Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review
Abstract
:1. Introduction
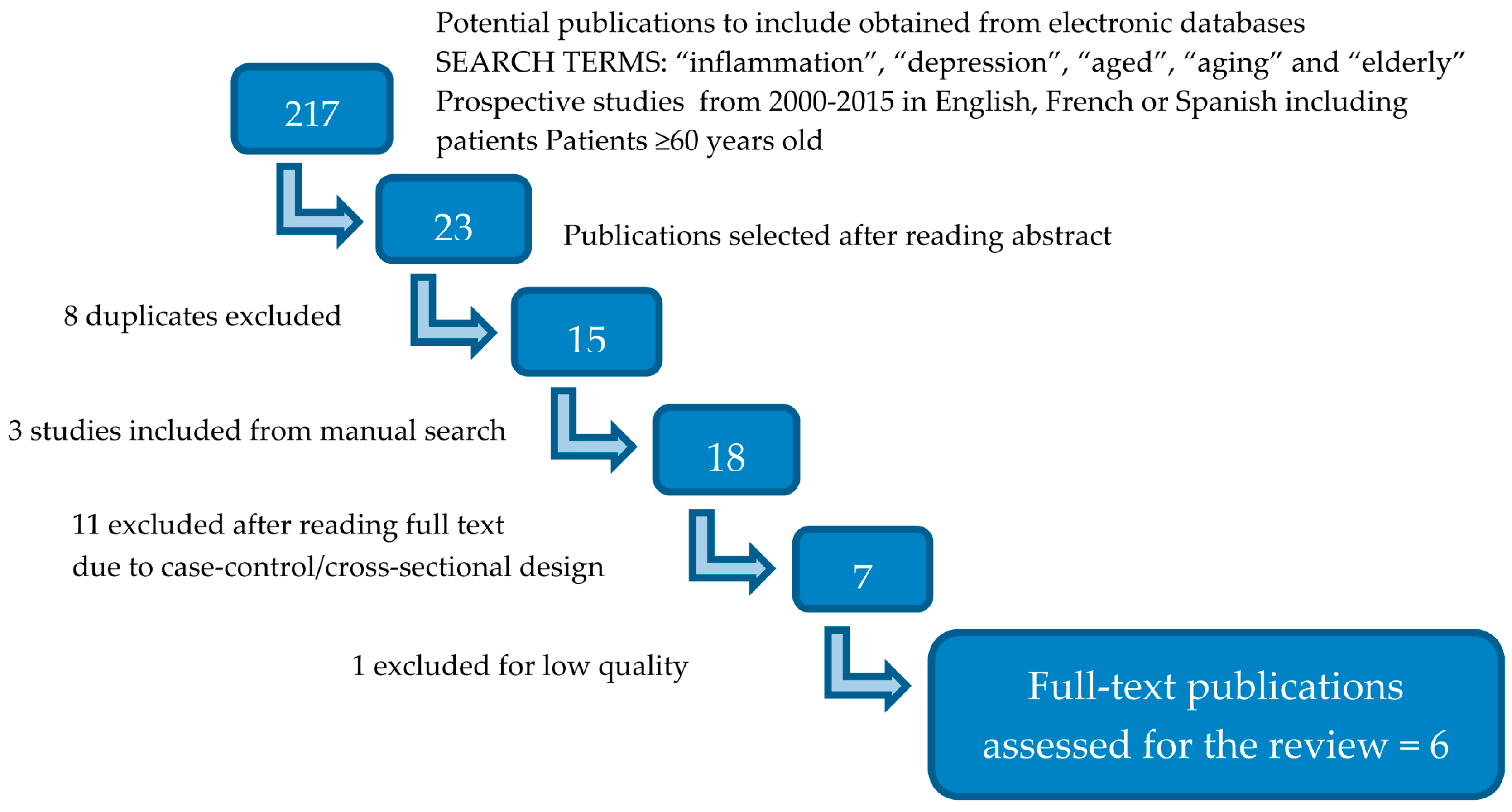
2. Literature Search
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laks, J.; Engelhardt, E. Peculiarities of geriatric psychiatry: A focus on aging and depression. CNS Neurosci. Ther. 2010, 16, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Naarding, P.; Schoevers, R.A.; Janzing, J.G.E.; Jonker, C.; Koudstaal, P.J.; Beekman, A.T.F. A study on symptom profiles of late-life depression: The influence of vascular, degenerative and inflammatory risk-indicators. J. Affect. Disord. 2005, 88, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Emery, V.O.; Oxman, T.E. Update on the dementia spectrum of depression. Am. J. Psychiatry 1992, 149, 305–317. [Google Scholar] [PubMed]
- Taylor, W.D.; Zhao, Z.; Ashley-Koch, A.; Payne, M.E.; Steffens, D.C.; Krishnan, R.R.; Hauser, E.; MacFall, J.R. Fiber tract-specific white matter lesion severity Findings in late-life depression and by AGTR1 A1166C genotype. Hum. Brain Mapp. 2013, 34, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Yamawaki, S.; Touhouda, Y. Incidence of silent cerebral infarction in patients with major depression. Stroke J. Cereb. Circ. 1993, 24, 1631–1634. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Morimoto, S.S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatry 2011, 26, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Dilger, R.N.; Johnson, R.W. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008, 84, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, S.; Marino, V.; Biondi, M.; Grimaldi, F.; Ippoliti, F.; Maes, M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J. Affect. Disord. 2002, 72, 237–241. [Google Scholar] [CrossRef]
- Wichers, M.; Maes, M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int. J. Neuropsychopharmacol. 2002, 5, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Gumnick, J.F.; Musselman, D.L.; Lawson, D.H.; Reemsnyder, A.; Nemeroff, C.B.; Miller, A.H. Neurobehavioral effects of interferon-α in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002, 26, 643–652. [Google Scholar] [CrossRef]
- Kenis, G.; Maes, M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 2002, 5, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.D.; Aizenstein, H.J.; Alexopoulos, G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry 2013, 18, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Smagula, S.F.; Lotrich, F.E.; Aizenstein, H.J.; Diniz, B.S.; Krystek, J.; Wu, G.F.; Mulsant, B.H.; Butters, M.A.; Reynolds, C.F.; Lenze, E.J. Immunological biomarkers associated with brain structure and executive function in late-life depression: Exploratory pilot study. Int. J. Geriatr. Psychiatry 2016. [Google Scholar] [CrossRef] [PubMed]
- Disabato, B.M.; Sheline, Y.I. Biological basis of late life depression. Curr. Psychiatry Rep. 2012, 14, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Associations of very high C-reactive protein concentration with psychosocial and cardiovascular risk factors in an ageing population. Atherosclerosis 2009, 206, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Teixeira, A.L.; Talib, L.L.; Mendonça, V.A.; Gattaz, W.F.; Forlenza, O.V. Increased soluble TNF receptor 2 in antidepressant-free patients with late-life depression. J. Psychiatr. Res. 2010, 44, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Tiemeier, H.; Hofman, A.; van Tuijl, H.R.; Kiliaan, A.J.; Meijer, J.; Breteler, M.M.B. Inflammatory proteins and depression in the elderly. Epidemiology 2003, 14, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Davis, S.; Morris, C.; Jackson, E.; Harrison, R.; O’Brien, J.T. Increase in interleukin-1β in late-life depression. Am. J. Psychiatry 2005, 162, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Smagula, S.F.; Ancoli-Israel, S.; Barrett-Connor, E.; Lane, N.E.; Redline, S.; Stone, K.L.; Cauley, J.A. Inflammation, sleep disturbances, and depressed mood among community-dwelling older men. J. Psychosom. Res. 2014, 76, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Dentino, A.N.; Pieper, C.F.; Rao, M.K.; Currie, M.S.; Harris, T.; Blazer, D.G.; Cohen, H.J. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J. Am. Geriatr. Soc. 1999, 47, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Kritchevsky, S.B.; Yaffe, K.; Newman, A.B.; Simonsick, E.M.; Rubin, S.; Ferrucci, L.; Harris, T.; Pahor, M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol. Psychiatry 2003, 54, 566–572. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; Eisel, U.L.M.; Comijs, H.C.; Groenewold, N.A.; de Deyn, P.P.; Bosker, F.J.; Luiten, P.G.; Den Boer, J.A.; Voshaar, R.O. Neutrophil gelatinase-associated lipocalin: A novel inflammatory marker associated with late-life depression. J. Psychosom. Res. 2013, 75, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Tools Web 2.0 Platform. Available online: http://www.lecturacritica.com/en/ (accessed on 18 May 2015).
- Baune, B.T.; Smith, E.; Reppermund, S.; Air, T.; Samaras, K.; Lux, O.; Brodaty, H.; Sachdev, P.; Trollor, J.N. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney Memory and Aging Study. Psychoneuroendocrinology 2012, 37, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, M.A.; Beekman, A.T.F.; Deeg, D.J.H.; Penninx, B.W.J.H.; Dik, M.G.; Hack, C.E.; Hoogendijk, W.J. Inflammatory markers in late-life depression: Results from a population-based study. J. Affect. Disord. 2008, 106, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Forti, P.; Rietti, E.; Pisacane, N.; Olivelli, V.; Mariani, E.; Chiappelli, M.; Licastro, F.; Ravaglia, G. Blood inflammatory proteins and risk of incident depression in the elderly. Dement. Geriatr. Cogn. Disord. 2010, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Corsi, A.M.; Penninx, B.W.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: The InCHIANTI study. Biol. Psychiatry 2009, 65, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Van den Biggelaar, A.H.J.; Gussekloo, J.; de Craen, A.J.M.; Frölich, M.; Stek, M.L.; van der Mast, R.C.; Westendorp, R.G. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 2007, 42, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.; Rand, K.L.; Muldoon, M.F.; Kamarck, T.W. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 2009, 23, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. J. Appl. Psychol. Meas. 1997, 1, 385–401. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), Text Revision, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1987. [Google Scholar]
- Bortolato, B.; Carvalho, A.F.; Soczynska, J.K.; Perini, G.I.; McIntyre, R.S. The involvement of TNF-α in cognitive dysfunction associated with major depressive disorder: An opportunity for domain specific treatments. Curr. Neuropharmacol. 2015, 13, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Neznanov, N.G.; Kozlova, S.N.; Mazo, G.E.; Shlyakhto, N.G.; Smirnov, B.I. Comorbidity of depressive disorders and coronary heart disease: General aspects of pathogenesis. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova Minist. Zdr. Meditsinskoĭ Promyshlennosti Ross. Fed. Vserossiĭskoe Obshchestvo Nevrol. Vserossiĭskoe Obshchestvo Psikhiatrov. 2015, 115, 20–26. [Google Scholar] [CrossRef]
- Kallaur, A.P.; Lopes, J.; Oliveira, S.R.; Reiche, E.M.; de Almeida, E.R.; Morimoto, H.K.; de Pereira, W.L.; Alfieri, D.F.; Borelli, S.D.; Kaimen-Maciel, D.R.; et al. Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple sclerosis: Increased peripheral inflammation but less acute neuroinflammation. Mol. Neurobiol. 2016, 53, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Appenzeller, S. The importance of cytokines and autoantibodies in depression. Autoimmun. Rev. 2015, 14, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stewart, R.; Kim, S.-W.; Kim, S.Y.; Bae, K.Y.; Kang, H.J.; Jang, J.E.; Shin, I.S.; Yoon, J.S. Physical health and incident late-life depression: Modification by cytokine genes. Neurobiol. Aging 2013, 34, 356.e1–356.e9. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.C.; Claiborne, J.; Sidoryk-Wegrzynowicz, M.; Reddy, R.; Aschner, M.; Lewis, D.A.; Mirnics, K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 2011, 16, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.J.; Baune, B.T. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014, 42, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Suarez, E.C.; Krishnan, R.R.; Lewis, J.G. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom. Med. 2003, 65, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Skoog, I.; Börjesson-Hanson, A.; Blennow, K.; Zetterberg, H.; Ostling, S.; Kern, J.; Gudmundsson, P.; Marlow, T.; Rosengren, L.; et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav. Immun. 2014, 41, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Endrighi, R.; Steptoe, A.; Hamer, M. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br. J. Psychiatry 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastorakos, G.; Chrousos, G.P.; Weber, J.S. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin. Endocrinol. Metab. 1993, 77, 1690–1694. [Google Scholar] [PubMed]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ganança, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2015, 63, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.-M.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.M.; Niskanen, L.; Miettola, J.; Tolmunen, T.; Viinamäki, H.; Mäntyselkä, P. Serum anti-inflammatory markers in general population subjects with elevated depressive symptoms. Neurosci. Lett. 2010, 484, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Song, C.; Yirmiya, R. Targeting IL-1 in depression. Expert Opin. Ther. Targets 2012, 16, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, Y.; Koponen, H.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Kumpusalo, E.; Vanhala, M. Depressive symptomatology is associated with decreased interleukin-1 beta and increased interleukin-1 receptor antagonist levels in males. Psychiatry Res. 2009, 167, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Patterson, C.C.; Appleton, K.M.; Blankenberg, S.; Woodside, J.V.; Donnelly, M.; Linden, G.; Zeller, T.; Esquirol, Y.; Kee, F. Predictive Value of Depressive Symptoms for All-Cause Mortality: Findings from the PRIME Belfast Study Examining the Role of Inflammation and Cardiovascular Risk Markers. Psychosom. Med. 2016, 78, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, C.; Numakawa, T.; Odaka, H.; Ooshima, Y.; Kiyama, Y.; Manabe, T.; Kunugi, H.; Iwakura, Y. IL-1 receptor-antagonist (IL-1ra) knockout mice show anxiety-like behavior by aging. Neurosci. Lett. 2015, 599, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Immunology of major depression. Neuroimmunomodulation 2014, 21, 123–130. [Google Scholar] [PubMed]
| Study | Country | Follow-Up | Sample | Age Mean (SD) | Inflammatory Parameters Assessed * | Directionality of the Explored Relationship | Measure of Depression ** | Change Score | Confounders Considered | Results | Potential Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baune, 2012 [26] | Australia | 2 years | 1037 non-depressed and non-demented individuals | 78.8 (4.8) | IL-1β, IL-6, IL-8, IL-10, IL-12p70 CRP, PAI-1, sVCAM-1, SAA, TNF-α |  | GDS [32] |
|
|
|
|
| Bremmer, 2008 [27] | The Netherlands | 1 year | 1285 elderly people | 75.4 (6.6) | Il-6, CRP |  | CES-D [33] and NIMH-Diagnostic Interview Schedule [34] |
|
|
| |
| Forti, 2010 [28] | Italy | 4 years | 704 elderly people | 73.4 (6.1) | IL-6, CRP, ICAM-1, ACT, TNF-α |  | GDS, DSM-IV criteria |
|
|
|
|
| Milaneschi, 2009 [29] | Italy | 3 and 6 years | 778 elderly people | 75 (7) | IL-6, sIL-6r, IL-1β, IL-1ra, TNF-α, IL-18, CRP |  | CES-D |
|
|
|
|
| Stewart, 2009 [31] | USA | 6 years | 263 healthy adults (50–70 years) | 61 (4.8) | IL-6, CRP |  | BDI-II [35] |
|
|
|
|
| Van den Biggelaar, 2007 [30] | The Netherlands | 5 years | 599 aged people | 85 (0) | IL-1β, IL-1ra, IL-6, IL-10, CPR, TNF-α |  | GDS |
|
|
|
|
| Proinflammatory parameters | IL-1β: Interleukin 1β |
| IL-6: Interleukin 6 | |
| sIL-6r: soluble IL-6 receptor | |
| IL-8: Interleukin 8 | |
| IL-12p70: IL-12 heterodimer | |
| IL-18: Interleukin 18 | |
| CRP: C-reactive protein | |
| TNF-α: Tumor necrosis factor α | |
| ICAM-1: Intercellular adhesion molecule 1 | |
| ACT: α 1-antichymotrypsin | |
| PAI-1: Plasminogen activator inhibitor-1 | |
| sVCAM-1: Soluble vascular cell adhesion molecule 1 | |
| Anti-inflammatory parameters | IL-1ra: IL-1 receptor antagonist |
| IL-10: Interleukin 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cengotitabengoa, M.; Carrascón, L.; O’Brien, J.T.; Díaz-Gutiérrez, M.-J.; Bermúdez-Ampudia, C.; Sanada, K.; Arrasate, M.; González-Pinto, A. Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review. Int. J. Mol. Sci. 2016, 17, 2022. https://doi.org/10.3390/ijms17122022
Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, Díaz-Gutiérrez M-J, Bermúdez-Ampudia C, Sanada K, Arrasate M, González-Pinto A. Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review. International Journal of Molecular Sciences. 2016; 17(12):2022. https://doi.org/10.3390/ijms17122022
Chicago/Turabian StyleMartínez-Cengotitabengoa, Mónica, Lucía Carrascón, John T. O’Brien, María-José Díaz-Gutiérrez, Cristina Bermúdez-Ampudia, Kenji Sanada, Marta Arrasate, and Ana González-Pinto. 2016. "Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review" International Journal of Molecular Sciences 17, no. 12: 2022. https://doi.org/10.3390/ijms17122022





