Adapting, Pilot Testing and Evaluating the Kick.it App to Support Smoking Cessation for Smokers with Severe Mental Illness: A Study Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. About the Kick.it App
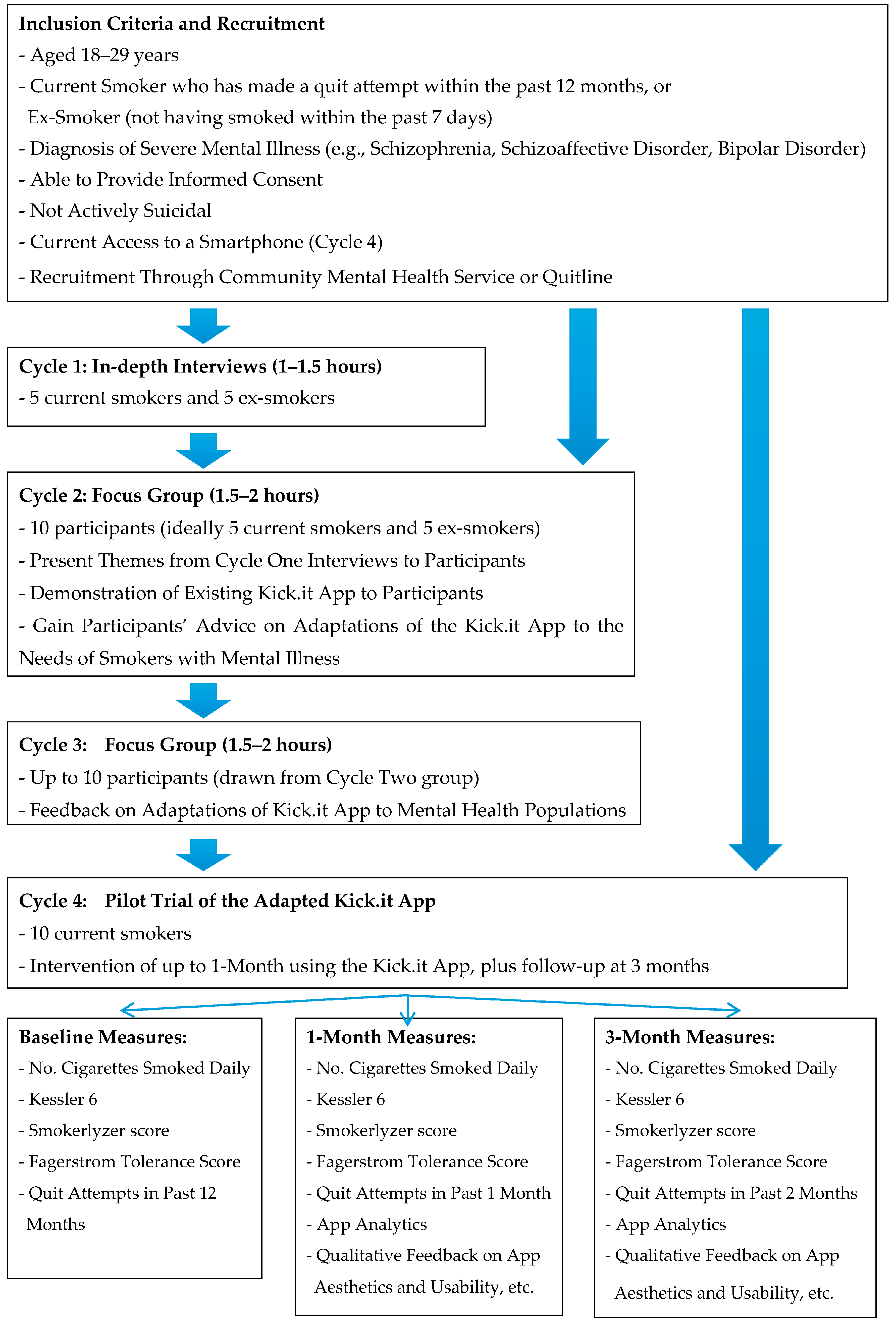
2.2. Participants
2.3. Recruitment
2.4. Data Collection
- Nicotine dependence: measured by a 2-item short form of the Fagerstrom Tolerance [77] Questionnaire. Scores range from 0–6 and are calculated by summing the points for (1) time to first cigarette smoked after waking (in minutes) and (2) number of cigarettes smoked per day.
- Prolonged abstinence: “Since (date of two weeks following baseline survey) did you smoke at all, even part of a cigarette?” [78].
- Quit attempts: commonly used item that asks “How many serious attempts to stop smoking have you made in the last 12 months? By serious attempt I mean you decided that you would try to make sure you never smoked again...” [78].
- Psychological distress: Brief 6-item K6 screening scale with robust psychometric properties [75]; it asks respondents to report how often they feel “nervous”, “hopeless”, “restless or fidgety”, “so depressed that nothing could cheer you up”, “that everything was an effort”, “worthless” over one of two recall periods (past month, and worst month).
2.5. Data Analysis
3. Discussion—Strengths and Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. WHO Report on the Global Tobacco Epidemic. 2015. Available online: http://www.who.int/tobacco/global_report/2015/en/ (accessed on 24 November 2017).
- Limm, S.S.; Vos, T.; Flaxman, A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Banks, E.; Joshy, G.; Weber, M.F.; Liu, B.; Grenfell, R.; Egger, S.; Paige, E.; Lopez, A.D.; Sitas, F.; Beral, V. Tobacco smoking and all-cause mortality in a large Australian cohort study: Findings from a mature epidemic with current low smoking prevalence. BMC Med. 2015, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004, 328, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Siahpush, M.; Borland, R.; Scollo, M. Smoking and financial stress. Tob. Control 2003, 12, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lasser, K.; Boyd, J.W.; Woolhandler, S.; Himmelstein, D.U.; McCormick, D.; Bor, D.H. Smoking and Mental Illness. A Population-Based Prevalence Study. JAMA 2000, 284, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Parrott, A.C. Smoking Cessation Leads to Reduced Stress, but Why? Int. J. Addict. 1995, 30, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). Australia’s Health 2014; Australia’s health series no. 14; Cat. no. AUS 178; AIHW: Canberra, Australia, 2014.
- Jablensky, A.; McGrath, J.; Herman, H.; Castle, D.; Gureie, O.; Evans, M.; Carr, V.; Morgan, V.; Korten, A.; Harvey, C. Psychotic disorders in urban areas: An overview of the Study on Low Prevalence disorders. Aust. N. Z. J. Psychiatry 2000, 43, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Mancuso, S.; Borland, R.; Slade, T.; Galletly, C.; Castle, D. Tobacco smoking among people living with a psychotic illness: The second Australian survey of psychosis. Aust. N. Z. J. Psychiatry 2012, 46, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Access Economics. Smoking and Mental Illness: Costs. 2007. Available online: http://www.sane.org/images/stories/information/research/0712_info_smokecosts.pdf (accessed on 24 November 2017).
- McManus, S.; Meltzer, H.; Campion, J. Cigarette Smoking and Mental Health in England. Data from the Adult Psychiatric Morbidity Survey. National Centre for Social Research. 2010. Available online: http://www.natcen.ac.uk/media/21994/smoking-mental-health.pdf (accessed on 2 February 2018).
- Gfroerer, J.; Dube, S.R.; King, B.A.; Garrett, B.E.; Babb, S.; McAfee, T. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥18 Years with Mental Illness-United States, 2009–2011. Morb. Mortal. Wkly. Rep. 2013, 62, 1–7. [Google Scholar]
- Guydish, J.; Passalacqua, E.; Pagano, A.; Martinez, C.; Le, T.; Tajima, B.; Docto, L.; Garina, D.; Delucchi, K. An international systematic review of smoking prevalence in addiction treatment. Addiction 2015, 111, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: Retrospective analysis of population based registers. BMJ 2013, 346, f2539. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Committee on Drugs. National Tobacco Strategy 2012–2018; Commonwealth of Australia: Canberra, Australia, 2012. [Google Scholar]
- Government of South Australia. South Australian Tobacco Control Strategy 2017–2020; Drug and Alcohol Services South Australia: Adelaide, Australia, 2016.
- Ziedonis, D.; Hitsman, B.; Beckham, J.C.; Zvolensky, M.; Adler, L.E.; Audrain-McGovern, J.; Breslau, N.; Brown, R.A.; George, T.P.; Williams, J.; et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob. Res. 2008, 10, 1691–1715. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsson, T.; Kriebel, D.; Tynelius, P.; Rasmussen, F.; Lundberg, I. Adolescent mental health predicts quitting smoking in adulthood: A longitudinal analysis. Eur. J. Public Health 2008, 18, 66–70. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, L.; Strong, D.; Kahler, C.; Abrantes, A.; Ramsey, S.; Brown, R. Association of post-treatment smoking change with future smoking and cessation efforts among adolescents with psychiatric comorbidity. Nicotine Tob. Res. 2007, 9, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.D.; Giese, A.A.; Turnbull, J.J.; Dickison, M.; Johnson Nagel, N. Predictors of tobacco use among persons with mental illnesses in a statewide population. Psychiatr. Serv. 2006, 57, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- DeHay, T.; Morris, C.; May, M.G.; Devine, K.; Waxmonsky, J. Tobacco use in youth with mental illnesses. J. Behav. Med. 2012, 35, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.A.; Naiman, J.M.; Williams, G.M.; Clavarino, A.M. Childhood and adolescent psychopathology and subsequent tobacco smoking in young adults: Findings from an Australian birth cohort. Addiction 2012, 107, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Mangerud, W.L.; Bjerkeset, O.; Holme, T.L.; Lydersen, S.; Indredavik, M.S. Smoking, alcohol consumption, and drug use among adolescents with psychiatric disorders compared with a population based sample. J. Adolesc. 2014, 37, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Jacka, F.N.; Pasco, J.A.; Berk, M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: A critical review of biological pathways. Brain Behav. 2013, 3, 302–326. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, W.; Von Soest, T. Smoking, nicotine dependence and mental health among young adults: A 13-year population-based longitudinal study. Addiction 2009, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Mitrou, F.; Sawyer, M.G.; Zubrick, S.R. Smoking status, mental disorders and emotional and behavioural problems in young people: Child and adolescent component of the National Survey of Mental Health and Wellbeing. Aust. N. Z. J. Psychiatry 2010, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Chaiton, M.; Cohen, J.E.; Rehm, J.; Abdulle, M.; O’Loughlin, J. Confounders or intermediate variables? Testing mechanisms for the relationship between depression and smoking in a longitudinal cohort study. Addict. Behav. 2015, 42, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Dierker, L.; Rose, J.; Selya, A.; Piasecki, T.M.; Hedeker, D.; Mermelstein, R. Depression and nicotine dependence from adolescence to young adulthood. Addict. Behav. 2015, 41, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Raupach, T.; West, R.; Brown, J. The Most “Successful” Method for Failing to Quit Smoking Is Unassisted Cessation. Nicotine Tob. Res. 2012, 15, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, T.; Stead, L. Physician advice for smoking cessation. Cochrane Database Syst. Rev. 2004, CD000165. [Google Scholar] [CrossRef]
- Stead, L.F.; Perera, R.; Bellen, C.; Mant, D.; Harmann-Boyce, J.; Cahill, K.; Lancaster, T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2008, CD000146. [Google Scholar] [CrossRef]
- Walker, J.F.; Loprinzi, P.D. Longitudinal examination of predictors of smoking cessation in a national sample of US adolescent and young adult smokers. Nicotine Tob. Res. 2014, 16, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Fromont, S.C.; Wa, C.; Matlow, R.; Ramo, D.E.; Hall, S.M. Tobacco use and its treatment among young people in mental health settings: A qualitative analysis. Nicotine Tob. Res. 2013, 15, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Palfrey, J.; Gasser, U. Born Digital: Understanding the First Generation of Digital Natives; Basic Books: New York, NY, USA, 2015. [Google Scholar]
- Orlowski, S.K.; Lawn, S.; Venning, A.; Winsall, M.; Jones, G.; Wyld, K.; Damarell, R.; Antezana, G.; Schrader, G.; Smith, D.; et al. Participatory research as one piece of the puzzle: A systematic review of consumer involvement in design of technology-based youth mental health and wellbeing interventions. JMIR Hum. Factors 2015, 2, e12. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, C.; Delucchi, K.; Lakon, C.M.; Prochaska, J.J. Randomised controlled trial evaluation of Tweet2Quit: A social network quit-smoking intervention. Tob. Control 2015, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramo, D.E.; Thrul, J.; Delucchi, K.L.; Ling, P.M.; Hall, S.M.; Prochaska, J.J. The Tobacco Status Project (TSP): Study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health 2015, 15, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J. Smoking and mental illness: Breaking the link. N. Engl. J. Med. 2011, 365, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Tidey, J.W.; Miller, M.E. Smoking cessation and reduction in people with chronic mental illness. BMJ 2015, 351, h4065. [Google Scholar] [CrossRef] [PubMed]
- Evins, A.E.; Cather, C.; Laffer, A. Treatment of tobacco use disorders in smokers with serious mental illness: Toward clinical best practices. Harv. Rev. Psychiatry 2015, 23, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.; Rigby, A.; Galletly, C. Evaluation of a community-based smoking cessation programme for people with severe mental illness. Tob. Control 2013, 24, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.; Rigby, A.; Galletly, C. What do 1000 smokers with mental illness say about their tobacco use? Aust. N. Z. J. Psychiatry 2013, 47, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.; Miller, C.L.; Bowden, J.A.; Bertossa, S. People with mental illness can tackle tobacco. Aust. N. Z. J. Psychiatry 2010, 44, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, D.T.; Porwal, M.; Webster, A.C. Review Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst. Rev. 2013, CD007253. [Google Scholar] [CrossRef]
- Kotz, D.; West, R. Explaining the social gradient in smoking cessation: It’s not in the trying, but in the succeeding. Tob. Control 2009, 18, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Ferrer, G.M.; Bernal, R.; Gallardo, M.; Romero, R.B.; Saez, D.A.; Romero, F.A. Predictors of 10-year smoking abstinence in smokers abstinent for 1 year after treatment. Addiction 2016, 111, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.L.; Wayne, G.F.; Kafali, E.N.; Liu, Z.; Shu, C.; Flores, M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA 2014, 311, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.; Campion, J. Achieving smoke-free mental health services: Lessons from the past decade of implementation research. Int. J. Environ. Public Health 2013, 10, 4224–4244. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M.; Prochaska, J.J. Treatment of smokers with co-occurring disorders: Emphasis on integration in mental health and addiction treatment settings. Annu. Rev. Clin. Psychol. 2009, 5, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.J.; Pols, R.G.; Barber, J.G. Smoking and quitting: A qualitative study with community-living psychiatric clients. Soc. Sci. Med. 2002, 54, 93–104. [Google Scholar] [CrossRef]
- Lawn, S.J. Systemic barriers to quitting smoking among institutionalised public mental health service populations: A comparison of two Australian sites. Int. J. Soc. Psychiatry 2004, 50, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians, Royal College of Psychiatrists. Smoking and Mental Health; Royal College of Psychiatrists Council Report CR178; RCP: London, UK, 2013. [Google Scholar]
- Lawn, S.J.; Pols, R.G. Nicotine withdrawal: Pathway to aggression and assault in the locked psychiatric ward. Australas. Psychiatyry 2003, 11, 199–203. [Google Scholar] [CrossRef]
- Ashton, M.; Lawn, S.; Hosking, J.R. Mental health workers’ views on addressing patients’ tobacco use. Aust. N. Z. J. Psychiatry 2010, 44, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, S.; Matthews, B.; Bidargaddi, N.; Jones, G.; Lawn, S.; Venning, A.; Collin, P. Mental Health Technologies: Designing with consumers. JMIR Hum. Factors 2016, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.A.; Aschbrenner, K.A.; Kim, S.J.; McHugo, G.J.; Unützer, J.; Bartels, S.J.; Marsch, L.A. Health behavior models for informing digital technology interventions for individuals with mental illness. Psychiatr. Rehabilit. J. 2017, 40, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Rubanovich, C.K.; Mohr, D.C.; Schueller, S.M. Health app use among individuals with symptoms of depression and anxiety: A survey study with thematic coding. JMIR Ment. Health 2017, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, J.W. Educational Research; Planning, Conduction and Evaluating Quantitative and Qualitative Research, 3rd ed.; Pearson Education: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Eldredge, L.K.B.; Markham, C.M.; Ruiter, R.A.C.; Fernandez, M.E.; Kok, G.; Parcel, G.S. Planning Health Promotion Programs: An Intervention Mapping Approach; John Wiley & Sons: San Francisco, CA, USA, 2016. [Google Scholar]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Oinas-Kukkonen, H.; Harjumaa, M. Persuasive systems design: Key issues, process model, and system features. Commun. Assoc. Inf. Syst. 2009, 24, 485–500. [Google Scholar]
- Van Agteren, J.E.M.; Lawn, S.; Bonevski, B.; Smith, B. Kick.it: The development of an evidence-based smoking cessation smartphone app. Transl. Behav. Med. 2018, in press. [Google Scholar]
- Westmaas, J.L.; Bontemps-Jones, J.; Bauer, J.E. Social support in smoking cessation: Reconciling theory and evidence. Nicotine Tob. Res. 2010, 12, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J.; Cobb, N.K. Social influence as a driver of engagement in a web-based health intervention. J. Med. Internet Res. 2012, 14, e36. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Eyal, N. Hooked: How to Build Habit-Forming Products. 2016. Available online: http://www.nirandfar.com/hooked (accessed on 16 January 2018).
- National Heart Foundation. National Heart Foundation of Australia Risk Factor Prevalence Study Survey 3 1989; National Heart Foundation of Australia and Australian Institute of Health: Canberra, Australia, 1990. [Google Scholar]
- Knowles, S.; Planner, C.; Bradshaw, T.; Peckham, E.; Man, M.; Gilbody, S. Making the journey with me: A qualitative study of experiences of a bespoke mental health smoking cessation intervention for service users with serious mental illness. BMC Psychiatry 2016, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rae, J.; Pettey, D.; Aubry, T.; Stol, J. Factors affecting smoking cessation efforts of people with severe mental illness: A qualitative study. J. Dual Diagn. 2015, 11, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.L.; Turner, A.; Kelly, P.J.; Spring, B.; Callister, R.; Collins, C.E.; Woodcock, K.L.; Kay-Lambkin, F.J.; Devir, H.; Lewin, T.J. Better Health Choices’ by telephone: A feasibility trial of improving diet and physical activity in people diagnosed with psychotic disorders. Psychiatry Res. 2014, 220, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.L.; Richmond, R.; Kay-Lambkin, F.J.; Filia, S.L.; Castle, D.; Williams, J.M.; Lewin, T.J.; Clark, V.; Callister, R.; Weaver, N. Randomized controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders. Nicotine Tob. Res. 2015, 17, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Hall, S.E.; Delucchi, K.; Hall, S.M. Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: A randomized controlled trial. Am. J. Public Health 2014, 104, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Bromet, E.; Bromet, E.; Cuitan, M.; Furukawa, T.A.; Gureje, O.; Hinkov, H.; et al. Screening for Serious Mental Illness in the General Population with the K6 screening scale: Results from the WHO World Mental Health (WMH) Survey Initiative. Int. J. Methods Psychiatr. Res. 2010, 19, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Bedfont Ltd. Smokerlyzer. Available online: https://www.bedfont.com/shop/smokerlyzer (accessed on 16 January 2018).
- John, U.; Meyer, C.; Schumann, A.; Hapke, U.; Rumpf, H.J.; Adam, C.; Alte, D.; Ludemann, J. A short form of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index in two adult population samples. Addict. Behav. 2004, 29, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Tombor, I.; Shahab, L.; Brown, J.; Notley, C.; West, R. Does non-smoker identity following quitting predict long-term abstinence? Evidence from a population survey in England. Addict. Behav. 2015, 45, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Qualitative research analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Liamputtong, P. Qualitative Research Methods, 3rd ed.; Oxford University Press: Melbourne, Australia, 2009. [Google Scholar]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.; Lewis, J. Qualitative Research Practice: A Guide for Social Science Students and Researchers; Sage: London, UK, 2003. [Google Scholar]
- Zhao, J.; Freeman, B.; Li, M. Can mobile phone apps influence people’s health behavior change? An evidence review. J. Med. Internet Res. 2016, 18, e287. [Google Scholar] [CrossRef] [PubMed]
- Gueorguieva, K. Move over ANOVA. Arch. Gen. Psychiatry 2004, 61, 3010–3017. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.W. A note on interpretation of the paired-samples t test. J. Educ. Behav. Stat. 1997, 22, 349–360. [Google Scholar] [CrossRef]
- Fisher, R.A. On the Interpretation of chi-squared from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Eysenbach, G.; CONSORT-EHEALTH Group. CONSORT-EHEALTH: Improving and standardizing evaluation reports of Web-based and mobile health interventions. J. Med. Internet Res. 2011, 13, e126–e135. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; LeFevre, A.E.; Lee, J.; L’Engle, K.; Mehl, G.; Sinha, C.; WHO mHealth Technical Evidence Review Group. Guidelines for reporting of health interventions using mobile phones: Mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016, 352, i1174. [Google Scholar] [CrossRef] [PubMed]
- My QuitBuddy. Available online: http://www.quitnow.gov.au/internet/quitnow/publishing.nsf/Content/quit-buddy (accessed on 16 January 2018).
- Oliver, J.A.; Hallyburton, M.B.; Pacek, L.R.; Mitchell, J.T.; Vilardaga, R.; Fuemmeler, B.F.; McClernon, F.J. What do smokers want in a smartphone-based cessation application? Nicotine Tob. Res. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawn, S.; Van Agteren, J.; Zabeen, S.; Bertossa, S.; Barton, C.; Stewart, J. Adapting, Pilot Testing and Evaluating the Kick.it App to Support Smoking Cessation for Smokers with Severe Mental Illness: A Study Protocol. Int. J. Environ. Res. Public Health 2018, 15, 254. https://doi.org/10.3390/ijerph15020254
Lawn S, Van Agteren J, Zabeen S, Bertossa S, Barton C, Stewart J. Adapting, Pilot Testing and Evaluating the Kick.it App to Support Smoking Cessation for Smokers with Severe Mental Illness: A Study Protocol. International Journal of Environmental Research and Public Health. 2018; 15(2):254. https://doi.org/10.3390/ijerph15020254
Chicago/Turabian StyleLawn, Sharon, Joseph Van Agteren, Sara Zabeen, Sue Bertossa, Christopher Barton, and James Stewart. 2018. "Adapting, Pilot Testing and Evaluating the Kick.it App to Support Smoking Cessation for Smokers with Severe Mental Illness: A Study Protocol" International Journal of Environmental Research and Public Health 15, no. 2: 254. https://doi.org/10.3390/ijerph15020254
APA StyleLawn, S., Van Agteren, J., Zabeen, S., Bertossa, S., Barton, C., & Stewart, J. (2018). Adapting, Pilot Testing and Evaluating the Kick.it App to Support Smoking Cessation for Smokers with Severe Mental Illness: A Study Protocol. International Journal of Environmental Research and Public Health, 15(2), 254. https://doi.org/10.3390/ijerph15020254





