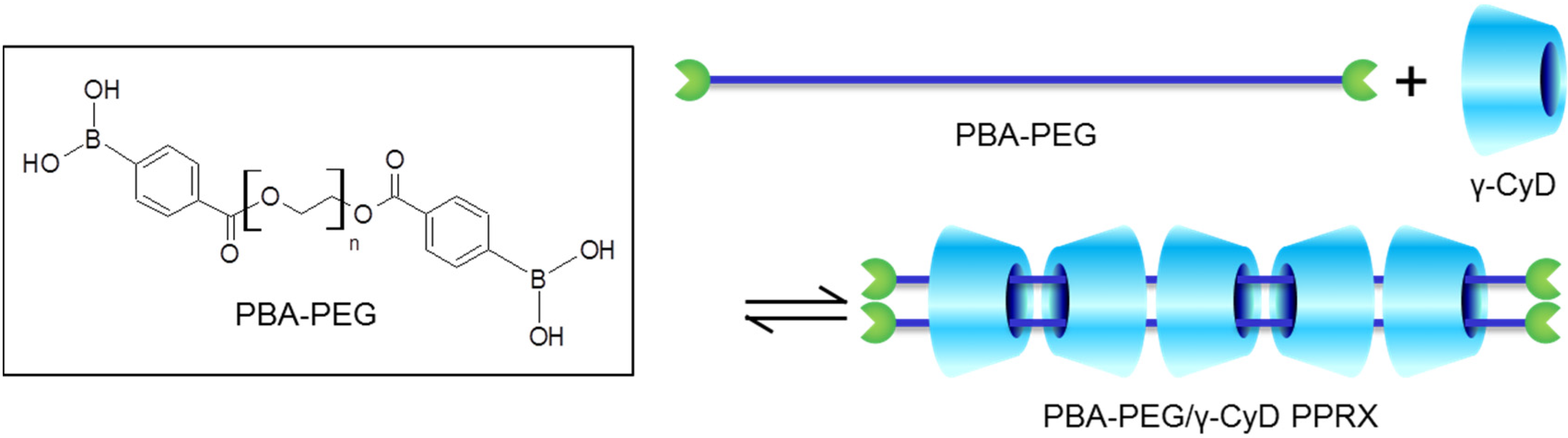
2. Results and Discussion
2.1. Preparation of PBA–PEG/γ-CyD PPRX

2.2. Sugar Response of PBA–PEG/γ-CyD PPRX
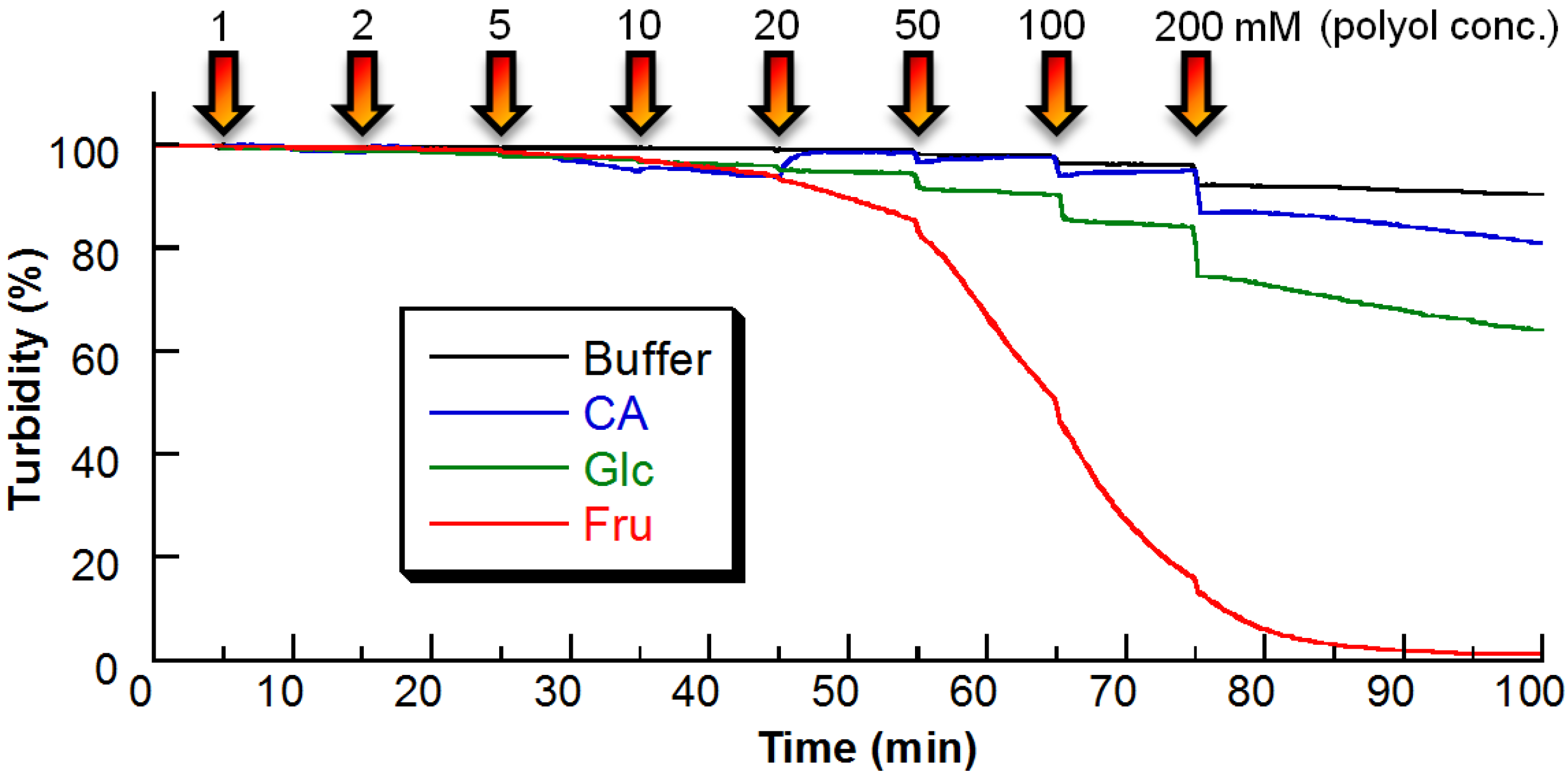
2.3. Response Mechanism of PBA–PEG/γ-CyD PPRX

3. Experimental Section
3.1. Materials
3.2. Apparatus
3.3. Preparation
3.3.1. Synthesis of PBA–PEG
3.3.2. Preparation of PBA–PEG/γ-CyD PPRX
3.4. Turbidity Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uekama, K. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 2004, 52, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Double-stranded inclusion complexes of cyclodextrin threaded on poly (ethylene glycol). Nature 1994, 370, 126–128. [Google Scholar] [CrossRef]
- Huang, F.; Gibson, H.W. Polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 2005, 30, 982–1018. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhao, F.; Li, J. Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 2011, 90, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Harada, A. Cyclodextrin-based molecular machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, W.; Chipot, C.; Shao, X. Thermodynamic insights into the dynamic switching of a cyclodextrin in a bistable molecular shuttle. J. Phys. Chem. Lett. 2010, 1, 1776–1780. [Google Scholar] [CrossRef]
- Higashi, T.; Hirayama, F.; Arima, H.; Uekama, K. Polypseudorotaxanes of pegylated insulin with cyclodextrins: Application to sustained release system. Bioorg. Med. Chem. Lett. 2007, 17, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Hirayama, F.; Yamashita, S.; Misumi, S.; Arima, H.; Uekama, K. Slow-release system of pegylated lysozyme utilizing formation of polypseudorotaxanes with cyclodextrins. Int. J. Pharm. 2009, 374, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Takamido, S.; Nagahama, K.; Ouchi, T.; Katoono, R.; Yui, N. Polyrotaxane composed of poly-L-lactide and alpha-cyclodextrin exhibiting protease-triggered hydrolysis. Biomacromolecules 2009, 10, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- James, T.D.; Samankumara Sandanayake, K.R.A.; Shinkai, S. Saccharide sensing with molecular receptors based on boronic acid. Angew. Chem. Int. Ed. Engl. 1996, 35, 1910–1922. [Google Scholar] [CrossRef]
- Fossey, J.S.; D’Hooge, F.; van den Elsen, J.M.H.; Pereira Morais, M.P.; Pascu, S.I.; Bull, S.D.; Marken, F.; Jenkins, A.T.A.; Jiang, Y.-B.; James, T.D. The development of boronic acids as sensors and separation tools. Chem. Rec. 2012, 12, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Chen, X.-X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 2013, 42, 8032–8048. [Google Scholar] [CrossRef] [PubMed]
- Egawa, Y.; Seki, T.; Takahashi, S.; Anzai, J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater. Sci. Eng. C 2011, 31, 1257–1264. [Google Scholar] [CrossRef]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials 2014, 7, 1201–1220. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yoshida, R.; Kataoka, K. Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules 2004, 5, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.G.; Jonas, A.M.; Demeester, J.; de Smedt, S.C. Glucose-responsive polyelectrolyte capsules. Langmuir 2006, 22, 5070–5074. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre, V.; Ancla, C.; Catargi, B.; Ravaine, V. Glucose-responsive microgels with a core-shell structure. J. Colloid Interface Sci. 2008, 327, 316–323. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 2014, 37 (Suppl. 1), S14–S80. [Google Scholar]
- Ozawa, R.; Hayashita, T.; Matsui, T.; Nakayama, C.; Yamauchi, A.; Suzuki, I. Effects of cyclodextrins and saccharides on dual fluorescence of N,N-dimethyl-4-aminophenylboronic acid in water. J. Incl. Phenom. Macrocycl. Chem. 2007, 60, 253–261. [Google Scholar] [CrossRef]
- Suzuki, I.; Yamauchi, A.; Sakashita, Y.; Hirose, K.; Miura, T.; Hayashita, T. Fluorescence response mechanism of D-glucose selectivity for supramolecular probes composed of phenylboronic-acid-modified beta-cyclodextrin and styrylpyridinium dyes. Anal. Sci. 2007, 23, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, L.; Huang, Y.; Li, Z.; Jiang, Y. A 2: 2 stilbeneboronic acid–γ-cyclodextrin fluorescent ensemble highly selective for glucose in aqueous solutions. Chem. Commun. 2012, 4362–4364. [Google Scholar] [CrossRef]
- Nakamura, K.; Seki, T.; Egawa, Y.; Miki, R.; Oda, Y.; Yamanoi, T.; Seki, T. Sugar-sensitive supramolecular structures based on phenylboronic acid modified cyclodextrins. Chem. Pharm. Bull. 2013, 61, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Tabushi, I. Cyclodextrin catalysis as a model for enzyme action. Acc. Chem. Res. 1982, 15, 66–72. [Google Scholar] [CrossRef]
- Abu Hashim, I.I.; Higashi, T.; Anno, T.; Motoyama, K.; Abd-elgawad, A.-E.H.; El-shabouri, M.H.; Borg, T.M.; Arima, H. Potential use of γ-cyclodextrin polypseudorotaxane hydrogels as an injectable sustained release system for insulin. Int. J. Pharm. 2010, 392, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- James, T.D.; Samankumara Sandanayake, K.R.A.; Iguchi, R.; Shinkai, S. Novel saccharide-photoinduced electron transfer sensors based on the interaction. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar] [CrossRef]
- Eggert, H.; Frederiksen, J.; Morin, C.; Norrild, J.C. A new glucose-selective fluorescent bisboronic acid. First report of strong r-furanose complexation in aqueous solution at physiological pH. J. Org. Chem. 1999, 64, 3846–3852. [Google Scholar] [CrossRef]
- Fang, H.; Kaur, G.; Wang, B. Progress in boronic acid-based fluorescent glucose sensors. J. Fluoresc. 2004, 14, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Chen, B.; Mendes, P.M.; Fossey, J.S.; Tony, D.; Long, Y.-T.; James, T.D.; Long, Y.-T.; Tony, D.; et al. A bis-boronic acid modified electrode for the sensitive and selective determination of glucose concentrations. Analyst 2013, 138, 7146–7151. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, T.; Namiki, M.; Egawa, Y.; Miki, R.; Juni, K.; Seki, T. Sugar-Responsive Pseudopolyrotaxane Composed of Phenylboronic Acid-Modified Polyethylene Glycol and γ-Cyclodextrin. Materials 2015, 8, 1341-1349. https://doi.org/10.3390/ma8031341
Seki T, Namiki M, Egawa Y, Miki R, Juni K, Seki T. Sugar-Responsive Pseudopolyrotaxane Composed of Phenylboronic Acid-Modified Polyethylene Glycol and γ-Cyclodextrin. Materials. 2015; 8(3):1341-1349. https://doi.org/10.3390/ma8031341
Chicago/Turabian StyleSeki, Tomohiro, Misato Namiki, Yuya Egawa, Ryotaro Miki, Kazuhiko Juni, and Toshinobu Seki. 2015. "Sugar-Responsive Pseudopolyrotaxane Composed of Phenylboronic Acid-Modified Polyethylene Glycol and γ-Cyclodextrin" Materials 8, no. 3: 1341-1349. https://doi.org/10.3390/ma8031341