Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention
Abstract
:1. Introduction
2. Results
2.1. GSE Extraction, Characterization and Antioxidant Activity
2.2. Effect on Cell Viability
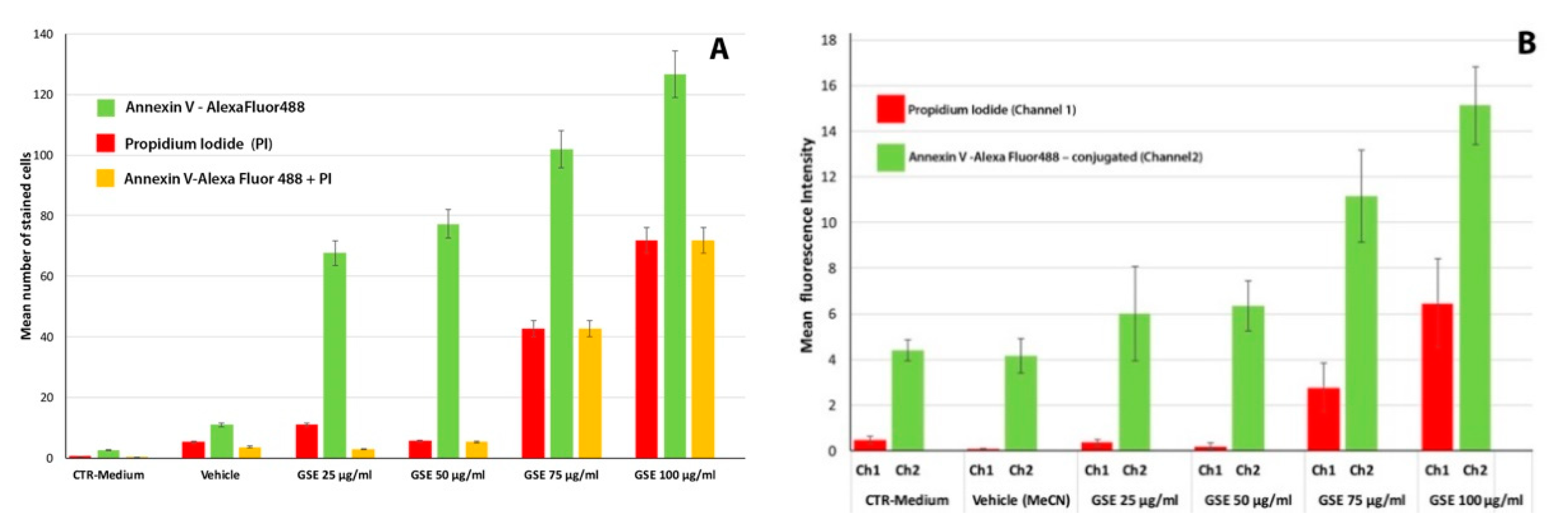
Apoptosis
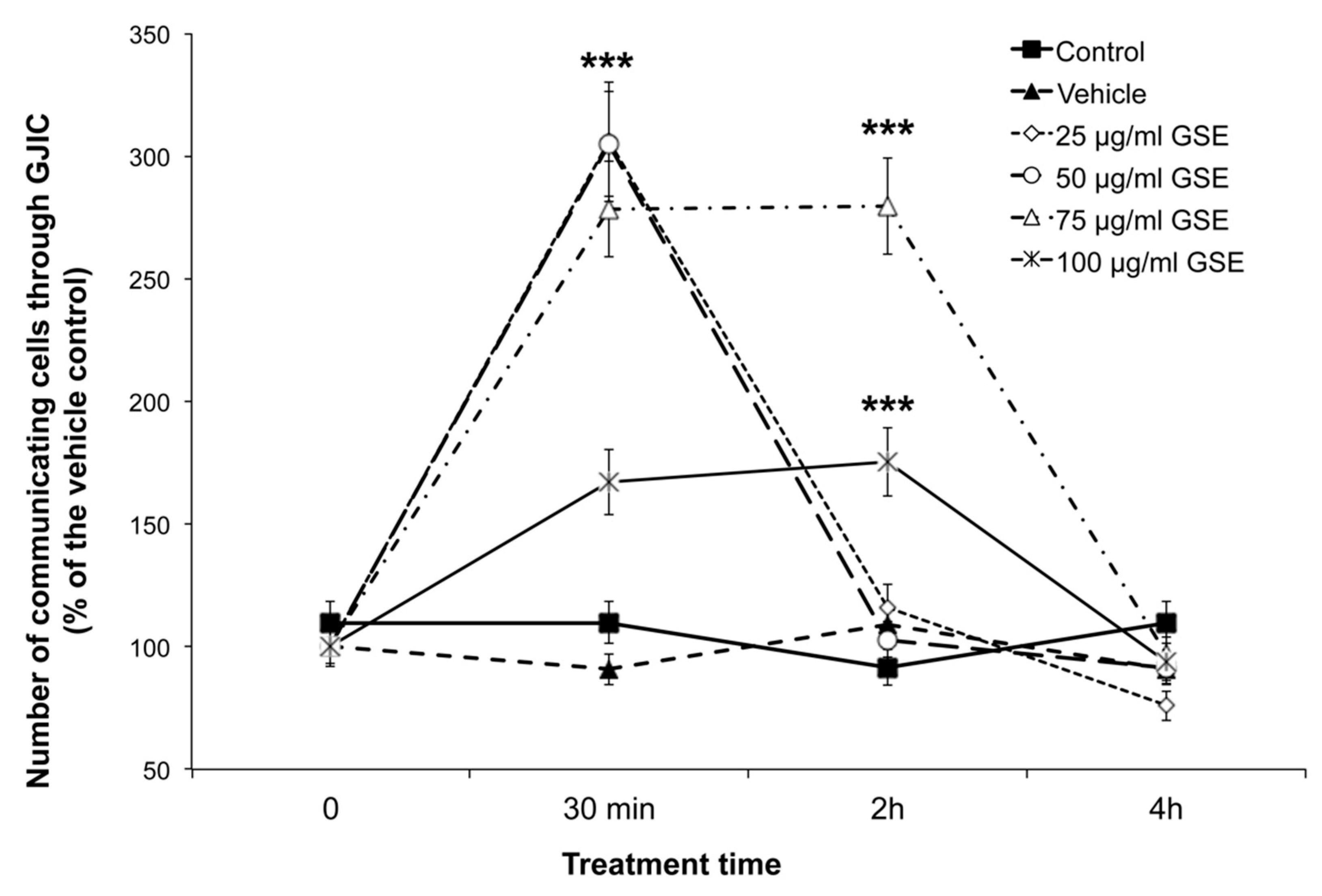
2.3. Effect on GJIC
2.3.1. GJIC Functionality
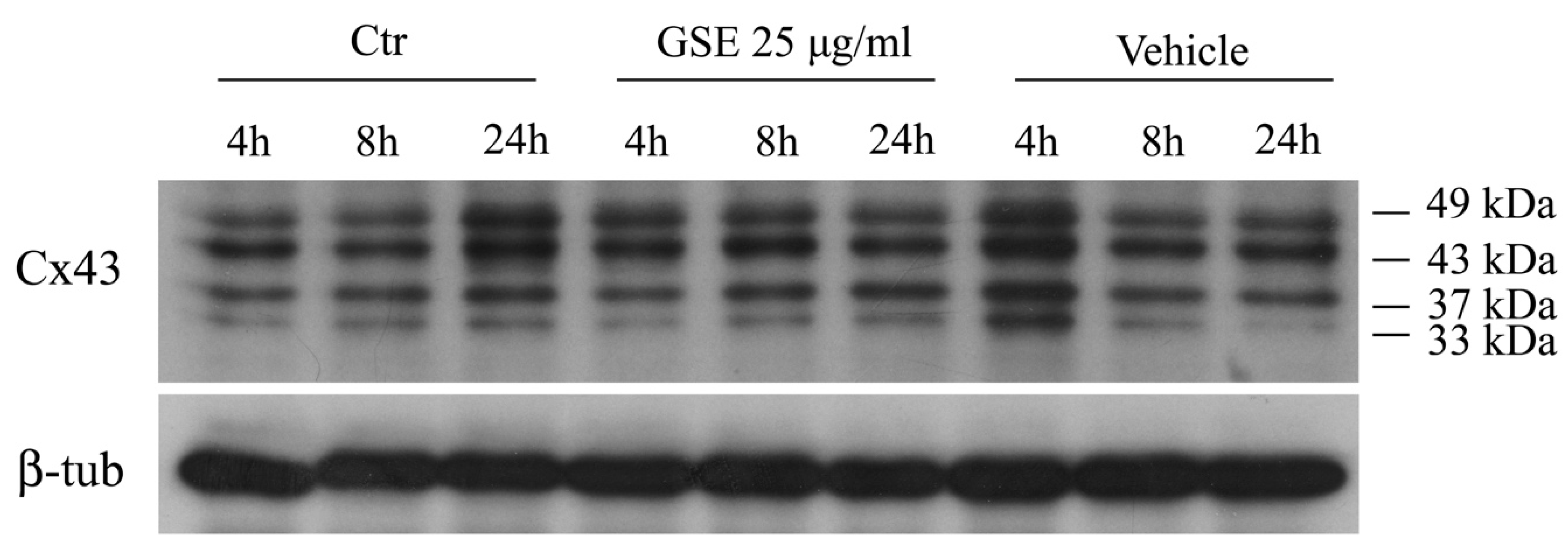
2.3.2. Western blot Analysis of Cx43 Protein
2.3.3. Cx43 Localization
2.3.4. Cx43 mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Source of Material
4.2. Extraction
4.3. Total Phenols
4.4. HPLC Analysis
4.5. Antioxidant Activity
4.6. Cell Cultures
4.7. Cell Viability and Treatments
4.8. Apoptosis
4.9. Estimation of GJIC by Scrape-Loading/Dye-Transfer (SL/DT) Assay
4.10. Western Blot Analisys of Connexin 43
4.11. Immunohistochemistry and Confocal Microscopy
4.12. Extraction of mRNA from MCF-7 Cells Treated with GSE
4.13. Quantitative Real-Time PCR
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GJIC | Gap-junction-intercellular communications |
| Cx43 | Connexin-43 protein |
| cx43 | Connexin-43 gene |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| SL/DT | Scrape-Loading/Dye-Transfer assay |
| GSE | Grape seeds extracts |
| GAE | Gallic Acid Equivalents |
| ROS | Reactive oxygen species |
| TE | Trolox Equivalents |
| GS | Grape Seeds |
| GJ | Gap junction |
References
- World Cancer Research Fund International: Breast Cancer Statistics. Available online: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics (accessed on 5 June 2019).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 22, 515. [Google Scholar] [CrossRef] [PubMed]
- Gollucke, A.P.B.; Aguiar, O.; Barbisan, L.F.; Ribeiro, D.A. Use of grape polyphenols against carcinogenesis: Putative molecular mechanisms of action using in vitro and in vivo test systems. J. Med. Food 2013, 16, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [PubMed]
- Feringa, H.H.H.; Laskey, D.A.; Dickson, J.E.; Coleman, C.I. The effect of grape seed extract on cardiovascular risk markers: A meta-analysis of randomized controlled trials. J. Am. Diet. Assoc. 2011, 111, 1173–1181. [Google Scholar] [CrossRef]
- Lecci, R.M.; Logrieco, A.; Leone, A. Pro-oxidative action of polyphenols as action mechanism for their pro-apoptotic activity. Anticancer Agents Med. Chem. 2014, 14, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef]
- Reddivari, L.; Charepalli, V.; Radhakrishnan, S.; Vadde, R.; Elias, R.J.; Lambert, J.D.; Vanamala, J.K. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complement. Altern. Med. 2016, 16, 278. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Health aspects of functional grape seed constituents. Trends Food Sci. Technol. 2004, 15, 422–433. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Gollücke, A.P.B. Recent applications of grape polyphenols in foods, beverages and supplements. Recent Pat. Food Nutr. Agric. 2010, 2, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C]procyanidin B2 in male rats. Drug Metab. Dispos. 2010, 38, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Derry, M.M.; Raina, K.; Balaiya, V.; Jain, A.K.; Shrotriya, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R.; Agarwal, C. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: Interlinking miRNA with cytokine signaling and inflammation. Cancer Prev. Res. (Phila) 2013, 6, 625–633. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Ouédraogo, M.; Charles, C.; Ouédraogo, M.; Guissou, I.P.; Stévigny, C.; Duez, P. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr. Cancer 2011, 63, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis 2018, 39, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Baliga, M.S.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2006, 27, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Vanden Berghe, W.; Boby, C.; Leroux, C.; Declerck, K.; Szarc vel Szic, K.; Heyninck, K.; Laukens, K.; Bizet, M.; Defrance, M.; et al. Dietary flavanols modulate the transcription of genes associated with cardiovascular pathology without changes in their DNA methylation state. PLoS ONE 2014, 9, e95527. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. Dietary modulation of the multistage, multimechanisms of human carcinogenesis: Effects on initiated stem cells and cell-cell communication. Nutr. Cancer 2006, 54, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. Cancer Prevention and Therapy of Two Types of Gap Junctional Intercellular Communication–Deficient “Cancer Stem Cell”. Cancers (Basel) 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Close, T.W.; Trosko, J.E. Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp. Cell Res. 2000, 254, 257–268. [Google Scholar] [CrossRef]
- Leone, A.; Longo, C.; Trosko, J.E. The chemopreventive role of dietary phytochemicals through gap junctional intercellular communication. Phytochem. Rev. 2012, 11, 285–307. [Google Scholar] [CrossRef]
- Leone, A.; Zefferino, R.; Longo, C.; Leo, L.; Zacheo, G. Supercritical CO(2)-extracted tomato Oleoresins enhance gap junction intercellular communications and recover from mercury chloride inhibition in keratinocytes. J. Agric. Food Chem. 2010, 58, 4769–4778. [Google Scholar] [CrossRef]
- Fornelli, F.; Leone, A.; Verdesca, I.; Minervini, F.; Zacheo, G. The influence of lycopene on the proliferation of human breast cell line (MCF-7). Toxicol. In Vitro 2007, 21, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Jung, J.-W.; Lee, Y.-S.; Kang, K.-S. Indole-3-carbinol prevents H(2)O(2)-induced inhibition of gap junctional intercellular communication by inactivation of PKB/Akt. J. Vet. Med. Sci. 2008, 70, 1057–1063. [Google Scholar] [PubMed]
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamins, phytochemicals, diets, and their implementation in cancer chemoprevention. Crit. Rev. Food Sci. Nutr. 2004, 44, 437–452. [Google Scholar] [PubMed]
- Trosko, J.E. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J. Biochem. Mol. Biol. 2003, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef] [PubMed]
- Sovadinova, I.; Babica, P.; Böke, H.; Wilke, A.; Kumar, E.; Park, J.S.; Trosko, J.E.; Upham, B.L. Phosphatidylcholine specific PLC-induced dysregulation of gap junctions, a robust cellular response to environmental toxicants, and prevention by resveratrol. PLoS ONE 2016, 3, e0124454. [Google Scholar] [CrossRef]
- Romo, D.; Velmurugan, K.; Upham, B.L.; Dwyer-Nield, L.-D.; Bauer, A.K. Dysregulation of Gap Junction Function and Cytokine Production in Response to Non-Genotoxic Polycyclic Aromatic Hydrocarbons in an In Vitro Lung Cell Model. Cancers 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Chang, C.C. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat. Res. 2001, 480–481, 219–229. [Google Scholar]
- Trosko, J.E. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed. Pharm. 2005, 59 (Suppl. 2), S326–S331. [Google Scholar] [CrossRef]
- Trosko, J.E.; Chang, C.-C.; Upham, B.L.; Tai, M.-H. The role of human adult stem cells and cell-cell communication in cancer chemoprevention and chemotherapy strategies. Mutat. Res. 2005, 591, 187–197. [Google Scholar] [CrossRef]
- Trosko, J.E.; Ruch, R.J. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr. Drug Targets 2002, 3, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Krutovskikh, V.A.; Piccoli, C.; Yamasaki, H.; Yamasaki, H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 2002, 21, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Kang, N.J.; Lee, K.M.; Lee, B.K.; Kim, J.H.; Lee, K.W.; Lee, H.J. Cocoa polyphenols attenuate hydrogen peroxide-induced inhibition of gap-junction intercellular communication by blocking phosphorylation of connexin 43 via the MEK/ERK signaling pathway. J. Nutr. Biochem. 2010, 21, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, L.; Xu, S.; Qiu, F.; Fan, Y.; Fu, G. Down-regulation of connexin43 gap junction by serum deprivation in human endothelial cells was improved by (-)-Epigallocatechin gallate via ERK MAP kinase pathway. Biochem. Biophys. Res. Commun. 2011, 404, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Yang, J.; Pan, Y.; Chen, Q.; Yan, X.; Wang, D.; Zhou, X.; Wu, Y. Up-regulation of the gap junction intercellular communication by tea polyphenol in the human metastatic lung carcinoma cell line. J. Cancer Ther. 2012, 3, 64–70. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis. Mech. Ageing Dev. 2006, 127, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Ale-Agha, N.; Stahl, W.; Sies, H. (-)-Epicatechin effects in rat liver epithelial cells: Stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Biochem. Pharmacol. 2002, 63, 2145–2149. [Google Scholar] [CrossRef]
- Chaumontet, C.; Droumaguet, C.; Bex, V.; Heberden, C.; Gaillard-Sanchez, I.; Martel, P. Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells. Cancer Lett. 1997, 114, 207–210. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Kanno, Y. Intercellular Communication and the Control of Tissue Growth: Lack of Communication between Cancer Cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef]
- Mesnil, M.; Crespin, S.; Avanzo, J.-L.L.; Zaidan-Dagli, M.-L.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandouz, M.; Batist, G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Ther. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.W.; Kaneda, M.; Morita, I. The gap junction-independent tumor-suppressing effect of connexin 43. J. Biol. Chem. 2003, 278, 44852–44856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Jiang, J.X. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions–an update. FEBS Lett. 2014, 588, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Moorby, C.; Patel, M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp. Cell Res. 2001, 271, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M.; Decrock, E.; Leybaert, L.; Bultynck, G.; Himpens, B.; Vanhaecke, T.; Rogiers, V. Non-channel functions of connexins in cell growth and cell death. Biochim. Biophys. Acta 2012, 1818, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Fistouris, P.; Batist, G.; Alpert, L.; Huynh, H.T.; Carystinos, G.D.; Alaoui-Jamali, M.A. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 1999, 59, 4104–4110. [Google Scholar] [PubMed]
- Kanczuga-Koda, L.; Sulkowska, M.; Koda, M.; Resze’c, J.; Famulski, W.; Baltaziak, M.; Sulkowski, S. Expression of connexin 43 in breast cancer in comparison with mammary dysplasia and the normal mammary gland. Folia Morphol. 2003, 62, 439–442. [Google Scholar]
- Yeh, E.S.; Williams, C.J.; Williams, C.B.; Bonilla, I.V.; Klauber-DeMore, N.; Phillips, S.L. Dysregulated connexin 43 in HER2-positive drug resistant breast cancer cells enhances proliferation and migration. Oncotarget 2017, 8, 109358. [Google Scholar] [CrossRef]
- Nicolson, G.; Lichtner, R.; Trosko, J. Cytoskeletal and junctional heterogeneity in mammary tumor cells and their possible significance in tumor progression. Adv. Exp. Med. Biol. 1988, 233, 21–26. [Google Scholar] [CrossRef]
- Fostok, S.; El-Sibai, M.; Bazzoun, D.; Lelièvre, S.; Talhouk, R. Connexin 43 Loss Triggers Cell Cycle Entry and Invasion in Non-Neoplastic Breast Epithelium: A Role for Noncanonical Wnt Signaling. Cancers 2019, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Ferrati, S.; Gadok, A.K.; Brunaugh, A.D.; Zhao, C.; Heersema, L.A.; Smyth, H.D.C.; Stachowiak, J.C. Connexin membrane materials as potent inhibitors of breast cancer cell migration. J. R. Soc. Interface 2017, 14, 20170313. [Google Scholar] [CrossRef] [PubMed]
- Chasampalioti, M.; Green, A.R.; Ellis, I.O.; Rakha, E.A.; Jackson, A.M.; Spendlove, I.; Ramage, J.M. Connexin 43 is an independent predictor of patient outcome in breast cancer patients. Breast Cancer Res. Treat. 2018, 174, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Xu, C.; Tsukamoto, T.; Sager, R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. Publ. Am. Assoc. Cancer Res. 1996, 7, 861–870. [Google Scholar]
- Upham, B.L.; Trosko, J.E. Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression. Antioxid. Redox Signal. 2009, 11, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Tyagi, A.K.; Singh, R.P.; Chan, D.C.F.; Agarwal, R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res. Treat. 2004, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Pasqualato, A.; Cucina, A.; Coluccia, P.; Ferranti, F.; Canipari, R.; Catizone, A.; Proietti, S.; D’Anselmi, F.; Ricci, G.; et al. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur. J. Nutr. 2014, 53, 421–431. [Google Scholar] [CrossRef]
- Salameh, A.; Dhein, S. Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta 2005, 1719, 36–58. [Google Scholar] [CrossRef]
- Gakhar, G.; Hua, D.H.; Nguyen, T.A. Combinational treatment of gap junctional activator and tamoxifen in breast cancer cells. Anticancer Drugs 2010, 21, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, X.; Li, P.; Li, Y.; Wang, H. Comparative Study of Antioxidant Activity of Grape (Vitis vinifera) Seed Powder Assessed by Different Methods. J. Food Drug Anal. 2008, 16, 67–73. [Google Scholar]
- Zefferino, R.; Leone, A.; Piccaluga, S.; Cincione, R.; Ambrosi, L. Mercury modulates interplay between IL-1beta, TNF-alpha, and gap junctional intercellular communication in keratinocytes: Mitigation by lycopene. J. Immunotoxicol. 2008, 5, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.P.; Hossain, M.Z.; Huang, R.; Gano, J.; Fan, Y.; Boynton, A.L. Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human glioblastoma cells. Int. J. Cancer 2001, 92, 130–138. [Google Scholar] [CrossRef]
- Laird, D.W. The life cycle of a connexin: Gap junction formation, removal, and degradation. J. Bioenerg. Biomembr. 1996, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Gouw, J.W.; Naus, C.C.; Foster, L.J. Connexin multi-site phosphorylation: Mass spectrometry-based proteomics fills the gap. Biochim. Biophys. Acta 2013, 1828, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorini, C.; Gilleron, J.; Carette, D.; Valette, A.; Tilloy, A.; Chevalier, S.; Segretain, D.; Pointis, G. Accelerated internalization of junctional membrane proteins (connexin 43, N-cadherin and ZO-1) within endocytic vacuoles: An early event of DDT carcinogenicity. Biochim. Biophys. Acta 2008, 1778, 56–67. [Google Scholar] [Green Version]
- Laird, D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta 2005, 1711, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Solan, J.L.; Lampe, P.D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta 2005, 1711, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, F.H.; Zarghi, A.; Kobarfard, F.; Zendehdel, R.; Nakhjavani, M.; Arfaiee, S.; Zebardast, T.; Mohebi, S.; Anjidani, N.; Ashtarinezhad, A.; et al. Remarks in Successful Cellular Investigations for Fighting Breast Cancer Using Novel Synthetic Compounds. In Breast Cancer—Focusing Tumor Microenvironment, Stem Cells and Metastasis; Gunduz, M., Gunduz, E., Eds.; InTech: Rijeka, Croatia, 2011; pp. 85–102. [Google Scholar] [CrossRef]
- Comşa, S.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Banerjee, D. Connexin’s Connection in Breast Cancer Growth and Progression. Int. J. Cell Biol. 2016, 2016, 9025905. [Google Scholar] [CrossRef]
- Jiang, G.; Dong, S.; Yu, M.; Han, X.; Zheng, C.; Zhu, X.; Tong, X. Influence of gap junction intercellular communication composed of connexin 43 on the antineoplastic effect of adriamycin in breast cancer cells. Oncol. Lett. 2017, 13, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.-L.; Chen, S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahner-Roedler, D.L.; Bauer, B.A.; Loehrer, L.L.; Cha, S.S.; Hoskin, T.L.; Olson, J.E. The effect of grape seed extract on estrogen levels of postmenopausal women: A pilot study. J. Diet. Suppl. 2014, 11, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Krohn, R.L.; Liu, W.; Joshi, S.S.; Kuszynski, C.A.; McGinn, T.R.; Bagchi, M.; Preuss, H.G.; Stohs, S.J.; Bagchi, D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol. Cell. Biochem. 1999, 196, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis 2006, 27, 2018–2027. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Zhang, J.; Yang, Q.; Teng, F. Grape seed extract inhibit proliferation of breast cancer cell MCF-7 and decrease the gene expression of survivin. Zhongguo Zhong Yao Za Zhi 2009, 34, 433–437. [Google Scholar]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer Oxidative stress—its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 1. [Google Scholar] [CrossRef]
- Lee, Y.J.; Galoforo, S.S.; Berns, C.M.; Chen, J.C.; Davis, B.H.; Sim, J.E.; Corry, P.M.; Spitz, D.R. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J. Biol. Chem. 1998, 273, 5294–5299. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, H.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Raza, A.; Ghoshal, A.; Chockalingam, S.; Ghosh, S.S. Connexin-43 enhances tumor suppressing activity of artesunate via gap junction-dependent as well as independent pathways in human breast cancer cells. Sci. Rep. 2017, 7, 7580. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Fistouris, P.; Batist, G.; Alpert, L.; Huynh, H.T.; Carystinos, G.D.; Alaoui-Jamali, M.A. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 1999, 15, 4104–4110. [Google Scholar]
- Singal, R.; Tu, Z.J.; Vanwert, J.M.; Ginder, G.D.; Kiang, D.T. Modulation of the connexin26 tumor suppressor gene expression through methylation in human mammary epithelial cell lines. Anticancer Res. 2000, 20, 59–64. [Google Scholar] [PubMed]
- Kameritsch, P.; Khandoga, N.; Pohl, U.; Pogoda, K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 2013, 4, e584. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santelli, G.M.; Ionta, M. Gap Junction Intercellular Communication and Connexin Expression Profile in Normal Liver Cells and Hepatocarcinoma. In Hepatocellular Carcinoma—Basic Research; Lau, W.Y.J., Ed.; InTech: Shangai, China, 2012; p. 402. [Google Scholar]
- Davidov-Pardo, G.; Moreno, M.; Arozarena, I.; Marín-Arroyo, M.R.; Bleibaum, R.N.; Bruhn, C.M. Sensory and consumer perception of the addition of grape seed extracts in cookies. J. Food Sci. 2012, 77, S430–S438. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.; Leone, A.; Longo, C.; Lombardi, D.A.; Raimo, F.; Zacheo, G. Antioxidant compounds and antioxidant activity in ‘early potatoes’. J. Agric. Food Chem. 2008, 56, 4154–4163. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Dulski, K.M.; Trosko, J.E. Loss of intercellular junctional communication correlates with metastatic potential in mammary adenocarcinoma cells. Proc. Natl. Acad. Sci. USA 1988, 85, 473–476. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshikawa, N.; Hiroki, I.; Sato, K.; Ohtsuki, K.; Chang, C.-C.; Upham, B.L.; Trosko, J.E. Beta-sitosterol from psyllium seed husk (Plantago ovata Forsk) restores gap junctional intercellular communication in Ha-ras transfected rat liver cells. Nutr. Cancer 2005, 51, 218–225. [Google Scholar] [CrossRef]





| GSE Samples | Total Phenols | Antioxidant Activity | |
|---|---|---|---|
| g of GAE/100 g GS ± DS | mol TE/GAE ± DS | mol TE/100 g GS ± DS | |
| GSE methanol:water (80:20) | 1.64 ± 0.04 | 7.07 ± 0.54 | 11.6 ± 0.5 |
| GSE ethanol:water (80:20) | 0.55 ± 0.02 | 5.87 ± 0.70 | 3.3 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. https://doi.org/10.3390/ijms20133244
Leone A, Longo C, Gerardi C, Trosko JE. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. International Journal of Molecular Sciences. 2019; 20(13):3244. https://doi.org/10.3390/ijms20133244
Chicago/Turabian StyleLeone, Antonella, Cristiano Longo, Carmela Gerardi, and James E. Trosko. 2019. "Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention" International Journal of Molecular Sciences 20, no. 13: 3244. https://doi.org/10.3390/ijms20133244





