The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells
Abstract
:1. Introduction
2. Results
2.1. RF Treatment, But Not HT Treatment, Inhibited the Proliferation of Pancreatic Cancer Cells In Vitro
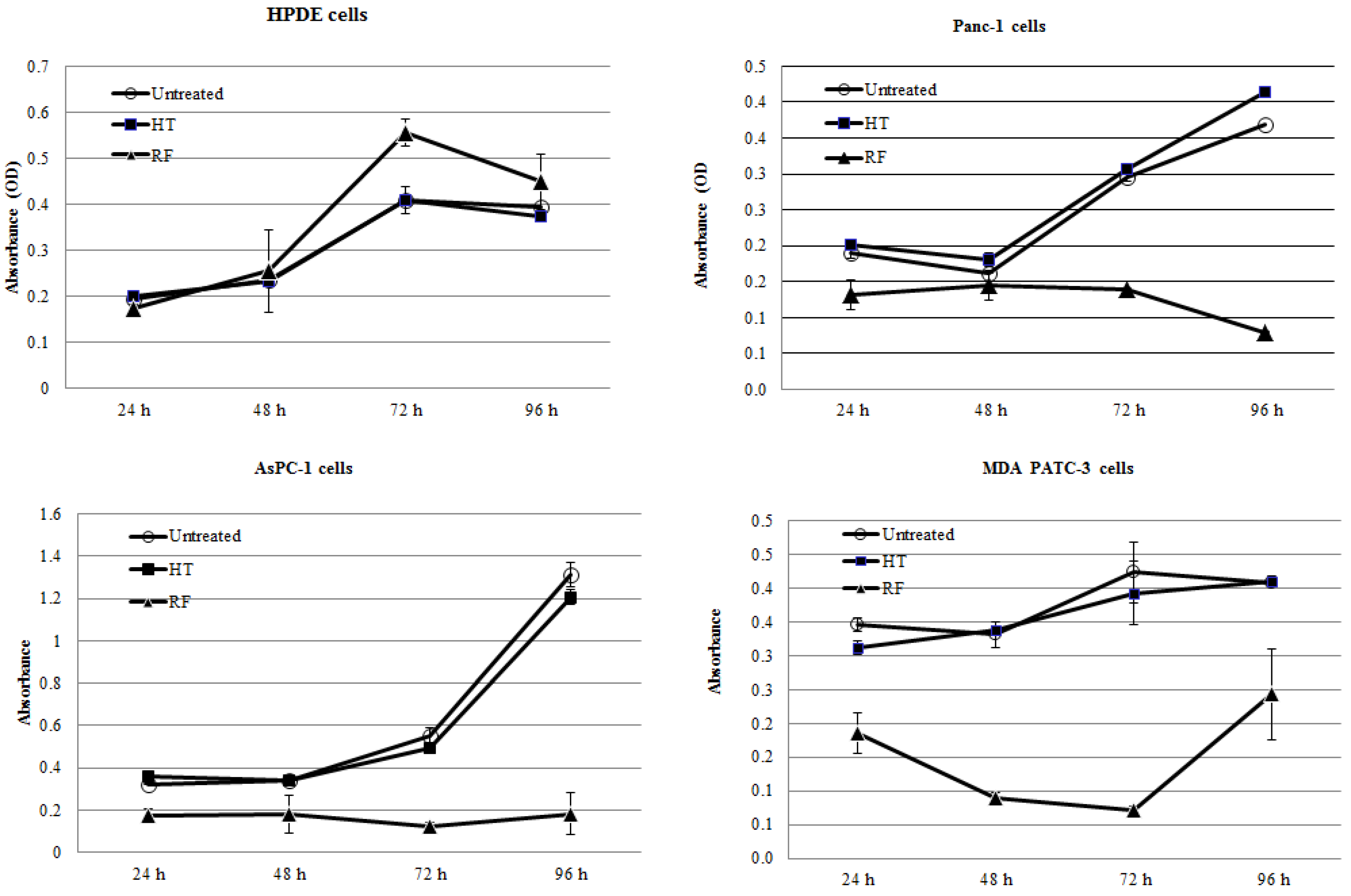

2.2. RF Treatment Decreases Oxygen Consumption Rates (OCR) in Cancer Cells More than HT Treatment

2.3. RF Treatment, But Not HT Treatment, Significantly Increased the Number of Autophagosomes in Cancer Cells

3. Discussion
4. Methods
4.1. Chemical Reagents and Cell Culture
4.2. RF and HT Treatments
4.3. Cell Proliferation and Viability Assays
4.4. OCR Measurement
4.5. Fluorescence Immunocytochemistry Analysis of Autophagy
4.6. Statistical Methods
5. Conclusions
Acknowledgments
Author Contributions
References
- Ding, J.; Jing, X.; Liu, J.; Wanf, Y.; Wang, F.; Wang, Y.; Du, Z. Complications of thermal ablation of hepatic tumours: Comparison of radiofrequency and microwave ablative techniques. Clin. Radiol. 2013, 68, 608–615. [Google Scholar] [CrossRef]
- Niu, L.; Xu, K.; Mu, F. Cryosurgery for lung cancer. J. Thorac. Dis. 2012, 4, 408–419. [Google Scholar]
- Zhou, G.; Chiu, D; Qin, D.; Niu, L.; Cai, J.; He, L.; Huang, W.; Xu, K. The efficacy evaluation of cryosurgery in pancreatic cancer patients with the expression of CD44v6, integrin-beta1, CA199, and CEA. Mol. Biotechnol. 2012, 52, 59–67. [Google Scholar] [CrossRef]
- Owusu-Agyemang, P.; Arunkumar, R.; Green, H.; Hurst, D.; Landoski, K.; Hayes-Jordan, A. Anesthetic management and renal functional in pediatric patients undergoing cytoreductive surgery with continous hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin. Ann. Surg. Oncol. 2012, 19, 2652–2656. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Briggs, K.; Palalon, F.; Curley, S.A. Autophagy and enhanced chemosensitivity in experimental pancreatic cancers induced by noninvasive radiofrequency field treatment. Cancer 2014, 120, 480–491. [Google Scholar] [CrossRef]
- Glazer, E.S.; Zhu, C.; Massey, K.L.; Thompson, C.S.; Kaluarachchi, W.D.; Hamir, A.N.; Curley, S.A. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin. Cancer Res.: An Offic. J. Am. Assoc. Cancer Res. 2010, 16, 5712–5721. [Google Scholar] [CrossRef]
- Raoof, M.; Cisneros, B.T.; Corr, S.J.; Palalon, F.; Curley, S.A.; Koshkina, N.V. Tumor selective hyperthermia induced by short-wave capacitively-coupled RF electric-fields. PloS ONE 2013, 8. [Google Scholar] [CrossRef]
- Gerweck, L.E. Hyperthermia in cancer therapy: The biological basis and unresolved questions. Cancer Res. 1985, 45, 3408–3414. [Google Scholar]
- Hayes-Jordan, A.; Green, H.; Ludwig, J.; Anderson, P. Toxicity of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric patients with sarcomatosis/carcinomatosis: Early experience and phase 1 results. Pediatr. Blood Cancer 2012, 59, 395–397. [Google Scholar] [CrossRef]
- Di Miceli, D.; Alfieri, S.; Caprino, P.; Menghi, R.; Quero, G.; Cina, C.; Ridolfini, M.P.; Doglietto, G.B. Complications related to hyperthermia during hypertermic intraoperative intraperitoneal chemiotherapy (HIPEC) treatment. Do they exist? Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 737–742. [Google Scholar]
- Glazer, E.S.; Curley, S.A. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer 2010, 116, 3285–3293. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Pennison, M.J.; Brezovich, I.; Yi, N.; Yang, C.T.; Ramaker, R.; Absher, D.; Myers, R.M.; Kuster, N.; Costa, F.P.; et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer 2012, 106, 307–313. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Sowers, A.E. Characterization of electric field-induced fusion in erythrocyte ghost membranes. J. Cell Biol. 1984, 99, 1989–1996. [Google Scholar] [CrossRef]
- Trosic, I.; Pavicic, I.; Marjanovic, A.M.; Busljeta, I. Non-thermal biomarkers of exposure to radiofrequency/microwave radiation. Arh. Hig. Rada Toksikol. 2012, 63 Suppl. 1, 67–73. [Google Scholar]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther. und Onkol. 2009, 185, 120–126. [Google Scholar] [CrossRef]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. Tumor cell redox state and mitochondria at the center of the non-canonical activity of telomerase reverse transcriptase. Mol. Asp. Med. 2010, 31, 21–28. [Google Scholar] [CrossRef]
- Curley, S.A.; Palalon, F.; Lu, X.; Koshkina, N.V. Noninvasive radiofrequency treatment effect on mitochondria in pancreatic cancer cells. Cancer 2014. [Google Scholar] [CrossRef]
- Raaphorst, G.P.; Freeman, M.L.; Dewey, W.C. Radiosensitivity and recovery from radiation damage in cultured CHO cells exposed to hyperthermia at 42.5 or 45.5 °C. Radiat. Res. 1979, 79, 390–402. [Google Scholar] [CrossRef]
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Jauchem, J.R. Effects of low-level radio-frequency (3 kHz to 300 GHz) energy on human cardiovascular, reproductive, immune, and other systems: A review of the recent literature. Int. J. Hyg. Environ. Health 2008, 211, 1–29. [Google Scholar] [CrossRef]
- Szmigielski, S. Reaction of the immune system to low-level RF/MW exposures. Sci. Total Environ. 2013, 454–455, 393–400. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Kirson, E.D.; Giladi, M.; Gurvich, Z.; Itzhaki, A.; Mordechovich, D.; Schneiderman, R.S.; Wasserman, Y.; Ryffel, B.; Goldsher, D.; Palti, Y. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin. Exp. Metastasis 2009, 26, 633–640. [Google Scholar] [CrossRef]
- Taghi, M.; Gholamhosein, R.; Saeed, R.Z. Effect of Radio Frequency Waves of Electromagnetic Field on the Tubulin. Recent Patents Endocr., Metab. Immune Drug Discov. 2013, 7, 252–256. [Google Scholar]
- Taghi, M.; Gholamhosein, R.; Saeed, R.Z. Effect of electromagnetic field on the polymerization of microtubules extracted from rat brain. Recent Patents Endocr., Metab. Immune Drug Discov. 2012, 6, 251–254. [Google Scholar]
- Zimmerman, J.W.; Pennison, M.J.; Brezovich, I.; Yi, N.; Yang, C.T.; Ramaker, R.; Absher, D.; Myers, R.M.; Kuster, N.; Costa, F.P. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer 2012, 106, 307–313. [Google Scholar] [CrossRef]
- Marchionni, I.; Paffi, A.; Pellegrino, M.; Liberti, M.; Apollonio, F.; Abeti, R.; Fontana, F.; D’lnzeo, G.; Mazzanti, M. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim. Biophys. Acta 2006, 1758, 597–605. [Google Scholar]
- Li, M.; Zhai, Q.; Bharadwaj, U.; Wang, H.; Li, F.; Fisher, W.E.; Chen, C.; Yao, Q. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer 2006, 106, 2284–2294. [Google Scholar]
- Koshkina, N.V.; Kleinerman, E.S. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int. J. Cancer 2005, 116, 458–463. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Curley, S.A.; Palalon, F.; Sanders, K.E.; Koshkina, N.V. The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells. Int. J. Environ. Res. Public Health 2014, 11, 9142-9153. https://doi.org/10.3390/ijerph110909142
Curley SA, Palalon F, Sanders KE, Koshkina NV. The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells. International Journal of Environmental Research and Public Health. 2014; 11(9):9142-9153. https://doi.org/10.3390/ijerph110909142
Chicago/Turabian StyleCurley, Steven A., Flavio Palalon, Kelly E. Sanders, and Nadezhda V. Koshkina. 2014. "The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells" International Journal of Environmental Research and Public Health 11, no. 9: 9142-9153. https://doi.org/10.3390/ijerph110909142



