Nutraceutical Supplements in the Management and Prevention of Osteoarthritis
Abstract
:1. Introduction
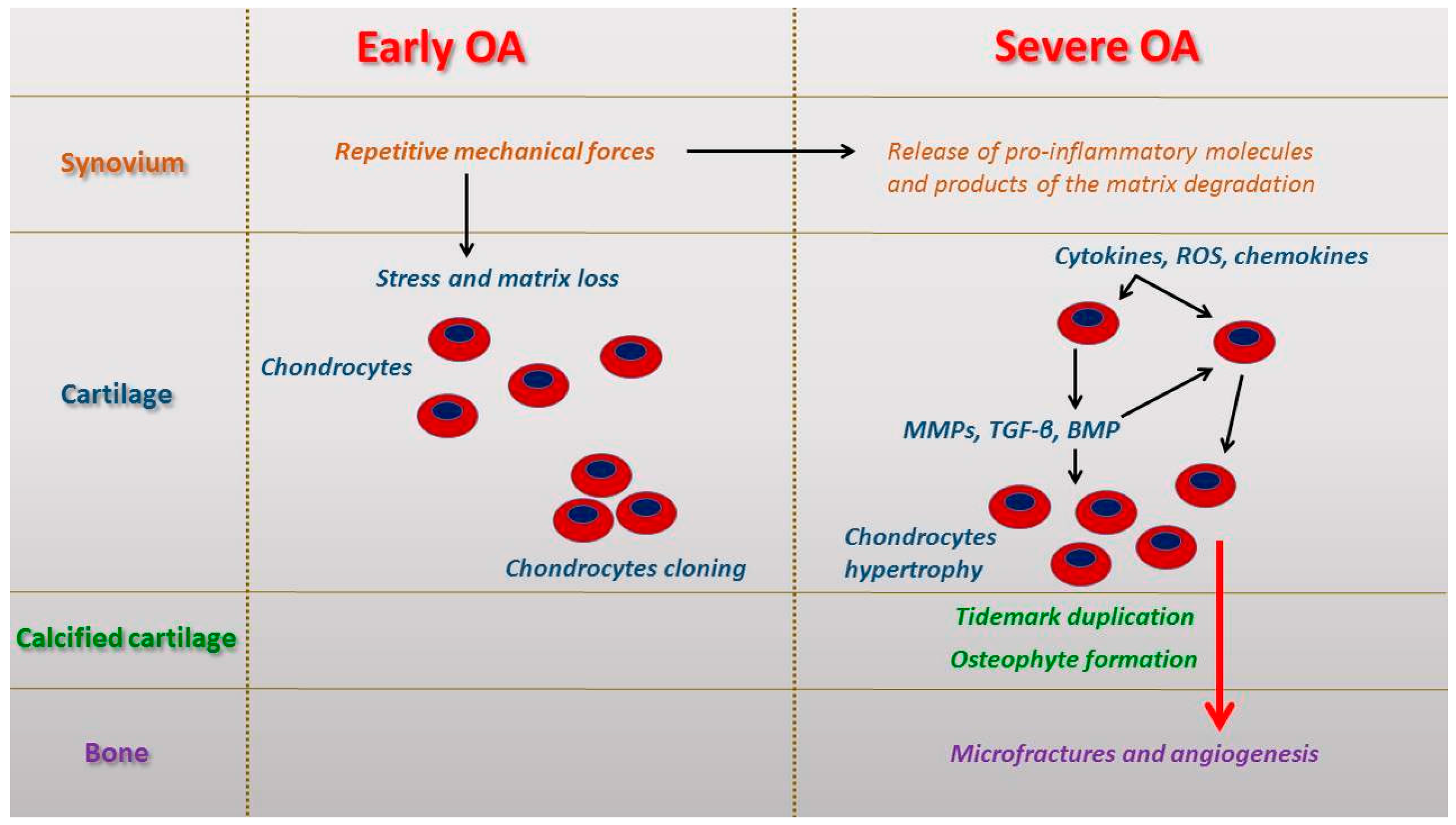
2. Micro and Macroscopic Features in Early and Severe Osteoarthritis (OA)
3. Nutraceuticals
3.1. Fish Oil
3.2. GAGs (Glucosamine Sulfate, Chondroitin Sulfate, and Hyaluronic Acid)
3.3. Olive Oil
3.4. Methionine
3.5. Undenatured Type II Collagen
3.6. Botanical Extracts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| MRI | Magnetic resonance imaging |
| ECM | Extracellular matrix |
| MMP | Matrix metalloproteinases |
| ROS | Reactive oxygen species |
| TGF-β | Transforming growth factor β |
| GAGs | Glycosaminoglycan glycans |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| HA | Hyaluronic acid |
| SAMe | S-adenosylmethionine |
| MUFA | Monounsaturated fatty acid |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| UC-II | Undenatured type II collagen |
| ASU | Avocado/soy unsaponifiable |
| PUFA | Polyunsaturated fatty acid |
| RNS | Reactive nitrogen species |
References
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The effects of exercise on physical limitations and fatigue in rheumatic diseases. World J. Orthop. 2015, 6, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Trovato, F.M.; di Rosa, M.; Malaguarnera, L.; Puzzo, L.; Leonardi, R.; Castrogiovanni, P.; Musumeci, G. Co-expression and co-localization of cartilage glycoproteins CHI3L1 and lubricin in osteoarthritic cartilage: Morphological, immunohistochemical and gene expression profiles. Int. J. Mol. Sci. 2016, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Imbesi, R.; Castrogiovanni, P. Post-operative rehabilitation and nutrition in osteoarthritis. F1000Research 2016, 3, 116. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Musumeci, G. Which is the best physical treatment for osteoarthritis? J. Funct. Morphol. Kinesiol. 2016, 1, 54–68. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Leonardi, R.; Al-Qahtani, M.; Mobasheri, A.; Musumeci, G. Altered joint tribology in osteoarthritis: Reduced lubricin synthesis due to the inflammatory process—New horizons for therapeutic approaches. Ann. Phys. Rehabil. Med. 2016, 59, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynard, L.N.; Loughlin, J. Genetics and epigenetics of osteoarthritis. Maturitas 2012, 71, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The effect of mechanical loading on articular cartilage. J. Funct. Morphol. Kinesiol. 2016, 1, 154–161. [Google Scholar] [CrossRef]
- Aiello, F.C.; Trovato, F.M.; Szychlinska, M.A.; Imbesi, R.; Castrogiovanni, P.; Borzì, F.; Loreto, C.; Musumeci, G. Molecular links between diabetes and osteoarthritis: The role of physical activity. Curr. Diabetes Rev. 2015. [Google Scholar] [PubMed]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis?—A narrative review from the lessons taken with five products. Osteoarthr. Cartil. 2011, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; Doherty, M. Guidelines for management of osteoarthritis published by the American College of Rheumatology and the European League against Rheumatism: Why are they so different? Rheum. Dis. Clin. N. Am. 2003, 29, 717–731. [Google Scholar] [CrossRef]
- Lorenz, H.; Richter, W. Osteoarthritis: Cellular and molecular changes in degenerating cartilage. Prog. Histochem. Cytochem. 2006, 40, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Imbesi, R.; Trovato, F.M.; di Giunta, A.; Lombardo, C.; Castorina, S.; Castrogiovanni, P. Advantages of exercise in rehabilitation, treatment and prevention of altered morphological features in knee osteoarthritis. A narrative review. Histol. Histopathol. 2014, 29, 707–719. [Google Scholar] [PubMed]
- Miosge, N.; Hartmann, M.; Maelicke, C.; Herken, R. Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem. Cell Biol. 2004, 122, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Shibakawa, A.; Aoki, H.; Masuko-Hongo, K.; Kato, T.; Tanaka, M.; Nishioka, K.; Nakamura, H. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthr. Cartil. 2003, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Miura, T.; Iwata, H.; Kikuchi, T.; Yamaguchi, T.; Yoshimi, T.; Itoh, H. Effect of high-molecular weight sodium hyaluronate on immobilized rabbit knee. Clin. Orthop. 1994, 299, 282–292. [Google Scholar] [CrossRef]
- Le Graverand, M.P.; Eggerer, J.; Vignon, E.; Otterness, I.G.; Barclay, L.; Hart, D.A. Assessment of specific mRNA levels in cartilage regions in a lapine model of osteoarthritis. J. Orthop. Res. 2002, 20, 535–544. [Google Scholar] [CrossRef]
- Kraus, V.B.; Huebner, J.L.; Stabler, T.; Flahiff, C.M.; Setton, L.A.; Fink, C.; Vilim, V.; Clark, A.G. Ascorbic acid increase the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004, 50, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Huebner, J.L.; DeGroot, J.; Bendele, A. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr. Cartil. 2010, 18, S35–S52. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D′Lima, D.D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Di Giunta, A.; Loreto, C.; Castorina, S. Microscopic and macroscopic anatomical features in healthy and osteoarthritic knee cartilage. OA Anat. 2013, 1, 30. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Castorina, S.; Imbesi, R.; Leonardi, R.; Castrogiovanni, P. Current concepts in the treatment of cartilage damage. A review. Ital. J. Anat. Embryol. 2013, 118, 189–203. [Google Scholar] [PubMed]
- Musumeci, G.; Loreto, C.; Castorina, S.; Imbesi, R.; Leonardi, R.; Castrogiovanni, P. New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review. Ital. J. Anat. Embryol. 2013, 118, 204–210. [Google Scholar] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.Z.; Patel, R.M.; Dawson, B.C.; Jiang, M.M.; Lee, B.H. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthr. Cartil. 2013, 21, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Aigner, T. Terminology of osteoarthritis cartilage and bone histopathology—A proposal for a consensus. Osteoarthr. Cartil. 2010, 18, S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef] [PubMed]
- Boe, C.; Vangsness, C.T. Fish oil and osteoarthritis: Current evidence. Am. J. Orthop. 2015, 44, 302–305. [Google Scholar] [PubMed]
- Hill, C.L.; March, L.M.; Aitken, D.; Lester, S.E.; Battersby, R.; Hynes, K.; Fedorova, T.; Proudman, S.M.; James, M.; Cleland, L.G.; et al. Fish oil in knee osteoarthritis: A randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis. 2016, 75, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.G.; Zeng, C.; Wei, J.; Wang, Y.L.; Lei, G.H. Paying attention to the safety and efficacy of fish oil in treatment of knee osteoarthritis. Ann. Rheum. Dis. 2016, 75, e13. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.G.; Samarasinghe, R.K. Review article: Glucosamine. J. Orthop. Surg. 2009, 17, 72–76. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res. Ther. 2012, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.A.; Stucki, G.; Frey, D.; de Vathaire, F.; Vignon, E.; Bruehlmann, P.; Uebelhart, D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: A randomized, controlled trial. Arthritis Rheum. 2005, 52, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kahan, A.; Uebelhart, D.; de Vathaire, F.; Delmas, P.D.; Reginster, J.Y. Long-term effects of chondroitin sulfate on knee osteoarthritis: The study on osteoarthritis progression prevention, a two-year, randomized, double- blind, placebo-controlled trial. Arthritis Rheum. 2009, 60, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.G.; Plaas, A.H.; Sandy, J.D.; Hua, C.; Kim-Rolands, S.; Barnhill, J.G.; Harris, C.L.; Clegg, D.O. The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthr. Cartil. 2010, 19, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.L. Nutritional interventions to prevent and treat osteoarthritis—Part II: Focus on micronutrients and supportive nutraceuticals. PM R 2012, 4, S155–S168. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., 3rd; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Petzold, E.; Busch, R.; Petzold, H.P.; Graubaum, H.J. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv. Ther. 2009, 26, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, E.; de Andres, M.C.; Ferández-Sueiro, J.L.; Galdo, F.; Blanco, F.J. The biological action of hyaluronan on human osteoarthritic articular chondrocytes: The importance of molecular weight. Clin. Exp. Rheumatol. 2004, 22, 307–312. [Google Scholar] [PubMed]
- Adams, M.E.; Lussier, A.J.; Peyron, J.G. A risk-benefit assessment of injections of hyaluronan and its derivatives in the treatment of knee osteoarthritis. Drug Saf. 2000, 23, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Polyak, A.; Matheetal, D. Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, S.; Jastan, M.; Nakhostin-Roohi, B.; Mohammadi, S.; Baghaei, Z. A pilot double-blinded, randomized, clinical trial of topical virgin olive oil versus piroxicam gel in osteoarthritis of the knee. J. Clin. Rheumatol. 2012, 18, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Packer, L. S-Adenosylmethionine: Molecular, biological, and clinical aspects: An introduction. Am. J. Clin. Nutr. 2002, 76, 1148S–1150S. [Google Scholar] [PubMed]
- Hosea Blewett, H.J. Exploring the mechanisms behind S-adenosylmethionine (SAMe) in the treatment of osteoarthritis. Crit. Rev. Food Sci. Nutr. 2008, 48, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; van Dijk, N.; Della Villa, S. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef] [PubMed]
- De Silva, V.; El-Metwally, A.; Ernst, E.; Lewith, G.; Macfarlane, G.J.; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: A systematic review. Rheumatology 2011, 50, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Najm, W.I.; Reinsch, S.; Hoehler, F.; Tobis, J.S.; Harvey, P.W. S-Adenosyl methionine versus celecoxib for the treatment of osteoarthritis symptoms: A double-blind cross-over trial. BMC Musculoskelet. Disord. 2004, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative therapeutic efficacy and safety of type-II collagen (UC-II®), glucosamine and chondroitin in arthritic dogs: Pain evaluation by ground force plate. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, X.Y.; Wang, H.K.; Jia, J.F.; Zheng, Z.H.; Ding, J.; Fan, C.M. Oral administration of type-II collagen peptide 250–270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin. Immunol. 2007, 122, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, M.J.; Cho, M.L.; Kwok, S.K.; Ju, J.H.; Ko, H.J.; Park, S.H.; Kim, H.Y. Type II collagen oral tolerance: Mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lippiello, L.; Nardo, J.V.; Harlan, R.; Chiou, T. Metabolic effects of avocado/soy unsaponifiables on articular chondrocytes. Evid. Based Complement. Altern. Med. 2008, 5, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Sanchez, C.; Deberg, M.A.; Piccardi, N.; Guillou, G.B.; Msika, P.; Reginster, J.Y. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J. Rheumatol. 2003, 30, 1825–1834. [Google Scholar] [PubMed]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclooxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011, 6, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Huang, Y.; Kong, A.N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: A randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; El-Farahaty, T.; Shabana, A.A.; Hawas, S.A.; El-Batoty, M.F. Boswellia-curcumin preparation for treating knee osteoarthritis. Altern. Complement. Ther. 2002, 8, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; de La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 β-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Song, M.; Katsumata, S.; Uehara, M.; Suzuki, K.; Ohigashi, H. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: Possible involvement of receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 2007, 30, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: Progress and promise. Arthritis Res. Ther. 2010, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Bitto, A.; Aguennouz, A.M.; Mazzeo, A.; Migliorato, A.; Polito, F.; Irrera, N.; Altavilla, D.; Vita, G.L.; Russo, M.; et al. Flavocoxid inhibits NFkappaB, MAPKs and COX/5-LOX pathways and improves muscle function and morphology in mdx mice: A comparison study with methylprednisolone. Exp. Neurol. 2009, 220, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Tilak, J.C.; Devasagayam, T.P. Free radical reactions of a naturally occurring flavone baicalein and possible mechanisms towards its membrane protective properties. Indian J. Biochem. Biophys. 2011, 48, 275–282. [Google Scholar] [PubMed]
- Tseng-Crank, J.; Sung, S.; Jia, Q.; Zhao, Y.; Burnett, B.; Park, D.R.; Woo, S.S. A medicinal plant extract of Scutellaria baicalensis and Acacia catechu reduced LPS-stimulated gene expression in immune cells: A comprehensive genomic study using QPCR, ELISA, and microarray. J. Diet. Suppl. 2010, 7, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; di Renzo, A.; Stuard, S.; Dugall, M.; et al. Treatment of osteoarthritis with Pycnogenol. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother. Res. 2008, 22, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtassák, J.; Duraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Hansen, C.; Shakibaei, M. Effect of a Harpagophytum procumbens DC extract on matrix metalloproteinases in human chondrocytes in vitro. Arzneimittel-Forschung 2004, 54, 213–220. [Google Scholar] [PubMed]
- Fiebich, B.L.; Muñoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular targets of the anti-inflammatory Harpagophytum procumbens (Devil′s claw): Inhibition of TNFα and COX-2 gene expression by preventing activation of AP-1. Phytother. Res. 2011, 10, 36–45. [Google Scholar]
- Wegener, T.; Lupke, N.P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil′s claw (Harpagophytum procumbens DC). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Chrubasik, S.; Manheimer, E. Harpagophytum procumbens for osteoarthritis and low back pain: A systematic review. BMC Complement. Altern. Med. 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Brien, S.; Lewith, G.; Walker, A.; Hicks, S.M.; Middleton, D. Bromelain as a treatment for osteoarthritis: A review of clinical studies. Evid. Based Complement. Altern. Med. 2004, 1, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tilwe, G.H.; Beria, S.; Turakhia, N.H.; Daftary, G.V.; Schiess, W. Efficacy and tolerability of oral enzyme therapy as compared to diclofenac in active osteoarthrosis of knee joint: An open randomized controlled clinical trial. J. Assoc. Physicians India 2001, 49, 617–621. [Google Scholar] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell. Dev. Biol. Anim. 2004, 40, 95–101. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Bliddal, H.; Rosetzky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wigler, I.; Grotto, I.; Caspi, D.; Yaron, M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthr. Cartil. 2003, 11, 783–789. [Google Scholar] [CrossRef]
| Analyzed Articles | Nutraceuticals | Potential Role | ||||
|---|---|---|---|---|---|---|
| Antiinflammatory | Redox Balance/Antioxidant | Anabolic | Anticatabolic | Structural Substrates | ||
| Boe and Vangsness; Am. J. Orthop. 2015, 44, 302–305. | Fish Oil EPA + DHA (2–4 g/day) | +++ | − | − | +++ | ++ |
| Hill et al.; Ann. Rheum. Dis. 2016, 75, 23–29. | ||||||
| Gao et al.; Ann. Rheum. Dis. 2016, 75, e13. | ||||||
| Lopez; PM R 2012, 4, S155–S168. | ||||||
| Kirkham and Samarasinghe; J. Orthop. Surg. 2009, 17, 72–76. | GAGs glucosamine sulfate (20 mg/kg body weight/day); chondroitin sulfate (1200 mg/d); hyaluronic acid (50–100 mg/d) | + | + | ++ | +++ | +++ |
| Michel et al.; Arthritis Rheum. 2005, 52, 779–786. | ||||||
| Kahan et al.; Arthritis Rheum. 2009, 60, 524–533. | ||||||
| Jackson et al.; Osteoarthr. Cartil. 2010, 19, 297–302. | ||||||
| Lopez; PM R 2012, 4, S155–S168. | ||||||
| Gruenwald et al.; Adv. Ther. 2009, 26, 858–871. | ||||||
| Maneiro et al.; Clin. Exp. Rheumatol. 2004, 22, 307–312. | ||||||
| Adams et al.; Drug Saf. 2000, 23, 115–130. | ||||||
| Balogh et al.; J. Agric. Food Chem. 2008, 56, 10582–10593. | ||||||
| Musumeci et al.; J. Nutr. Biochem. 2013, 24, 2064–2075. | Olive oil phenolic compounds, MUFAs (500–2000 mg/d) | +++ | ++ | + | − | + |
| Lopez; PM R 2012, 4, S155–S168. | ||||||
| Bohlooli et al.; J. Clin. Rheumatol. 2012, 18, 99–101. | ||||||
| Lieber and Packer; Am. J. Clin. Nutr. 2002, 76, 1148S–1150S. | Methionine (800–1200 mg/d) | − | +++ | + | + | ++ |
| Hosea Blewett; Crit. Rev. Food Sci. Nutr. 2008, 48, 458–463. | ||||||
| Kon et al.; Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. | ||||||
| Lopez; PM R 2012, 4, S155–S168. | ||||||
| Lugo et al.; Nutr. J. 2016, 15, 14. | Undenatured type II collagen (40 mg/d) | + | − | + | +++ | +++ |
| Gupta et al.; J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. | ||||||
| Zhu et al.; Clin. Immunol. 2007, 122, 7584. | ||||||
| Park et al.; Mod. Rheumatol. 2009, 19, 581–589. | ||||||
| Lopez; PM R 2012, 4, S155–S168. | ||||||
| Lippiello et al.; Evid. Based Complement Alternat. Med. 2008, 5, 191–197. | Botanical extracts ASU (300–600 mg/d); polyphenols (300–2000 mg/d) | ++ | ++ | + | ++ | ++ |
| Henrotin et al.; J. Rheumatol. 2003, 30, 1825–1834. | ||||||
| Hong et al.; Carcinogenesis 2004, 25, 1671–1679. | ||||||
| Shakibaei et al.; Biochem. Pharmacol. 2007, 73, 1434–1445. | ||||||
| Shakibaei et al.; Genes Nutr. 2011, 6, 171–179. | ||||||
| Saw et al.; Biochem. Pharmacol. 2010, 79, 421–430. | ||||||
| -Siddiqui; Indian J. Pharm. Sci. 2011, 73, 255–261. | ||||||
| Abdel-Tawab et al.; Clin. Pharmacokinet. 2011, 50, 349–369. | ||||||
| Kimmatkar et al.; Phytomedicine 2003, 10, 3–7. | ||||||
| Badria et al.; Altern. Complement. Ther. 2002, 8, 341–348. | ||||||
| Visioli et al.; Crit. Rev. Food Sci. Nutr. 2011, 51, 524546. | ||||||
| Fraga and Oteiza; Free Radic. Biol. Med. 2011, 51, 813–823. | ||||||
| González et al.; Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. | ||||||
| Russo et al.; Biochem. Pharmacol. 2012, 83, 6–15. | ||||||
| Ahmed et al.; J. Pharmacol. Exp. Ther. 2004, 308, 767–773. | ||||||
| Murakami et al.; Biofactors 2007, 30, 179–192. | ||||||
| Ahmed; Arthritis Res. Ther. 2010, 12, 208. | ||||||
| Messina et al.; Exp. Neurol. 2009, 220, 349–358. | ||||||
| Adhikari et al.; Indian J. Biochem. Biophys. 2011, 48, 275–282. | ||||||
| Tseng-Crank et al.; J. Diet. Suppl. 2010, 7, 253–272. | ||||||
| Belcaro et al.; Phytother. Res. 2008, 22, 518–523. | ||||||
| Cisár et al.; Phytother. Res. 2008, 22, 1087–1092. | ||||||
| Schulze-Tanzil et al.; Arzneimittelforschung 2004, 54, 213–220. | ||||||
| Fiebich et al.; Phytother. Res. 2011, 10, 36–45. | ||||||
| Wegener and Lupke; Phytother. Res. 2003, 17, 1165–1172. | ||||||
| Gagnier et al.; BMC Complement. Altern. Med. 2004, 4, 13–23. | ||||||
| Brien et al.; Evid. Based Complement. Altern. Med. 2004, 1, 251–257. | ||||||
| Tilwe et al.; J. Assoc. Physicians. India 2001, 49, 617–621. | ||||||
| Semwal et al.; Phytochemistry 2015, 117, 554–568. | ||||||
| Frondoza et al.; In Vitro Cell Dev. Biol. Anim. 2004, 40, 95–101. | ||||||
| Altman and Marcussen; Arthritis. Rheum. 2001, 44, 2531–2538. | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. https://doi.org/10.3390/ijms17122042
Castrogiovanni P, Trovato FM, Loreto C, Nsir H, Szychlinska MA, Musumeci G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. International Journal of Molecular Sciences. 2016; 17(12):2042. https://doi.org/10.3390/ijms17122042
Chicago/Turabian StyleCastrogiovanni, Paola, Francesca Maria Trovato, Carla Loreto, Houda Nsir, Marta Anna Szychlinska, and Giuseppe Musumeci. 2016. "Nutraceutical Supplements in the Management and Prevention of Osteoarthritis" International Journal of Molecular Sciences 17, no. 12: 2042. https://doi.org/10.3390/ijms17122042








