Aedes aegypti (Diptera: Culicidae) Immune Responses with Different Feeding Regimes Following Infection by the Entomopathogenic Fungus Metarhizium anisopliae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Fungal Isolate and Preparation of Suspensions
2.4. Infection of Mosquitoes with M. anisopliae and Survival Assays
2.5. RNA Extraction and cDNA Synthesis
2.6. RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of Fungal Infection on the Survival of A. aegypti Females
3.2. The Immune Response of A. aegypti to Infection by M. anisopliae
3.3. Fungal Infection Induces Upregulation of Antimicrobial Peptides
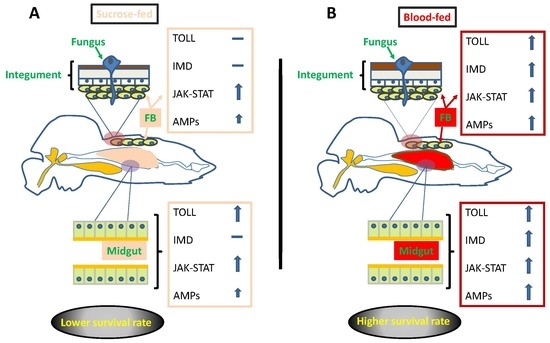
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Higgs, S.; Beaty, B.J. Natural cycles of vector borne pathogens. In Biology of Disease Vectors, 2nd ed.; Marquardt, W.C., Black, W.C., Hemingway, J., Freier, J.E., Higgs, S., Hagedorn, H., James, A., Moore, C., Kondratieff, B., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2004; Chapter 14; pp. 167–186. [Google Scholar]
- Benelli, G.; Romano, D. Mosquito Vectors of Zika Virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. Available online: www.Who.Int/News-Room/Fact-Sheets/Detail/Dengue-and-Severe-Dengue (accessed on 10 November 2019).
- Gatherer, D.; Kohl, A. Zika Virus: A Previously Slow Pandemic Spreads Rapidly through the Americas. J. Gen. Virol. 2016, 97, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikan, N.; Smith, D.R. Zika Virus: History of a Newly Emerging Arbovirus. Lancet Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.M.; Velozo, L.S.; Carvalho, M.A.; Serdeiro, M.T.; Honório, N.A.; Kaplan, M.A.C.; Maleck, M. Larvicidal Activity of Ottonia anisum Metabolites against Aedes aegypti: A Potential Natural Alternative Source for Mosquito Vector Control in Brazil. J. Vector Borne Dis. 2017, 8, 61–68, PMID:28352047. [Google Scholar]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Negl. Trop. Dis. 2017, 11, 1–20. [Google Scholar] [CrossRef]
- Lacey, L.A.; Lacey, C.M. The Medical Importance of Riceland Mosquitoes and Their Control Using Alternatives to Chemical Insecticides. J. Am. Mosq. Control Assoc. Suppl. 1990, 2, 1–93. [Google Scholar]
- Manning, J.E.; Morens, D.M.; Kamhawi, S.; Valenzuela, J.G.; Memoli, M. Mosquito Saliva: The Hope for a Universal Arbovirus Vaccine? J. Infect. Dis. 2018, 218, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. JEZS 2018, 6, 27–32. [Google Scholar]
- Dal Bello, G.; Padín, S.B.; López Lástra, C.C.; Fabrizio, M. Laboratory evaluation of chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. J. Stored Prod. Res. 2000, 37, 77–84. [Google Scholar] [CrossRef]
- Cherry, A.J.; Abalo, P.; Hell, K. A Laboratory Assessment of the Potential of Different Strains of the Entomopathogenic Fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to Control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in Stored Cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Lord, J.C. Desiccant Dusts Synergize the Effect of Beauveria bassiana (Hyphomycetes: Moniliales) on Stored-Grain Beetles. J. Econ. Entomol. 2001, 94, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.-J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic Fungi for Mosquito Control: A Review. J. Insect Sci. 2004, 24, 19. [Google Scholar] [CrossRef]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic Fungi: New Insights into Host-Pathogen Interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [PubMed]
- Lovett, B.; St. Leger, R.J. The Insect Pathogens. Fungal Kingd. 2017, 5, 925–943. [Google Scholar] [CrossRef]
- Fang, W.; Azimzadeh, P.; St. Leger, R.J. Strain Improvement of Fungal Insecticides for Controlling Insect Pests and Vector-Borne Diseases. Curr. Opin. Microbiol. 2012, 15, 232–238. [Google Scholar] [CrossRef]
- Paula, A.R.; Carolino, A.T.; Silva, C.P.; Samuels, R.I. Susceptibility of Adult Female Aedes aegypti (Diptera: Culicidae) to the Entomopathogenic Fungus Metarhizium anisopliae is Modified Following Blood Feeding. Parasit. Vectors 2011, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Whitehorn, J.; Simmons, C.P. The Pathogenesis of Dengue. Vaccine 2011, 29, 7221–7228. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.R. The Insect Cellular Immune Response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect Hemocytes and Their Role in Immunity. Insect Biochem. Mol. Biol. 2002, 15, 1295–1309. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H.; Yu, X.-Q. Innate Immune Responses of a Lepidopteran Insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Tanveer, A.; Biswas, S.; Ram, E.V.S.R.; Gupta, A.; Kumar, B.; Habib, S. Nuclear-Encoded DnaJ Homologue of Plasmodium falciparum Interacts with Replication Ori of the Apicoplast Genome. Mol. Microbiol. 2010, 75, 942–956. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Souza-Neto, J.; Cosme, R.T.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal Tripartite Interactions between the Aedes aegypti Midgut Microbiota, Innate Immune System and Dengue Virus Influences Vector Competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef]
- Lu, H.-L.; St. Leger, R.J. Chapter Seven—Insect Immunity to Entomopathogenic Fungi. In Advances in Genetics; Lovett, B., St. Leger, R.J., Eds.; Genetics and Molecular Biology of Entomopathogenic Fungi; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, pp. 251–285. [Google Scholar] [CrossRef]
- Blandin, S.; Levashina, E.A. Thioester-Containing Proteins and Insect Immunity. Mol. Immunol. 2004, 40, 903–908. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Dunlap, C.A.; Muturi, E.J.; Barletta, A.B.F.; Rooney, A.P. Entomopathogenic Fungal Infection Leads to Temporospatial Modulation of the Mosquito Immune System. PLoS Negl. Trop. Dis. 2018, 12, e0006433. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.L.; Muturi, E.J.; Barletta, A.B.F.; Rooney, A.P. The Aedes aegypti IMD Pathway Is a Critical Component of the Mosquito Antifungal Immune Response. Dev. Comp. Immunol. 2019, 95, 1–9. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.-M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette Spätzle/Toll/Cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Wrkato, D.; Andaraon, K.V. The Spiitzk Gene Encodes a Component of the Naling Attem. Cell 1994, 76, 677–688. [Google Scholar]
- Schneider, D.S.; Jin, Y.; Morisato, D.; Anderson, K.V. A Processed Form of the Spatzle Protein Defines Dorsal-Ventral Polarity in the Drosophila Embryo. Development 1994, 120, 1243–1250. [Google Scholar] [PubMed]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, G.; Shin, S.W.; Cheon, H.M.; Kokoza, V.; Raikhel, A.S. Transgenic Alteration of Toll Immune Pathway in the Female Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2005, 102, 13568–13573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garver, L.S.; Dong, Y.; Dimopoulos, G. Caspar Controls Resistance to Plasmodium falciparum in Diverse Anopheline Species. PLoS Pathog. 2009, 5, e1000335. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.W.; Kokoza, V.; Bian, G.; Cheon, H.-M.; Kim, Y.J.; Raikhel, A.S. REL1, a Homologue of Drosophila Dorsal, Regulates Toll Antifungal Immune Pathway in the Female Mosquito Aedes aegypti. J. Biol. Chem. 2005, 280, 16499–16507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhu, Y.; Pang, X.; Xiao, X.; Zhang, R.; Cheng, G. Regulation of Antimicrobial Peptides in Aedes aegypti Aag2 Cells. Front. Cell. Infect. Microbiol. 2017, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Hoa, N.T.; Lin, H.; Zhang, L.; Nguyen, H.L.A.; Kanzok, S.M.; Zheng, L. Expression of Immune Responsive Genes in Cell Lines from Two Different Anopheline Species. Insect Mol. Biol. 2006, 15, 721–729. [Google Scholar] [CrossRef]
- Barillas-Mury, C.; Charlesworth, A.; Gross, I.; Richman, A.; Hoffmann, J.A.; Kafatos, F.C. Immune Factor Gambif1, a New Rel Family Member from the Human Malaria Vector, Anopheles gambiae. EMBO J. 1996, 15, 4691–4701. [Google Scholar] [CrossRef]
- Antonova, Y.; Alvarez, K.S.; Kim, Y.J.; Kokoza, V.; Raikhel, A.S. The Role of NF-ΚB Factor REL2 in the Aedes aegypti Immune Response. Insect Biochem. Mol. Biol. 2009, 39, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Meister, S.; Kanzok, S.M.; Zheng, X.-L.; Luna, C.; Li, T.-R.; Hoa, N.T.; Clayton, J.R.; White, K.P.; Kafatos, F.C.; Christophides, G.K.; et al. Immune Signaling Pathways Regulating Bacterial and Malaria Parasite Infection of the Mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2005, 102, 11420–11425. [Google Scholar] [CrossRef] [Green Version]
- Meister, S.; Agianian, B.; Turlure, F.; Relógio, A.; Morlais, I.; Kafatos, F.C.; Christophides, G.K. Anopheles gambiae PGRPLC-Mediated Defense against Bacteria Modulates Infections with Malaria Parasites. PLoS Pathog. 2009, 5, e1000542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Morton, J.C.; Ramirez, J.L.; Souza-Neto, J.A.; Dimopoulos, G. The Entomopathogenic Fungus Beauveria bassiana Activate Toll and JAK-STAT Pathway-Controlled Effector Genes and Anti-Dengue Activity in Aedes aegypti. Insect Biochem. Mol. Biol. 2012, 42, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula, A.R.; Brito, E.S.; Pereira, C.R.; Carrera, M.P.; Samuels, R.I. Susceptibility of Adult Aedes aegypti (Diptera: Culicidae) to Infection by Metarhizium anisopliae and Beauveria bassiana: Prospects for Dengue Vector Control. Biocontrol Sci. Technol. 2008, 18, 1017–1025. [Google Scholar] [CrossRef]
- Paula, A.R.; Carolino, A.T.; Paula, C.O.; Samuels, R.I. The Combination of the Entomopathogenic Fungus Metarhizium anisopliae with the Insecticide Imidacloprid Increases Virulence against the Dengue Vector Aedes aegypti (Diptera: Culicidae). Parasit. Vectors 2011, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Dzaki, N.; Ramli, K.N.; Azlan, A.; Ishak, I.H.; Azzam, G. Evaluation of Reference Genes at Different Developmental Stages for Quantitative Real-Time PCR in Aedes aegypti. Sci. Rep. 2017, 7, 43618. [Google Scholar] [CrossRef] [Green Version]
- Barletta, A.B.F.; Silva, M.C.L.N.; Sorgine, M.H.F. Validation of Aedes aegypti Aag-2 Cells as a Model for Insect Immune Studies. Parasit. Vectors 2012, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Everitt, B.S. Statistical Methods for Medical Investigations; Halsted Press: New York, NY, USA, 1994. [Google Scholar]
- Schneider, D. Physiological Integration of Innate Immunity. In Insect Infection and Immunity: Evolution, Ecology, and Mechanisms; OUP: Oxford, UK, 2009. [Google Scholar]
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic Fungi and Insect Behaviour: From Unsuspecting Hosts to Targeted Vectors. BioControl 2010, 55, 89–102. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Dunn, W.A.; Campbell, C.L.; Olson, K.E.; Dimon, M.T.; Marinotti, O.; James, A.A. RNA-Seq Analyses of Blood-Induced Changes in Gene Expression in the Mosquito Vector Species, Aedes aegypti. BMC Genom. 2011, 12. [Google Scholar] [CrossRef] [Green Version]
- Bottino-Rojas, V.; Talyuli, O.A.C.; Jupatanakul, N.; Sim, S.; Dimopoulos, G.; Venancio, T.M.; Bahia, A.C.; Sorgine, M.H.; Oliveira, P.L.; Paiva-Silva, G.O. Heme Signaling Impacts Global Gene Expression, Immunity and Dengue Virus Infectivity in Aedes aegypti. PLoS ONE 2015, 10, e013598. [Google Scholar] [CrossRef] [Green Version]
- Upton, L.M.; Povelones, M.; Christophides, G.K. Anopheles gambiae Blood Feeding Initiates an Anticipatory Defense Response to Plasmodium berghei. J. Innate Immun. 2015, 7, 74–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of Aedes aegypti and Allows Proliferation of Intestinal Microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukutani, K.F.; Kasprzykowski, J.I.; Paschoal, A.R.; Gomes, M.S.; Barral, A.; de Oliveira, C.I.; Ramos, P.I.P.; de Queiroz, A.T.L. Meta-Analysis of Aedes aegypti Expression Datasets: Comparing Virus Infection and Blood-Fed Transcriptomes to Identify Markers of Virus Presence. Front. Bioeng. Biotechnol. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawidian, P.; Rhodes, V.L.; Michel, K. Mosquito-Fungus Interactions and Antifungal Immunity. Insect Biochem. Mol. Biol. 2019, 111, 103182. [Google Scholar] [CrossRef] [PubMed]
- Garver, L.S.; Bahia, A.C.; Das, S.; Souza-Neto, J.A.; Shiao, J.; Dong, Y.; Dimopoulos, G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. Available online: https://link-galegroup.ez29.capes.proxy.ufrj.br/apps/doc/A304535999/AONE?sid=lms (accessed on 27 November 2019). [CrossRef]
- Barletta, A.B.F.; Nascimento-Silva, M.C.L.; Talyuli, O.A.C.; Oliveira, J.H.M.; Pereira, L.O.R.; Oliveira, P.L.; Sorgine, M.H.F. Microbiota Activates IMD Pathway and Limits Sindbis Infection in Aedes aegypti. Parasit. Vectors 2017, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect Pathogenic Fungus Interacts with the Gut Microbiota to Accelerate Mosquito Mortality. Proc. Natl. Acad. Sci. 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [Green Version]
- Alkhaibari, A.M.; Carolino, A.T.; Yavasoglu, S.I.; Maffeis, T.; Mattoso, T.C.; Bull, J.C.; Samuels, R.I.; Butt, T.M. Metarhizium brunneum Blastospore Pathogenesis in Aedes aegypti Larvae: Attack on Several Fronts Accelerates Mortality. PLoS Pathog. 2016, 12, e1005715. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Pérez, C.; Clifton, M.E.; Noriega, F.G. How Micronutrients Influence the Physiology of Mosquitoes. Curr. Opin. Insect Sci. 2017, 23, 112–117. [Google Scholar] [CrossRef]





| Gene | Primer sequence (5′–3′) | AAEL# ID (GenBank BankIt No.) |
|---|---|---|
| Rps17 | FW GGGACAAATCGGCCAGGCTATC | AAEL009496 |
| RV TCGTGGACGCTTCTGCTTGTTG | ||
| AaREL1 | FW ATAGGCGAGATCAACATCAGCAGC | AAEL012164 |
| RV CGTTGCTGTTCCTGCTTCATATCG | ||
| AaREL2 | FW TTTGAATGTGCTGTTGGGTC | AAEL007624 |
| RV GAATGTTGTTTCCGTGCTTA | ||
| AaCACTUS | FW AGACAGCCGCACCTTCGATTCC | AAEL000709 |
| RV CGCTTCGGTAGCCTCGTGGAT | ||
| AaIMD | FW TGGTCAACCTGTTATGGCAA | AAEL010083 |
| RV GGGTTGACTTTGTCGTCGTT | ||
| AaCaspar | FW CCTTTCTCGACCTACTTGCG | AAEL027860 |
| RV CGATCCTTATCAGTGCCGTT | ||
| AaPIAS | FW GCTGCAACGCATGAAAACTA | AAEL000896 |
| RV CAGACGGGACAGTTCCAAGT | ||
| AaSTAT | FW ACCGGACCTTCACCTTCTG | AAEL020559 |
| RV CCAGCTCACTGTTCGGAGAA | ||
| AaAttacin | FW TTGGCAGGCACGGAATGTCTTG | AAEL003389 |
| RV TGTTGTCGGGACCGGGAAGTG | ||
| AaCecropin G | FW TCACAAAGTTATTTCTCCTGATCG | AAEL015515 |
| RV GCTTTAGCCCCAGCTACAAC | ||
| AaDefensin A | FW CTGCCGGAGGAAACCTATCAG | AAEL003841 |
| RV GCAATGCAATGAGCAGCACAAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, S.; de Paula, A.; Samuels, R.; da Fonseca, R.; Gomes, S.; Silva, J.R.; Mury, F. Aedes aegypti (Diptera: Culicidae) Immune Responses with Different Feeding Regimes Following Infection by the Entomopathogenic Fungus Metarhizium anisopliae. Insects 2020, 11, 95. https://doi.org/10.3390/insects11020095
Cabral S, de Paula A, Samuels R, da Fonseca R, Gomes S, Silva JR, Mury F. Aedes aegypti (Diptera: Culicidae) Immune Responses with Different Feeding Regimes Following Infection by the Entomopathogenic Fungus Metarhizium anisopliae. Insects. 2020; 11(2):95. https://doi.org/10.3390/insects11020095
Chicago/Turabian StyleCabral, Sara, Adriano de Paula, Richard Samuels, Rodrigo da Fonseca, Simone Gomes, José Roberto Silva, and Flávia Mury. 2020. "Aedes aegypti (Diptera: Culicidae) Immune Responses with Different Feeding Regimes Following Infection by the Entomopathogenic Fungus Metarhizium anisopliae" Insects 11, no. 2: 95. https://doi.org/10.3390/insects11020095