Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of Porous Anodic Alumina Membranes
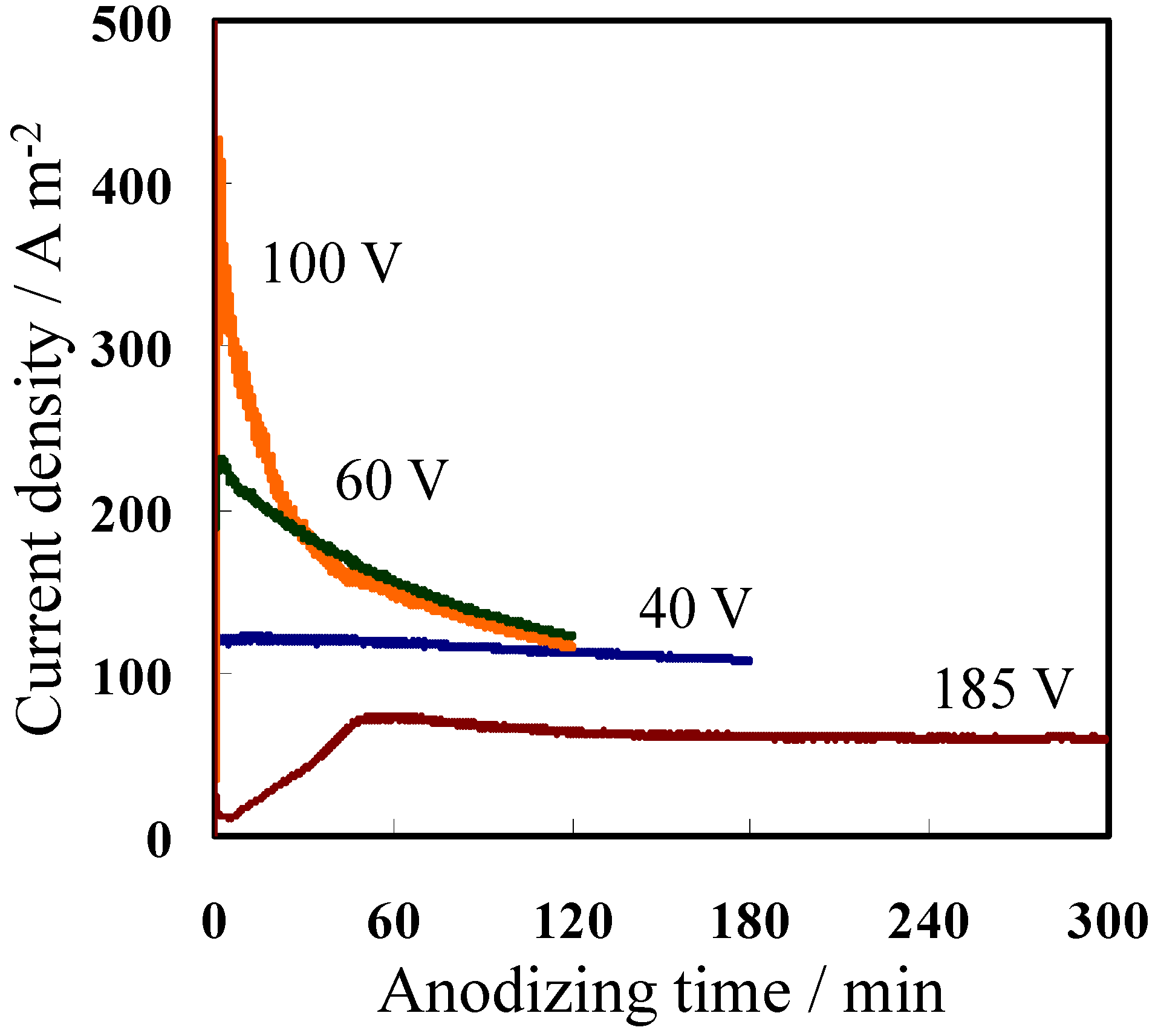
| Electrolytes | Temperature (°C) | Formation Voltage (V) | Anodizing Time (min) | |
|---|---|---|---|---|
| 1st | 2nd | |||
| 0.3 mol·dm−3 oxalic acid | 30 | 40 | 40 | 180 |
| 0.3 mol·dm−3 oxalic acid | 20 | 55 | 60 | 120 |
| 0.3 mol·dm−3 oxalic acid–0.2 mol·dm−3 phosphoric acid | 25 | 100 | 100 | 120 |
| 0.2 mol·dm−3 phosphoric acid | 5–15 | 185 | 185 | 300 |
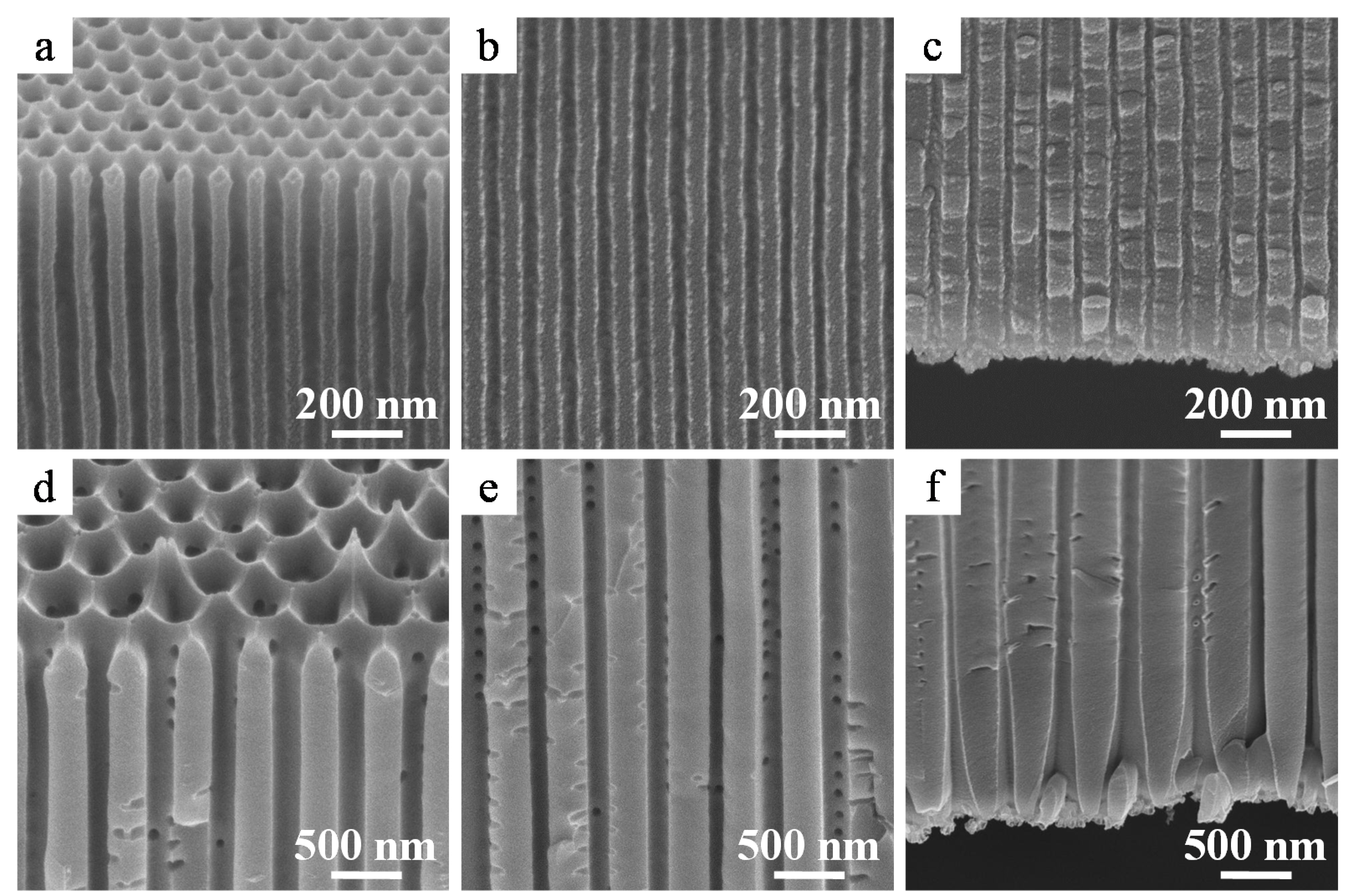
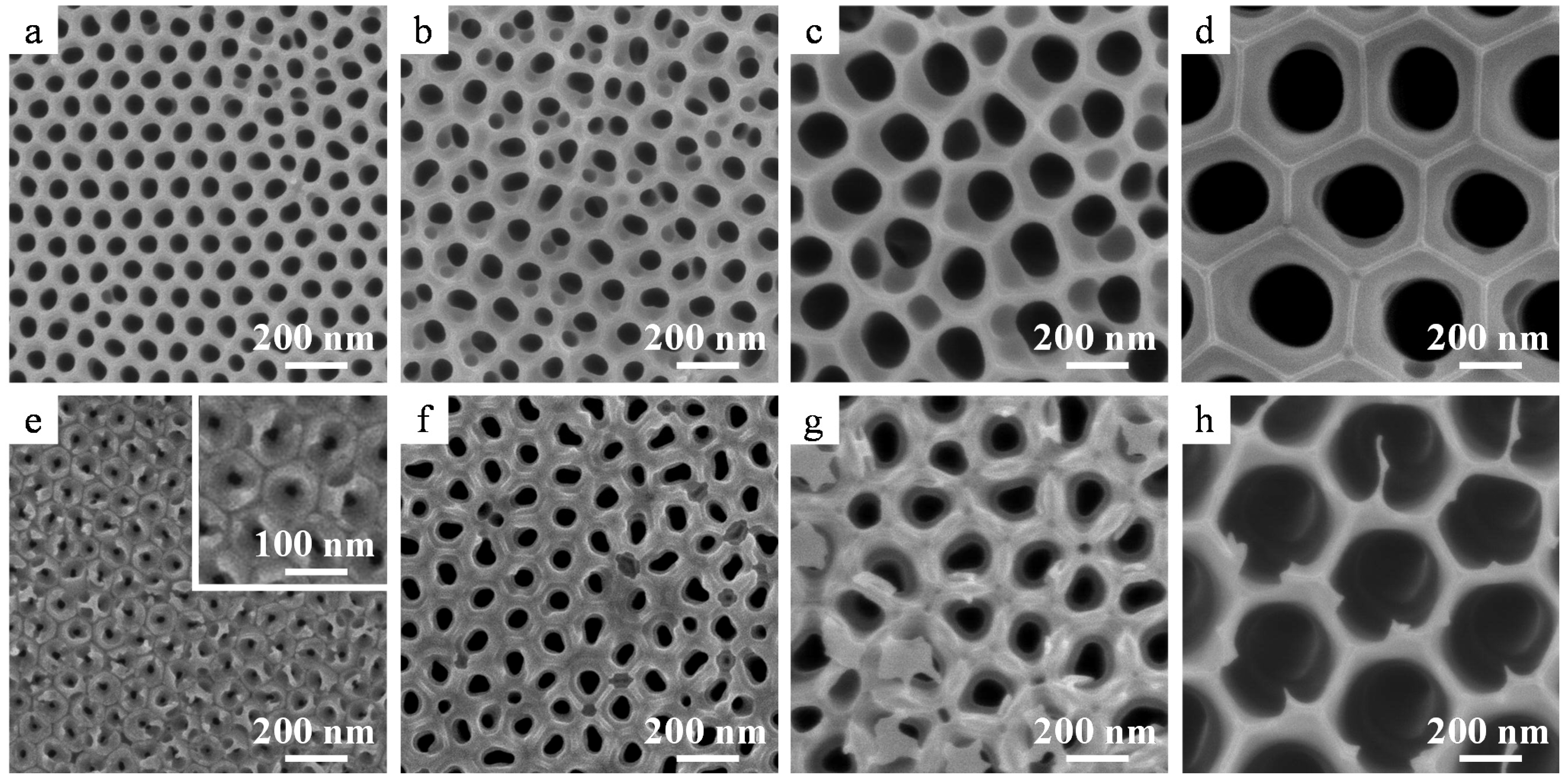
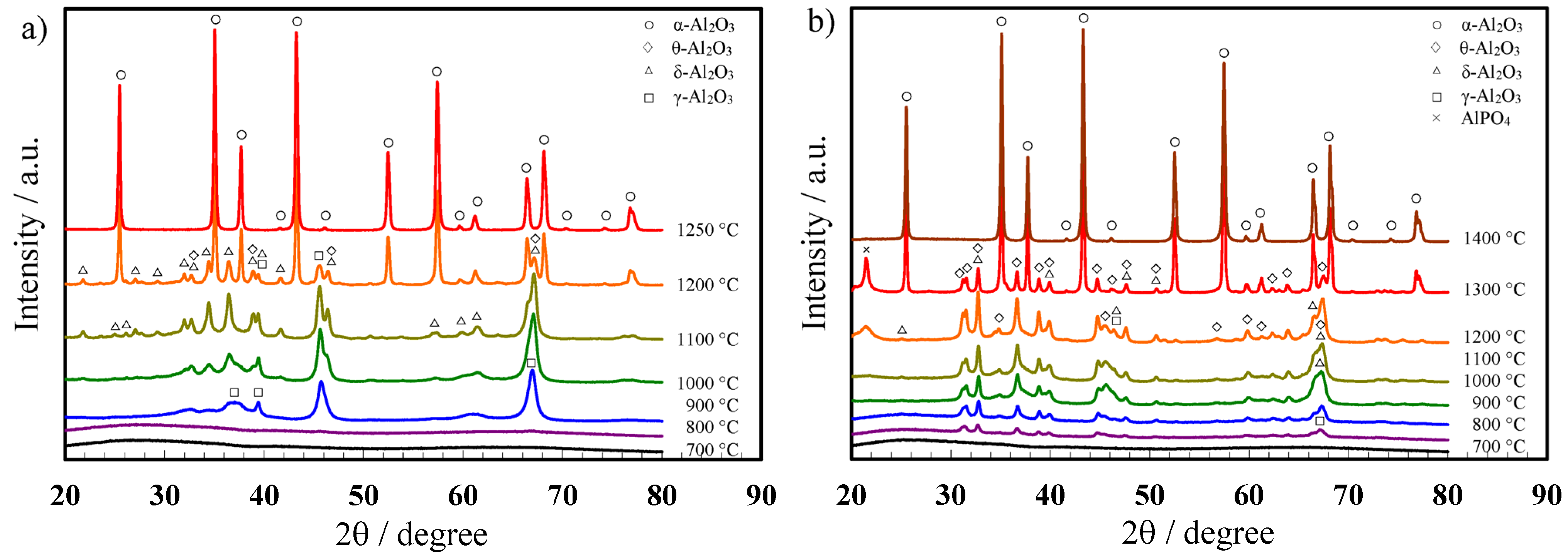
2.2. Crystallization of the Alumina Membranes via Heating
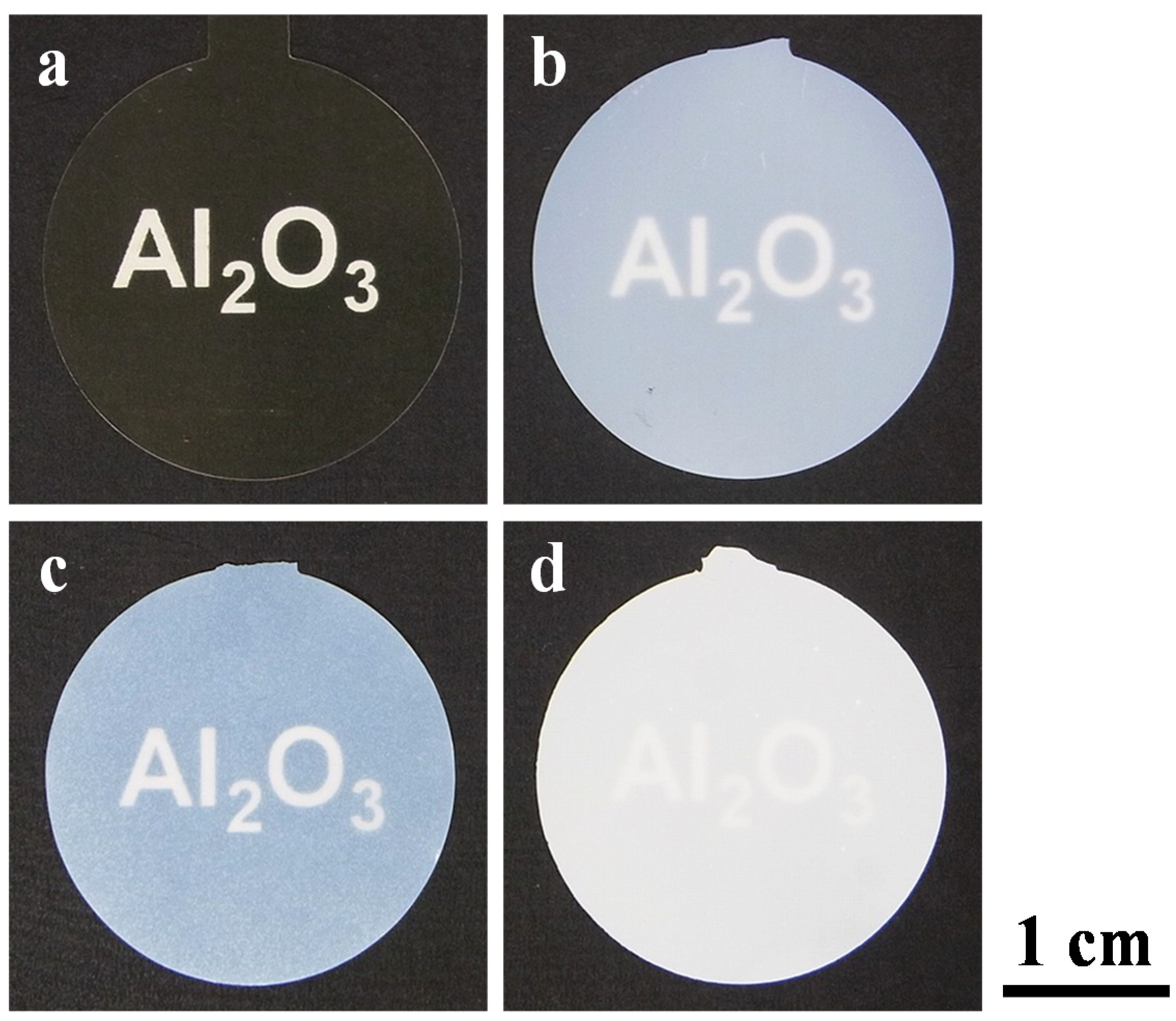
2.3. Nanostructure of the α-Alumina Membranes
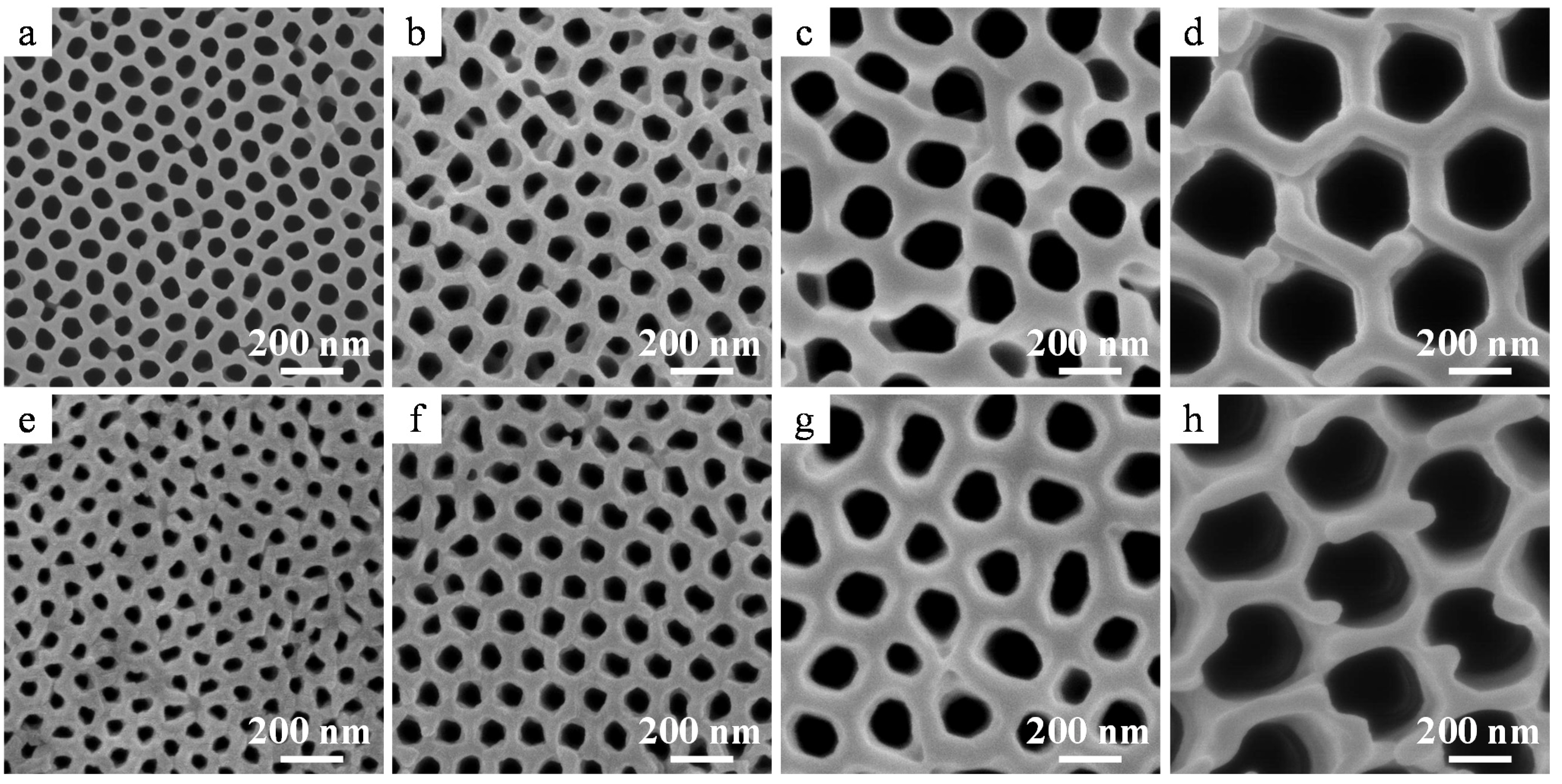
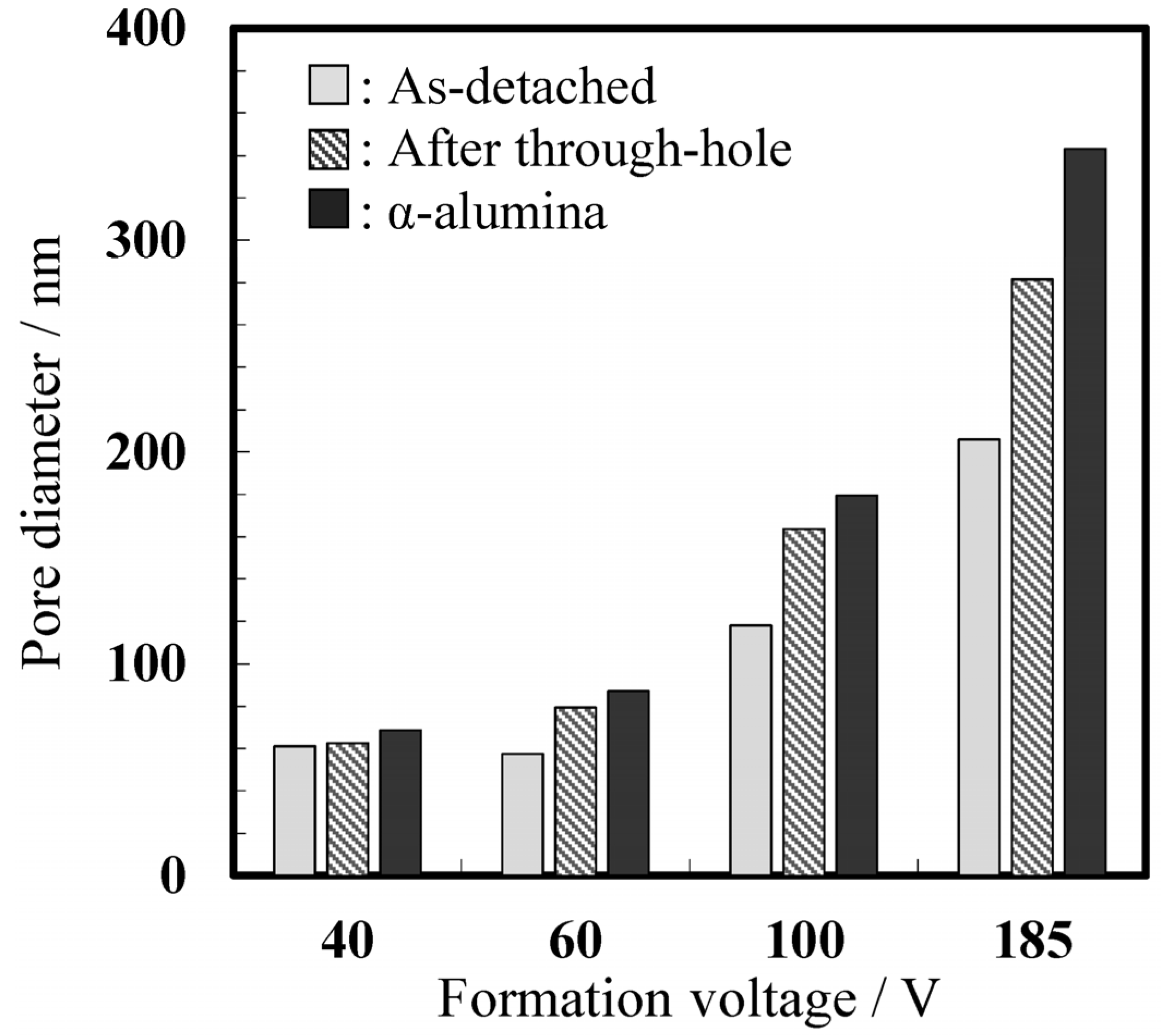
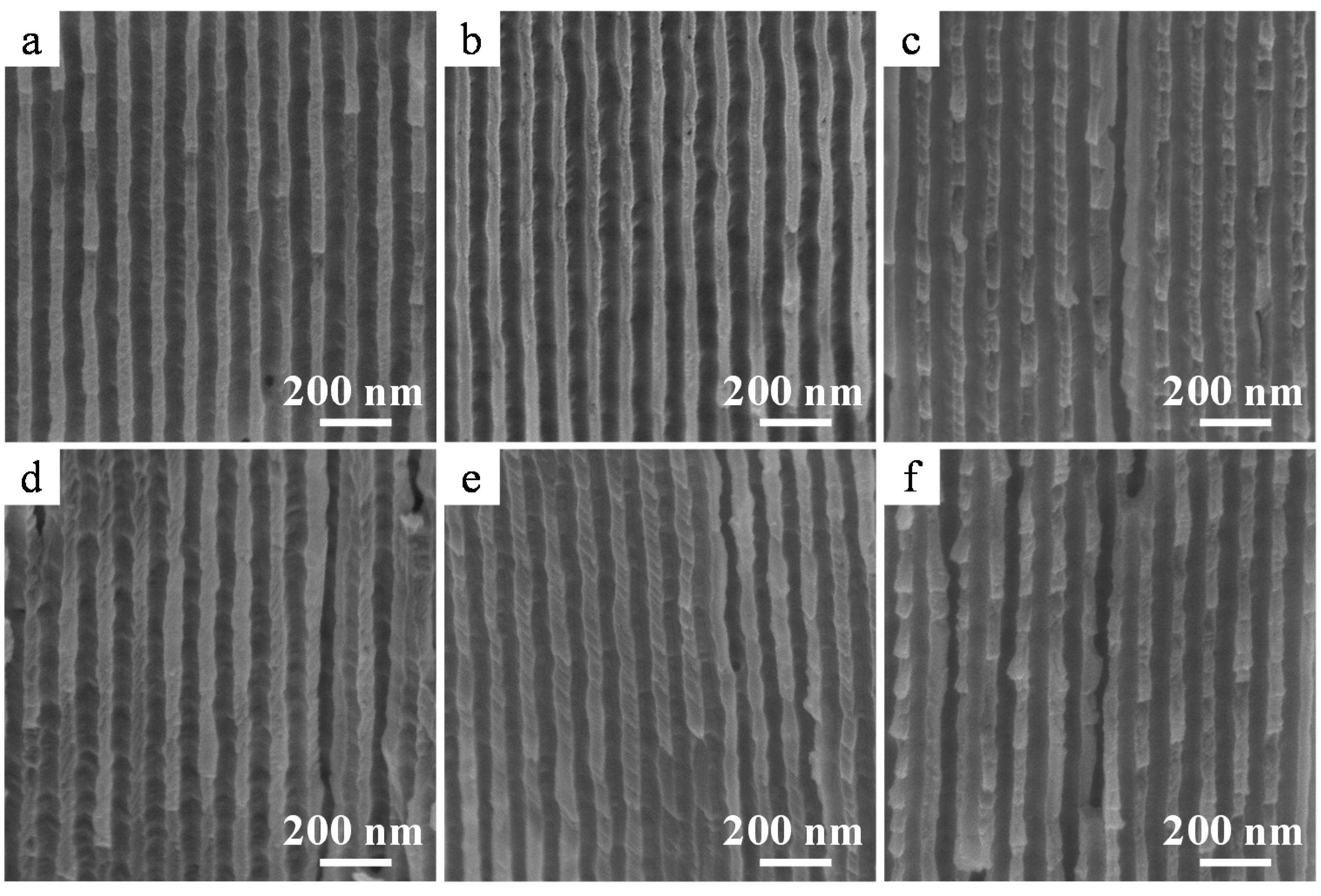
2.4. Properties of the α-Alumina Membranes
2.4.1. Chemical Resistance
2.4.2. Mechanical Properties
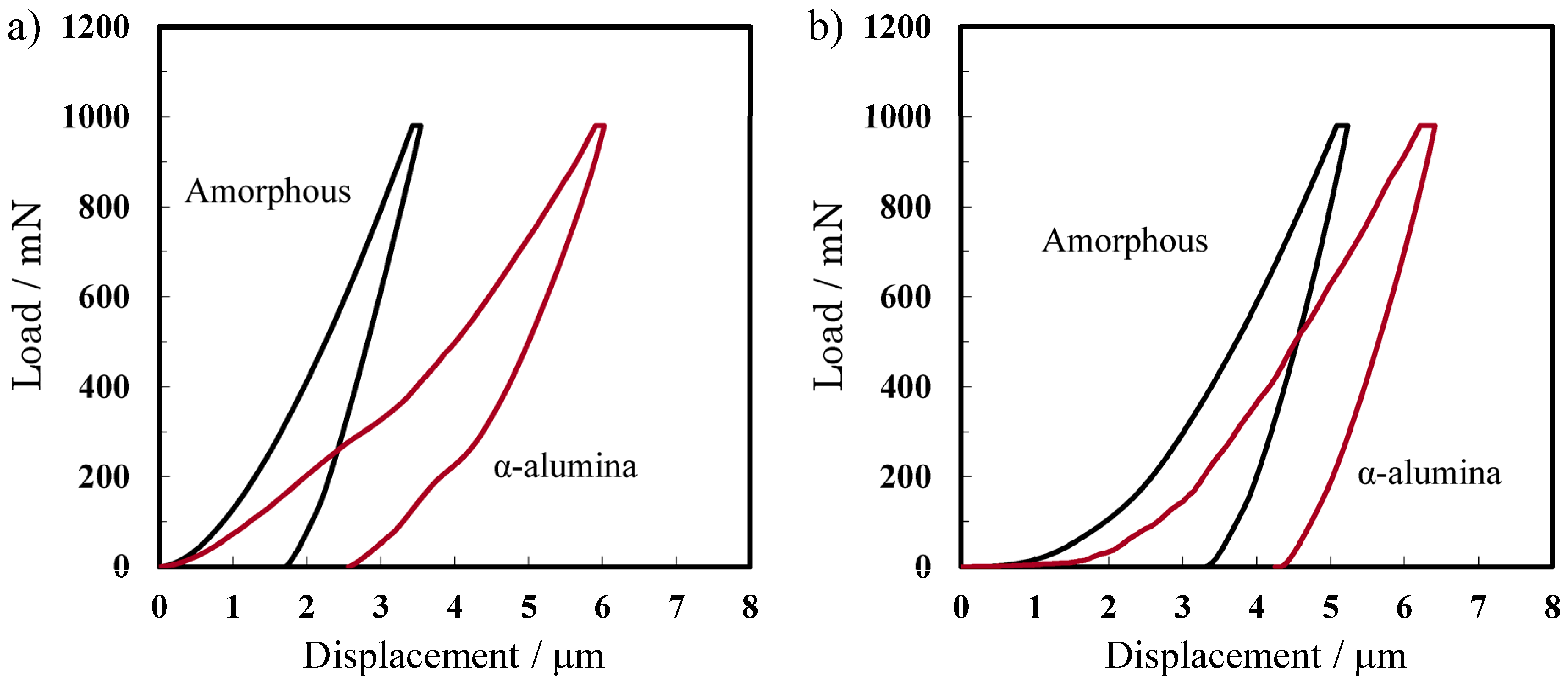
| Formation Voltages | 40 V | 185 V | ||
|---|---|---|---|---|
| Amorphous | α-alumina | Amorphous | α-alumina | |
| Young’s modulus (GPa) | 47.1 ± 1.4 | 16.3 ± 0.7 | 29.0 ± 0.5 | 19.7 ± 0.9 |
| Hardness (HV) | 444 ± 6 | 349 ± 18 | 533 ± 10 | 175 ± 13 |
| Porosity | 0.42 | 0.47 | 0.45 | 0.68 |
2.4.3. Filtration Properties
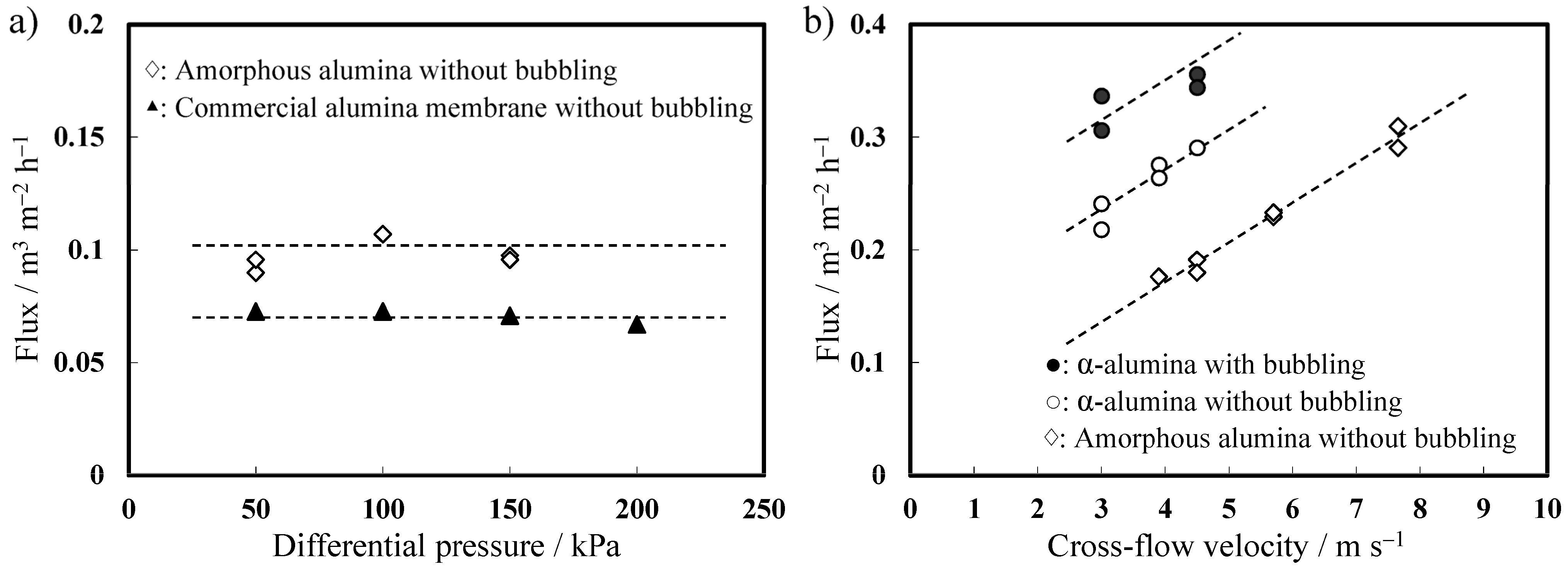
3. Experimental Section
3.1. Preparation of the Anodic Porous Alumina Membranes
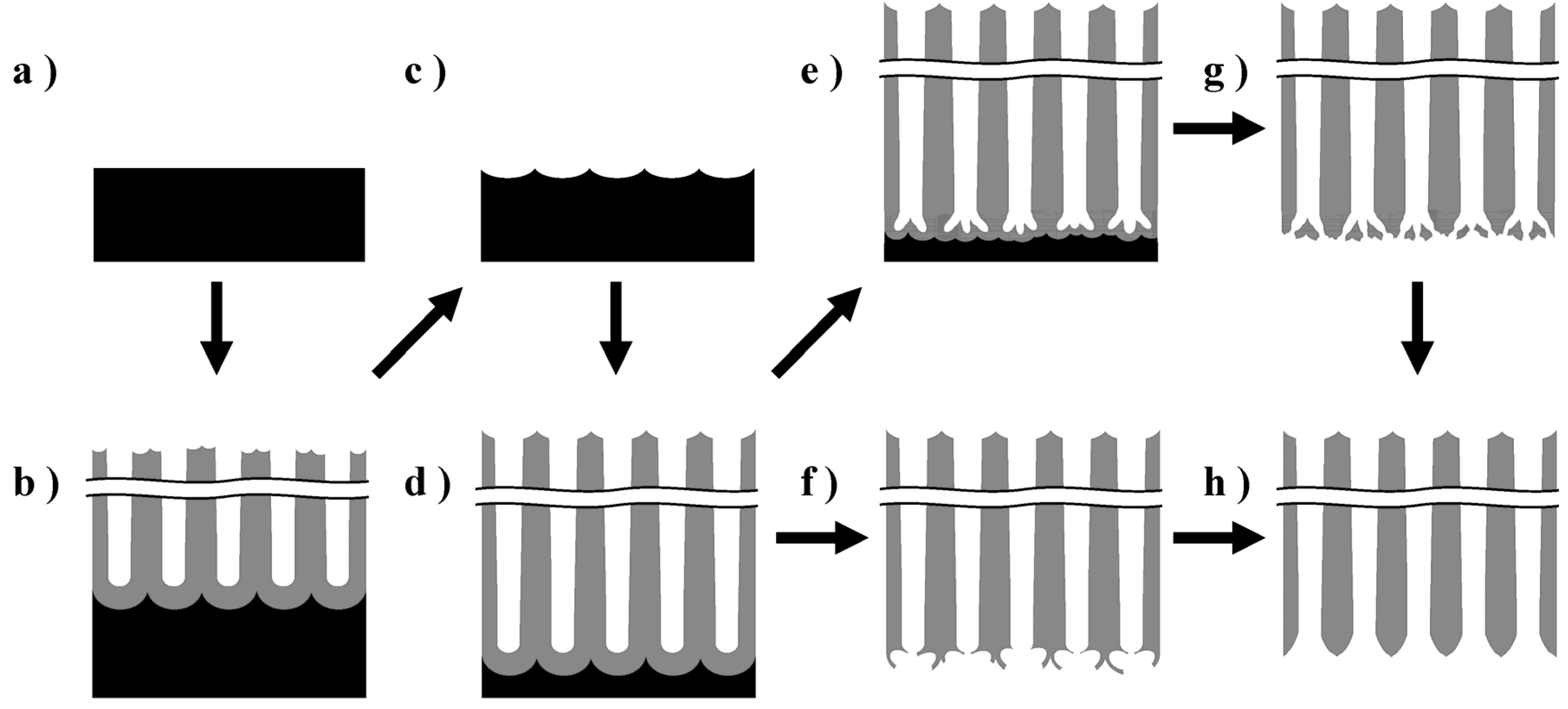
3.2. Crystallization of the Alumina Membrane via Heat Treatment
3.3. Characterization of the α-Alumina Membranes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- O’Sullivan, J.P.; Wood, G.C. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. Roy. Soc. Lond. 1970, A317, 511–543. [Google Scholar] [CrossRef]
- Dekker, A.; Middelhoek, A. Transport numbers and the structure of porous anodic films on aluminum. J. Electrochem. Soc. 1970, 117, 440–448. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Satoh, M. Fabrication of gold nanodot array using anodic porous alumina as an evaporation mask. Jpn. J. Appl. Phys. 1996, 35, L126–L129. [Google Scholar] [CrossRef]
- Masuda, H.; Hasegawa, F.; Ono, S. Self-ordering of cell arrangement of anodic porous alumina formed in sulfuric acid solution. J. Electrochem. Soc. 1997, 144, L127–L130. [Google Scholar] [CrossRef]
- Masuda, H.; Yada, K.; Osaka, A. Self-ordering of cell configuration of anodic porous alumina with large-size pores in phosphoric acid solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Ishiguro, M.; Asoh, H. Controlling factor of self-ordering of anodic porous Alumina. J. Electrochem. Soc. 2004, 151, B473–B478. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina formed in organic acid electrolytes. Electrochim. Acta 2005, 51, 827–833. [Google Scholar] [CrossRef]
- Furneaux, R.C.; Rigby, W.R.; Davidson, A.P. The formation of controlled-porosity membranes from anodically oxidized aluminium. Nature 1989, 337, 147–149. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef]
- Kouklin, N.; Menon, L.; Wong, A.Z.; Thompson, D.W.; Woollam, J.A.; Williams, P.F.; Bandyopadhyay, S. Giant photoresistivity and optically controlled switching in self-assembled nanowires. Appl. Phys. Lett. 2001, 79, 4423–4425. [Google Scholar] [CrossRef]
- Yanagishita, T.; Tomabechi, Y.; Nishio, K.; Masuda, H. Preparation of monodisperse SiO2 nanoparticles by membrane emulsification using ideally ordered anodic porous alumina. Langmuir 2004, 20, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Romero, L.; Montero-Moreno, J.M.; Pellicer, E.; Peiró, F.; Cornet, A.; Morante, J.R.; Sarret, M.; Müller, C. Assessment of the thermal stability of anodic alumina membranes at high temperatures. Mater. Chem. Phys. 2008, 111, 542–547. [Google Scholar] [CrossRef]
- Choudhari, K.S.; Sudheendra, P.; Udayashankar, N.K. Fabrication and high-temperature structural characterization study of porous anodic alumina membranes. J. Porous Mat. 2012, 19, 1053–1062. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; Govyadinov, A.N.; Mukhurov, N.I.; Rzhevskii, A.M.; Paterson, R. Assessment of the thermal stability of anodic alumina membranes at high temperatures. J. Membr. Sci. 1995, 98, 131–142. [Google Scholar] [CrossRef]
- Ozao, R.; Yoshida, H.; Ichikawa, Y.; Inada, T.; Ochiai, M. Crystallization of anodic alumina membranes studied by simultaneous TG-DTA/FTIR. J. Therm. Anal. Calorim. 2001, 64, 915–922. [Google Scholar] [CrossRef]
- McQuaing, M.K., Jr.; Toro, A.; Geertruyden, W.V.; Misiolek, W.Z. The effect of high temperature heat treatment on the structure and properties of anodic aluminum oxide. J. Mater. Sci. 2011, 46, 243–253. [Google Scholar] [CrossRef]
- Ebihara, K.; Takahashi, H.; Nagayama, M. Structure and density of anodic oxide films formed on aluminum in oxalic acid solutions. J. Surf. Finish. Soc. Jpn. 1983, 34, 548–553. [Google Scholar] [CrossRef]
- Chang, Y.; Ling, Z.; Liu, Y.; Hu, X.; Li, Y. A simple method for fabrication of highly ordered porous α-alumina ceramic membranes. J. Mater. Chem. 2012, 22, 7445–7448. [Google Scholar] [CrossRef]
- Ono, S.; Masuko, N. The duplex structure of cell walls of porous anodic films formed on aluminum. Corros. Sci. 1992, 33, 503–505. [Google Scholar] [CrossRef]
- Ono, S.; Masuko, N. Dissolution behavior of the barrier layer of porous anodic films formed on aluminum studied by pore-filling technique. J. Jpn. Inst. Light Met. 1993, 43, 447–452. [Google Scholar] [CrossRef]
- Ono, S.; Masuko, N. Electron microscopic study of the structure and dissolution behavior of porous anodic films formed on aluminum. J. Jpn. Inst. Light Met. 1993, 43, 453–458. [Google Scholar] [CrossRef]
- Kirchner, A.; MacKenzie, K.J.D.; Brown, I.W.M.; Kemmitt, T.; Bowden, M.E. Structural characterisation of heat-treated anodic alumina membranes prepared using a simplified fabrication process. J. Membr. Sci. 2007, 287, 264–270. [Google Scholar] [CrossRef]
- Takahashi, H.; Nagayama, M.; Akahori, H.; Kitahara, A. Electron-microscopy of porous anodic oxide films on aluminium by ultra-thin sectioning technique part 1. The structural change of the film during the current recovery period. J. Electron Microsc. 1973, 22, 149–157. [Google Scholar]
- Yuan, J.H.; He, F.Y.; Sun, D.C.; Xia, X.H. A simple method for preparation of through-hole porous anodic alumina membrane. Chem. Mater. 2004, 16, 1841–1844. [Google Scholar] [CrossRef]
- Ono, S.; Nakamura, M.; Masuda, T.; Asoh, H. Fabrication of nanoporous crystalline alumina membrane by anodization of aluminum. Mater. Sci. Forum 2014, 783–786, 1470–1475. [Google Scholar] [CrossRef]
- Masuda, T.; Asoh, H.; Haraguchi, S.; Ono, S. Nanoporous α-alumina membrane prepared by anodizing and heat treatment. Electrochemistry 2014, 82, 448–455. [Google Scholar] [CrossRef]
- Rashidi, F.; Masuda, T.; Asoh, H.; Ono, S. Metallographic effects of pure aluminum on properties of nanoporous anodic alumina (NPAA). Surf. Interface Anal. 2013, 45, 1490–1496. [Google Scholar] [CrossRef]
- Molchan, I.S.; Molchan, T.V.; Gaponenko, N.V.; Skeldon, P.; Thompson, G.E. Impurity-driven defect generation in porous anodic alumina. Electrochem. Commun. 2010, 12, 693–696. [Google Scholar] [CrossRef]
- Ono, S.; Ichinose, H.; Masuko, N. Defects in porous anodic films formed on high purity aluminum. J. Electrochem. Soc. 1991, 138, 3705–3710. [Google Scholar] [CrossRef]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Laboratories: New York, NY, USA, 1987; p. 47. [Google Scholar]
- Ono, S.; Masuko, N. The effect of incorporated anions to the sealing of porous anodic films on aluminum. J. Surf. Finish. Soc. Jpn. 1994, 45, 1070–1071. [Google Scholar] [CrossRef]
- Fukuda, Y.; Fukushima, T. High temperature hard anodizing with tartaric acid-oxalic acid-triethanolamine bath. Bull. Chem. Soc. Jpn. 1980, 53, 366–371. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina induced by local current concentration: Burning. Electrochem. Solid State Lett. 2004, 7, B21–B24. [Google Scholar] [CrossRef]
- Asoh, H.; Tanabe, K.; Ono, S. Effect of heat treatment on solubility of anodic porous alumina. J. Surf. Finish. Soc. Jpn. 2002, 53, 777–778. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, T.; Asoh, H.; Haraguchi, S.; Ono, S. Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters. Materials 2015, 8, 1350-1368. https://doi.org/10.3390/ma8031350
Masuda T, Asoh H, Haraguchi S, Ono S. Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters. Materials. 2015; 8(3):1350-1368. https://doi.org/10.3390/ma8031350
Chicago/Turabian StyleMasuda, Tatsuya, Hidetaka Asoh, Satoshi Haraguchi, and Sachiko Ono. 2015. "Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters" Materials 8, no. 3: 1350-1368. https://doi.org/10.3390/ma8031350
APA StyleMasuda, T., Asoh, H., Haraguchi, S., & Ono, S. (2015). Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters. Materials, 8(3), 1350-1368. https://doi.org/10.3390/ma8031350





