Formative Evaluation of Clinician Experience with Integrating Family History-Based Clinical Decision Support into Clinical Practice
Abstract
:1. Introduction
1.1. Description of Application
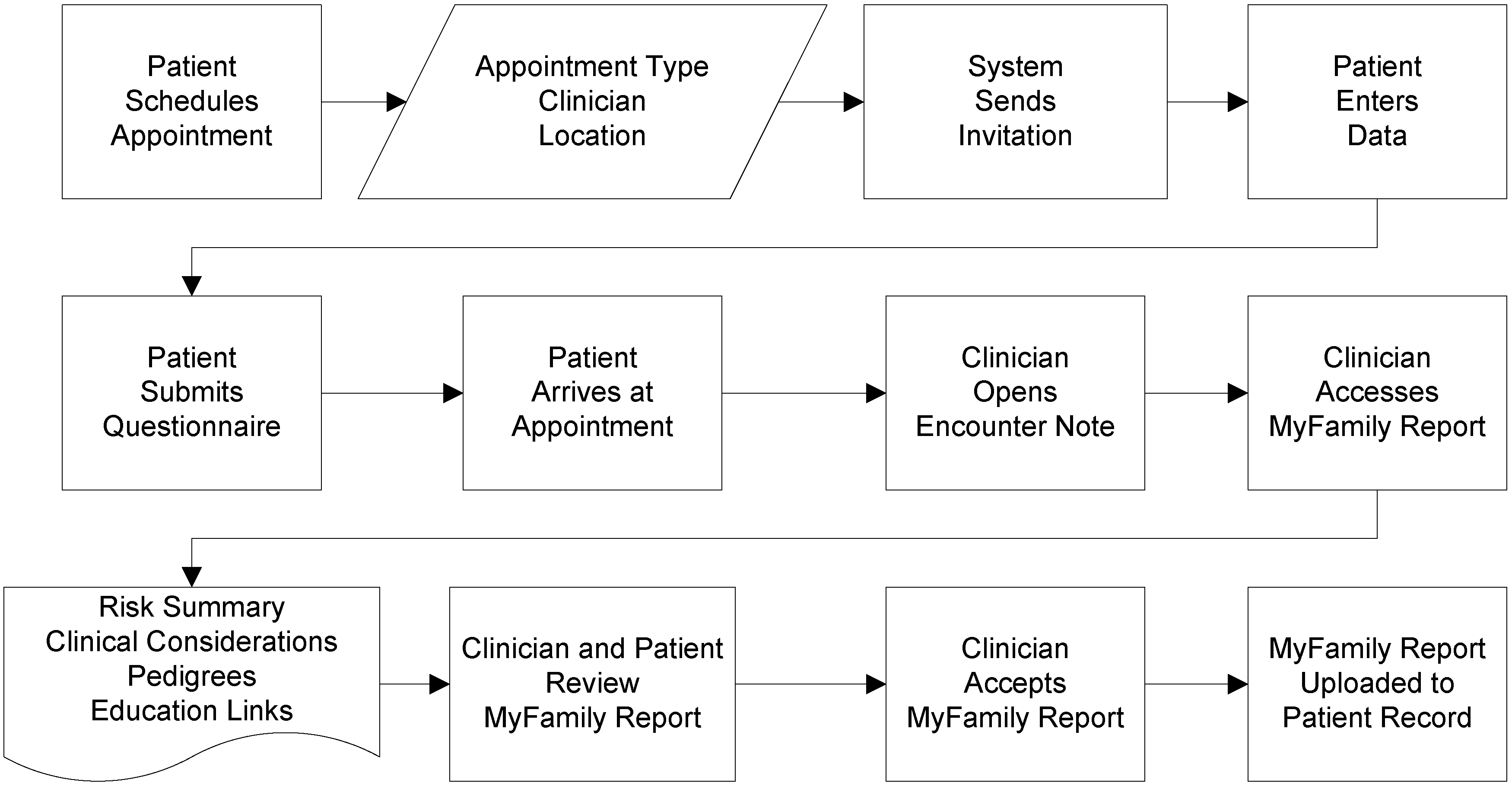
| Familial breast cancer |
| Familial colorectal cancer |
| Familial endometrial cancer |
| Familial ovarian cancer |
| Hereditary breast and ovarian cancer syndrome |
| Hereditary nonpolyposis colorectal cancer syndrome |
| Abdominal aortic aneurysm * |
| Diabetes * |
1.2. Aims of the Study
2. Methods
2.1. Design
2.2. Participant Selection
2.3. Interview Procedures
2.4. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Summary of Findings
| Themes | Impact on patient care | Impact on clinical encounter and clinician workflow | Process of implementation |
|---|---|---|---|
| Findings |
|
|
|
3.2.1. Impact on Patient Care
3.2.1.1. Quality
| Title | PC | GC |
|---|---|---|
| Provides standard and equal care to patients | “Often times that [family health history conversation] doesn’t happen especially if patients have a lot of medical problems, you know we might not spend as much time on preventative care… having that opportunity to sit down with them and their family history kinda gives that opportunity to have that discussion.” (PC101) | “I can see it helping a lot of people that… would never come to attention if they are healthy individuals with concerning family history… something like [MyFamily] would pick [these patients] up.” (GC107) “… [MyFamily is] a very good educational opportunity for providers who did not previously really understand who needed to be referred to genetics.” (GC108) |
| Accuracy of family health history | “…if that patient is starting that dialogue at home where they have access to the information, talking to families, friends, relatives, they could possibly do it more accurately.” (PC102) | “…it increases my confidence that we are getting accurate information because I think it forces patients to think about it ahead of time, and they ask family members for information and they come in better prepared to answer our questions, so in that sense, I think [MyFamily] improves [quality].” (GC108) |
| Supports cross-disciplinary care | “…having a more detailed history and having that analyzed and then given to me… helps me decide… should I send him off to a specialist sooner than later or should I really be on their case to get that colonoscopy done and that sort of stuff.” (PC104) | “…I think [the use of MyFamily] is really going to improve our referrals in terms of quantity and quality.” (GC108) “A patient “was referred to me from their primary care physician after completing [MyFamily]. [I reviewed the patient’s MyFamily report] because I was trying to get a better understanding of why that patient was referred to genetic counseling.” (GC105) |
“The people who take the time [to complete MyFamily], it’s going to treat them equally ‘cause everybody is going to be looked at as a blank slate, no names, no numbers, none of that attached to that.”(CS110)
“I would fear that if it does become [too] time consuming, that [patients] are going to be like, ‘You know what, I’m just going to put down the people who have cancer and the other people don’t really matter.’ Although the other people do matter…”(CS110)
“[MyFamily] definitely increases the quality of care. Again, I think that it brings out the fact that we are multi-disciplinary and that we are interested in the whole [patient]; every piece that we can bring to a patient we are bringing to them.”(CS110)
3.2.1.2. Patient Engagement
| PC | GC |
|---|---|
| “I think that it’s a great way to really engage patients and to really help them to see [that] what they are doing, the work that they are doing pre-visit, is really helping us to make this preventative [care] plan with them. So, I really like that aspect of it…. I think they enjoyed seeing their results of their efforts during the clinical encounter, I think they really appreciated that.” (PC101) | “[my favorite part is] the fact that the patients are more engaged…. They have been more active in their health care. They have been more active in their family history gathering. They come in having a better idea of what to expect.” (GC107) |
“I find [MyFamily] incredibly useful because it engages the patient in their health which ultimately [supports] the value based operations that the Cleveland Clinic is moving towards. If we don’t partner with our patients, their employers, our local governments, our communities, our churches, our neighbors, we will not be able to afford health care in this country…”(PC102)
“…if the patients are taking the effort… to make sure that the clinicians who are getting the information are discussing it. Because otherwise the patient will stop filling these things out, if they fill it out and they have to ask [their clinician] about it or it never even gets brought up [by the clinician].”(CS110)
3.2.1.3. Institutional Impact
The CS agreed,“…if we can do [family history risk assessment] in a uniform way and [patients] can understand that if someone has reviewed this, this is constantly being updated, I think it will save a lot of time for the system.”(PC101)
“…it allows me to tell the patient that [his/her family history has] already been reviewed and this is what it is. It’s not a gray area [where I would say] I will get back to you, let me review this with a genetic counselor—the decision has already been made. … I think it saves time because there is not the checking, double checking, calling the patient back.”(CS110)
“…having a more detailed history and having that analyzed and then given to me… it personalizes their care which is obviously the whole idea behind it.”(PC104)
3.2.2. Impact on Clinical Encounter and Clinician Workflow
3.2.2.1. Impact on Workflow
3.2.2.2. Time
3.2.2.4. Length and Detail
Length: “I think it is too long. I think…that is not reasonable to expect a [clinician] who sees a ton of patients every day to get through easily. I mean though there is a summary page and I think that is very helpful, but I think there is also really good information on the individual pages that, you know, they have to, you know, scroll through…”(GC108)
Detail: “There are certain parts, pieces of information that the patient says they inputted but does not pull through in to my screen when I open the MyFamily document… It seems like the output to me is not equivalent to what the patient inputs.”(GC108)
Length: “…I find that I just, I look at the scores and I pick maybe the top two and probably ignore the rest.”(PC101)
Detail: “…there is a section that says, ‘why is your person flagged’, but I don’t find that there is enough granular information in there to really counsel the patient about why they were at an increased risk.”(PC101)
3.2.2.5. Risk Assessment
“My hope [is that] we could get to the point where we have an individual plan for patients that would change the way we do screening. I would like to see it to the point where well, you know, this 50-year old woman maybe really doesn’t need a colonoscopy, maybe we could wait until 55 for this person.”
“So you would really be able to stratify high-risk populations from general risk populations and change your screening to meet those two populations?”
“Yes.”
3.2.2.6. Pedigrees
“…with the pedigree being so divided up that it really kinda compartmentalizes the patient’s care… Where if you have a pedigree that’s all on the same page, you can really get a grasp of what that patient is dealing with…if you are looking helping the patient, to look at only one problem in their health history, I’m not really sure it’s doing them a whole lot of good.”(CS110)
“…if I left it as all the separate pedigrees, I would not use it.”(GC107)
“…if the pedigree is good, I would accept [the MyFamily report]. If not, I would just not use [MyFamily for that patient].”(GC106)
“I do like the ability to look at things before the patient checks in… because I have had an opportunity to review it before they have arrived, it doesn’t interrupt at all. I am prepared to go in and have that discussion.”(CS110)
3.2.3. Process of Implementation
3.2.3.1. Barriers
“…particularly in regards to primary care, patients are taking the time to fill this out, but if the physician is too busy to look it over or review it, this might not be a physician that [MyFamily] should be sent out for…”(CS110)
3.2.3.2. Facilitators
“I have had a lot of one-on-one TLC from you guys.”(CS110)
3.2.3.3. Future Functions
“I have a better jumping off point and at least the family structure basically down. So it, I think, especially with the newly improved pedigrees, [using MyFamily for patient care] will be faster.”(GC108)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Downing, G.J.; Boyle, S.N.; Brinner, K.M.; Osheroff, J.A. Information management to enable personalized medicine: Stakeholder roles in building clinical decision support. BMC Med. Inform. Decis. Mak. 2009, 9, e44. [Google Scholar] [CrossRef]
- Berg, M. Implementing information systems in health care organizations: Myths and challenges. Int. J. Med. Inform. 2001, 64, 143–156. [Google Scholar]
- Sittig, D.F.; Krall, M.A.; Dykstra, R.H.; Russell, A.; Chin, H.L. A survey of factors affecting clinician acceptance of clinical decision support. BMC Med. Inform. Decis. Mak. 2006, 6, e6. [Google Scholar] [CrossRef]
- Kortteisto, T.; Komulainen, J.; Makela, M.; Kunnamo, I.; Kaila, M. Clinical decision support must be useful, functional is not enough: A qualitative study of computer-based clinical decision support in primary care. BMC Health Serv. Res. 2012, 12, e349. [Google Scholar] [CrossRef]
- Jones, J.B.; Stewart, W.F.; Darer, J.D.; Sittig, D.F. Beyond the threshold: Real-time use of evidence in practice. BMC Med. Inform. Decis. Mak. 2013, 13, e47. [Google Scholar] [CrossRef]
- Kousgaard, M.B.; Siersma, V.; Reventlow, S.; Ertmann, R.; Felding, P.; Waldorff, F.B. The effectiveness of computer reminders for improving quality assessment for point-of-care testing in general practice—A randomized controlled trial. Implement. Sci. 2013, 8, e47. [Google Scholar] [CrossRef]
- Acheson, L.S.; Wiesner, G.L.; Zyzanski, S.J.; Goodwin, M.A.; Stange, K.C. Family history-taking in community family practice: Implications for genetic screening. Genet. Med. 2000, 2, 180–185. [Google Scholar]
- Guttmacher, A.E.; Collins, F.S.; Carmona, R.H. The family history—More important than ever. N. Engl. J. Med. 2004, 351, 2333–2336. [Google Scholar] [CrossRef]
- Rich, E.C.; Burke, W.; Heaton, C.J.; Haga, S.; Pinsky, L.; Short, M.P.; Acheson, L. Reconsidering the family history in primary care. J. Gen. Intern. Med. 2004, 19, 273–280. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services 2020 Topics & Objectives: Genomics. Available online: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=15#two/ (accessed on 18 November 2013).
- Edelman, E.A.; Lin, B.K.; Doksum, T.; Drohan, B.; Edelson, V.; Dolan, S.M.; Hughes, K.; O’Leary, J.; Vasquez, L.; Copeland, S.; et al. Evaluation of a novel electronic genetic screening and clinical decision support tool in prenatal clinical settings. Matern. Child Health J. 2013. [Google Scholar] [CrossRef]
- Facio, F.M.; Feero, W.G.; Linn, A.; Oden, N.; Manickam, K.; Biesecker, L.G. Validation of my family health portrait for six common heritable conditions. Genet. Med. 2010, 12, 370–375. [Google Scholar] [CrossRef]
- Hulse, N.C.; Ranade-Kharkar, P.; Post, H.; Wood, G.M.; Williams, M.S.; Haug, P.J. Development and early usage patterns of a consumer-facing family health history tool. AMIA Annu. Symp. Proc. 2011, 2011, 578–587. [Google Scholar]
- Orlando, L.A.; Buchanan, A.H.; Hahn, S.E.; Christianson, C.A.; Powell, K.P.; Skinner, C.S.; Chesnut, B.; Blach, C.; Due, B.; Ginsburg, G.S.; et al. Development and validation of a primary care-based family health history and decision support program (metree). N. C. Med. J. 2013, 74, 287–296. [Google Scholar]
- IOM (Institute of Medicine). Clinical Practice Guidelines We Can Trust; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, e50. [Google Scholar] [CrossRef]
- Welch, B.M.; Kawamoto, K. Clinical decision support for genetically guided personalized medicine: A systematic review. J. Am. Med. Inform. Assoc. 2013, 20, 388–400. [Google Scholar] [CrossRef]
- Rubinstein, W.S.; Acheson, L.S.; O’Neill, S.M.; Ruffin, M.T.; Wang, C.; Beaumont, J.L.; Rothrock, N. Family Healthware Impact Trial, G., Clinical utility of family history for cancer screening and referral in primary care: A report from the family healthware impact trial. Genet. Med. 2011, 13, 956–965. [Google Scholar] [CrossRef]
- Scheuner, M.T.; Hamilton, A.B.; Peredo, J.; Sale, T.J.; Austin, C.; Gilman, S.C.; Bowen, M.S.; Goldzweig, C.L.; Lee, M.; Mittman, B.S.; et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet. Med. 2013. [Google Scholar] [CrossRef]
- Kawamoto, K.; Houlihan, C.A.; Balas, E.A.; Lobach, D.F. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. Br. Med. J. 2005, 330, e765. [Google Scholar] [CrossRef]
- McDermott, L.; Yardley, L.; Little, P.; Ashworth, M.; Gulliford, M. Developing a computer delivered, theory based intervention for guideline implementation in general practice. BMC Fam. Pract. 2010, 11, e90. [Google Scholar] [CrossRef]
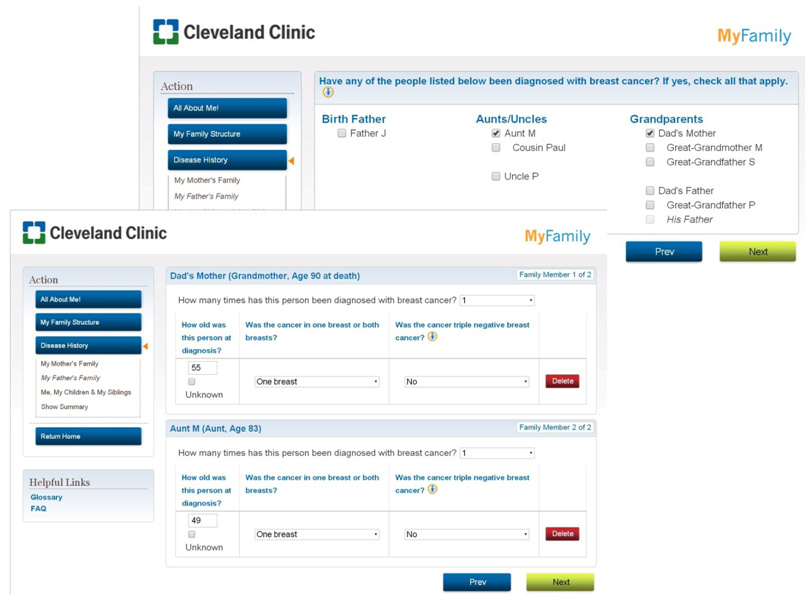
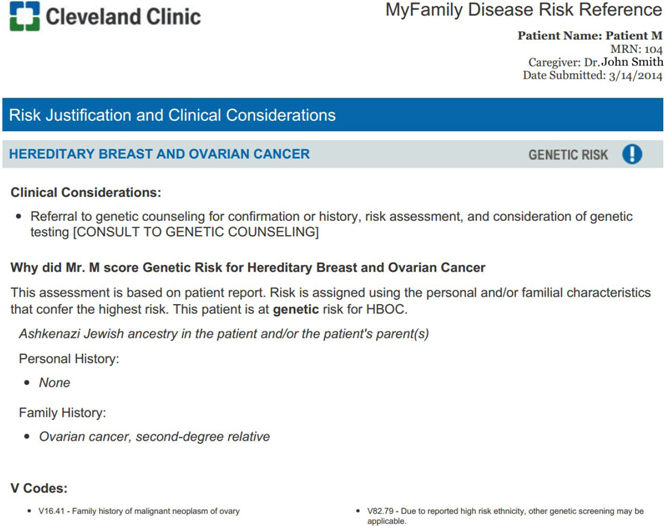
Appendix A: Screen Shots of MyFamily Application
Appendix B: Interview Guide
- 1.
- One a scale of 1 to 5, with 1 being very easy and 5 being very difficult, how easy is it to use the Risk Reference (RR) in the patient encounter?
- 2.
- Are there parts of the RR that are easier or harder to use?
- Which parts?
- Why?
- 3.
- One a scale of 1 (very easy) to 5 (very difficult), how easy or hard is it to access the RR in the EMR?
- 4.
- One a scale of 1 (very easy) to 5 (very difficult), how easy or hard is it to approve the RR in the EMR?
- 5.
- One a scale of 1 (very easy) to 5 (very difficult), how easy or hard is it to understand the information in the RR?
- 6.
- Are there aspects of the RR that are difficult to understand?
- Which parts?
- How could these parts be improved?
- 7.
- On a scale of 1 (very useful) to 5 (not very useful at all), how useful are MyFamily and the RR for your practice?
- Which aspects of the RR are most useful for your practice?
- Which aspects of the RR are least useful for your practice?
- 8.
- How useful will MyFamily be to other providers?
- 9.
- Is the length of the RR and amount of information okay, too little, or too long? Why or why not?
- 10.
- Is the level of difficulty of the information okay, too simple, or too complex?
- (if too simple or too complex) Which parts are too (simple/complex)
- How could this be improved?
- 11.
- Think about the clinical recommendations and risk assessment in the Risk Reference. On a scale of 1 to 5, with 1 being strongly agree and 5 being strongly disagree, how strongly do you agree with the clinical risk assessment and recommendations?
- (if disagree) Please tell me more about your concerns.
- Do you need additional information about how the recommendations were generated?
- 12.
- Do you think the RR increases or decreases quality of care?
- 13.
- On a scale of 1 (very helpful) to 5 (not very helpful at all), how helpful is the RR in meeting patient health maintenance requirements?
- 14.
- On a scale of 1 (very helpful) to 5 (not very helpful at all), how helpful is the RR in identifying patients appropriate for referral to subspecialties and other services?
- 15.
- Does the RR give you the information you need to appropriately identify and communicate with patients about a genetic risk?
- Does the RR include enough additional or supportive educational content (e.g., why the patient is at increased risk, why the recommendation is considered best practice)
- 16.
- Does MyFamily and the RR change your confidence in identifying, managing, or communicating about genetic risks? Please describe your answer.
- 17.
- What have your patients told you about the process or experience of using MyFamily before the appointment?
- 18.
- Are there some kinds of patients who have an easier or harder time using MyFamily? If so, please describe.
- 19.
- Do you think MyFamily benefits CCF patients equally, or are there some patient populations who benefit more or less?
- 20.
- Do you recommend any changes to the patient interface of MyFamily for improved patient access and usability?
- 21.
- Approximately how many patients have you seen that have completed the MyFamily web questionnaire and have a RR available in the clinical encounter?
- 22.
- Can you please briefly describe how you have been using the RR in your clinic?
- 23.
- How has your clinical encounter changed since using RR?
- 24.
- Does the RR align with your current work flow?
- If not, how did you change your work flow since the introduction of MyFamily?
- What could be changed in the system to improve work flow using MyFamily?
- 25.
- Do you think using the RR saves time, increases time, or does not change your time spent in the clinical encounter?
- 26.
- Do you always use the RR if it is available? Why or why not?
- 27.
- What resources were needed to get ready for launch? Examples: Staff time, meetings, IT help/training.
- Did you and your team have what they needed to launch? (Probe if needed: Is the level of MyFamily education and training that you received sufficient?)
- 28.
- What resources are needed to continue to use and maintain the program in the clinic?
- 29.
- What barriers or challenges has the team experienced in the implementation and use of MyFamily?
- 30.
- What facilitators have supported the successful implementation of MyFamily?
- 31.
- What is the impact of MyFamily on the clinical support staff (e.g., Medical Assistant)?
- 32.
- What is your overall impression of MyFamily and the RR?
- 33.
- What is your favorite part of the program?
- 34.
- What is your least favorite part of the program?
- 35.
- Are there features of MyFamily or the RR that should be changed or added for improved usability and patient care? Please describe.
- 36.
- Do you have any concerns about using My Family and the RR?
- 37.
- What conditions should the MyFamily staff focus on next?
- 38.
- Any other comments?
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doerr, M.; Edelman, E.; Gabitzsch, E.; Eng, C.; Teng, K. Formative Evaluation of Clinician Experience with Integrating Family History-Based Clinical Decision Support into Clinical Practice. J. Pers. Med. 2014, 4, 115-136. https://doi.org/10.3390/jpm4020115
Doerr M, Edelman E, Gabitzsch E, Eng C, Teng K. Formative Evaluation of Clinician Experience with Integrating Family History-Based Clinical Decision Support into Clinical Practice. Journal of Personalized Medicine. 2014; 4(2):115-136. https://doi.org/10.3390/jpm4020115
Chicago/Turabian StyleDoerr, Megan, Emily Edelman, Emily Gabitzsch, Charis Eng, and Kathryn Teng. 2014. "Formative Evaluation of Clinician Experience with Integrating Family History-Based Clinical Decision Support into Clinical Practice" Journal of Personalized Medicine 4, no. 2: 115-136. https://doi.org/10.3390/jpm4020115
APA StyleDoerr, M., Edelman, E., Gabitzsch, E., Eng, C., & Teng, K. (2014). Formative Evaluation of Clinician Experience with Integrating Family History-Based Clinical Decision Support into Clinical Practice. Journal of Personalized Medicine, 4(2), 115-136. https://doi.org/10.3390/jpm4020115




