A Retrospective Observational Study of Risk Factors for Denosumab-Related Osteonecrosis of the Jaw in Patients with Bone Metastases from Solid Cancers
Abstract
:1. Introduction
2. Results
2.1. Patient Demographics and Characteristics
2.2. Comparison of DRONJ and Non-DRONJ Patients
2.3. Risk Factors for DRONJ
3. Discussion
4. Patients and Methods
4.1. Data Sources and Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Study Variables
4.4. Study Outcomes
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gul, G.; Sendur, M.A.; Aksoy, S.; Sever, A.R.; Altundag, K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr. Med. Res. Opin. 2016, 32, 133–145. [Google Scholar] [CrossRef]
- Coleman, R.E. Bisphosphonates: Clinical experience. Oncologist. 2004, 9 (Suppl. 4), 14–27. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer. Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinoda, Y.; Sawada, R.; Yoshikawa, F.; Oki, T.; Hirai, T.; Kobayashi, H.; Matsudaira, K.; Oka, H.; Tanaka, S.; Kawano, H.; et al. Factors related to the quality of life in patients with bone metastases. Clin. Exp. Metastasis 2019, 36, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.J.; Raje, N.S. Denosumab for the treatment of bone disease in solid tumors and multiple myeloma. Future Oncol. 2018, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.; Somerfield, M.R.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Clemons, M.J.; Dhesy-Thind, S.K.; Dillmon, M.S.; Eisen, A.; Frank, E.S. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J. Clin. Oncol. 2017, 35, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Kiesel, L.; Kohl, A. Role of the RANK/RANKL pathway in breast cancer. Maturitas 2016, 86, 10–16. [Google Scholar] [CrossRef]
- de Groot, A.F.; Appelman-Dijkstra, N.M.; van der Burg, S.H.; Kroep, J.R. The anti-tumor effect of RANKL inhibition in malignant solid tumors—A systematic review. Cancer. Treat. Rev. 2018, 62, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Beaudoin, C.; Jean, S.; Bessette, L.; Ste-Marie, L.G.; Moore, L.; Brown, J.P. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Song, M. Dental care for patients taking antiresorptive drugs: A literature review. Restor. Dent. Endod. 2019, 44, e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Perianez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet. Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Wat, W.Z.M. Current Controversies on the Pathogenesis of Medication-Related Osteonecrosis of the Jaw. Dent. J. (Basel) 2016, 4, E38. [Google Scholar] [CrossRef] [Green Version]
- Shibahara, T. Imaging modalities for drug-related osteonecrosis of the jaw (2), Overview of the position paper on medication-related osteonecrosis of the jaw and the current status of the MRONJ in Japan. Jpn. Dent. Sci. Rev. 2019, 55, 71–75. [Google Scholar] [CrossRef]
- Yuan, A.; Munz, A.; Reinert, S.; Hoefert, S. Histologic analysis of medication-related osteonecrosis of the jaw compared with antiresorptive-exposed bone and other infectious, inflammatory, and necrotic jaw diseases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 133–140. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Caldroney, S.; Ghazali, N.; Dyalram, D.; Lubek, J.E. Surgical resection and vascularized bone reconstruction in advanced stage medication-related osteonecrosis of the jaw. Int. J. Oral. Maxillofac. Surg. 2017, 46, 871–876. [Google Scholar] [CrossRef]
- Oteri, G.; De Ponte, F.S.; Runci, M.; Peditto, M.; Marciano, A.; Cicciu, M. Oral-Health-Related Quality of Life After Surgical Treatment of Osteonecrosis of the Jaws. J. Craniofac. Surg. 2018, 29, 403–408. [Google Scholar] [CrossRef]
- Oteri, G.; Trifiro, G.; Peditto, M.; Lo Presti, L.; Marciano, I.; Giorgianni, F.; Sultana, J.; Marciano, A. Treatment of Medication-Related Osteonecrosis of the Jaw and its Impact on a Patient’s Quality of Life: A Single-Center, 10-Year Experience from Southern Italy. Drug Saf. 2018, 41, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2017, 10, CD012432. [Google Scholar] [CrossRef] [PubMed]
- Capocci, M.; Romeo, U.; Guerra, F.; Mannocci, A.; Tenore, G.; Annibali, S.; Ottolenghi, L. Medication-related osteonecrosis of the jaws (MRONJ) and quality of life evaluation: A pilot study. Clin. Ter. 2017, 168, e253–e257. [Google Scholar] [PubMed]
- Boquete-Castro, A.; Gomez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants. Res. 2016, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Soutome, S.; Hayashida, S.; Funahara, M.; Sakamoto, Y.; Kojima, Y.; Yanamoto, S.; Umeda, M. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: Is tooth extraction a risk factor? PLoS ONE 2018, 13, e0201343. [Google Scholar] [CrossRef] [Green Version]
- Peer, A.; Khamaisi, M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J. Dent. Res. 2015, 94, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Lo Muzio, L.; Sipmo Onj, B.; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. Biomed. Res. Int. 2018, 2018, 2684924. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kawakita, A.; Ueda, N.; Funahara, R.; Tachibana, A.; Kobayashi, M.; Kondou, E.; Takeda, D.; Kojima, Y.; Sato, S.; et al. A multicenter retrospective study of the risk factors associated with medication-related osteonecrosis of the jaw after tooth extraction in patients receiving oral bisphosphonate therapy: Can primary wound closure and a drug holiday really prevent MRONJ? Osteoporos. Int. 2017, 28, 2465–2473. [Google Scholar] [CrossRef]
- Narayanan, P. Denosumab: A comprehensive review. South Asian J. Cancer. 2013, 2, 272–277. [Google Scholar] [CrossRef]
- Haslbauer, F.; Petzer, A.; Safanda, M.; Tomova, A.; Porubska, M.; Bajory, Z.; Niepel, D.; Jaeger, C.; Bjorklof, K.; Kalinin, D. Prospective observational study to evaluate the persistence of treatment with denosumab in patients with bone metastases from solid tumors in routine clinical practice: Final analysis. Support Care Cancer. 2020, 28, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Brown-Glaberman, U.; Stopeck, A.T. Impact of denosumab on bone mass in cancer patients. Clin. Pharmacol. 2013, 5, 117–129. [Google Scholar] [PubMed] [Green Version]
- Scagliotti, G.V.; Hirsh, V.; Siena, S.; Henry, D.H.; Woll, P.J.; Manegold, C.; Solal-Celigny, P.; Rodriguez, G.; Krzakowski, M.; Mehta, N.D.; et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J. Thorac. Oncol. 2012, 7, 1823–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health. 2008, 11, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Anastasilakis, A.D.; Toulis, K.A.; Polyzos, S.A.; Anastasilakis, C.D.; Makras, P. Long-term treatment of osteoporosis: Safety and efficacy appraisal of denosumab. Ther. Clin. Risk Manag. 2012, 8, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittman, K.; Antill, Y.C.; Goldrick, A.; Goh, J.; de Boer, R.H. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac. J. Clin. Oncol. 2017, 13, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, K.; Ishikawa, K.; Tani, S.; Oshita, Y.; Kuroda, T.; Yamamura, R.; Emori, H.; Maruyama, H.; Matsuoka, A.; Kudo, Y.; et al. Analysis of three-dimensional bone mineral density and bone strength measured by quantitative computed tomography following denosumab discontinuation in a patient with postmenopausal osteoporosis. Clin. Interv. Aging. 2019, 14, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Egloff-Juras, C.; Gallois, A.; Salleron, J.; Massard, V.; Dolivet, G.; Guillet, J.; Phulpin, B. Denosumab-related osteonecrosis of the jaw: A retrospective study. J. Oral Pathol. Med. 2018, 47, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017, 12, CD003188. [Google Scholar] [CrossRef]
- Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Nagata, T.; Urade, M.; Shibahara, T.; et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2017, 35, 6–19. [Google Scholar] [CrossRef]
- Karna, H.; Gonzalez, J.; Radia, H.S.; Sedghizadeh, P.P.; Enciso, R. Risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: A systematic review with meta-analyses. Oral Oncol. 2018, 85, 15–23. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ. International Task Force on Osteonecrosis of the Jaw. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, S.; Song, M.; Lee, C.; Yagita, H.; Williams, D.W.; Sung, E.C.; Hong, C.; Shin, K.H.; Kang, M.K.; et al. Removal of Pre-Existing Periodontal Inflammatory Condition before Tooth Extraction Ameliorates Medication-Related Osteonecrosis of the Jaw-Like Lesion in Mice. Am. J. Pathol. 2018, 188, 2318–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiodt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]
- Diniz-Freitas, M.; Fernandez-Feijoo, J.; Diz Dios, P.; Pousa, X.; Limeres, J. Denosumab-related osteonecrosis of the jaw following non-surgical periodontal therapy: A case report. J. Clin. Periodontol. 2018, 45, 570–577. [Google Scholar] [CrossRef]
- Hallmer, F.; Bjornland, T.; Andersson, G.; Becktor, J.P.; Kristoffersen, A.K.; Enersen, M. Bacterial diversity in medication-related osteonecrosis of the jaw. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 436–444. [Google Scholar] [CrossRef]
- Charopoulos, I.; Orme, S.; Giannoudis, P.V. The role and efficacy of denosumab in the treatment of osteoporosis: An update. Expert Opin. Drug Saf. 2011, 10, 205–217. [Google Scholar] [CrossRef]
- Brown-Glaberman, U.; Stopeck, A.T. Role of denosumab in the management of skeletal complications in patients with bone metastases from solid tumors. Biologics. 2012, 6, 89–99. [Google Scholar]
- Silva-Fernandez, L.; Loza, E.; Martinez-Taboada, V.M.; Blanco, R.; Rua-Figueroa, I.; Pego-Reigosa, J.M.; Munoz-Fernandez, S.; Systemic Autoimmune Diseases Study Group of the Spanish Society for, R. Biological therapy for systemic vasculitis: A systematic review. Semin. Arthritis Rheum. 2014, 43, 542–557. [Google Scholar] [CrossRef]
- Ristow, O.; Otto, S.; Troeltzsch, M.; Hohlweg-Majert, B.; Pautke, C. Treatment perspectives for medication-related osteonecrosis of the jaw (MRONJ). J. Craniomaxillofac. Surg. 2015, 43, 290–293. [Google Scholar] [CrossRef]
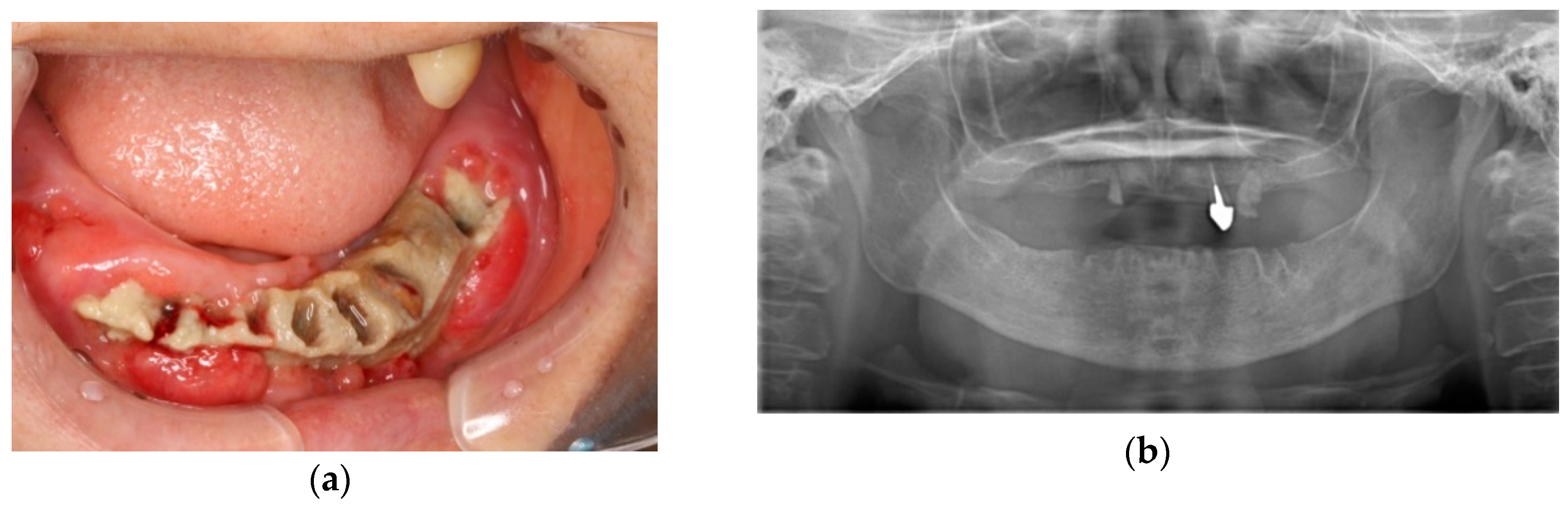
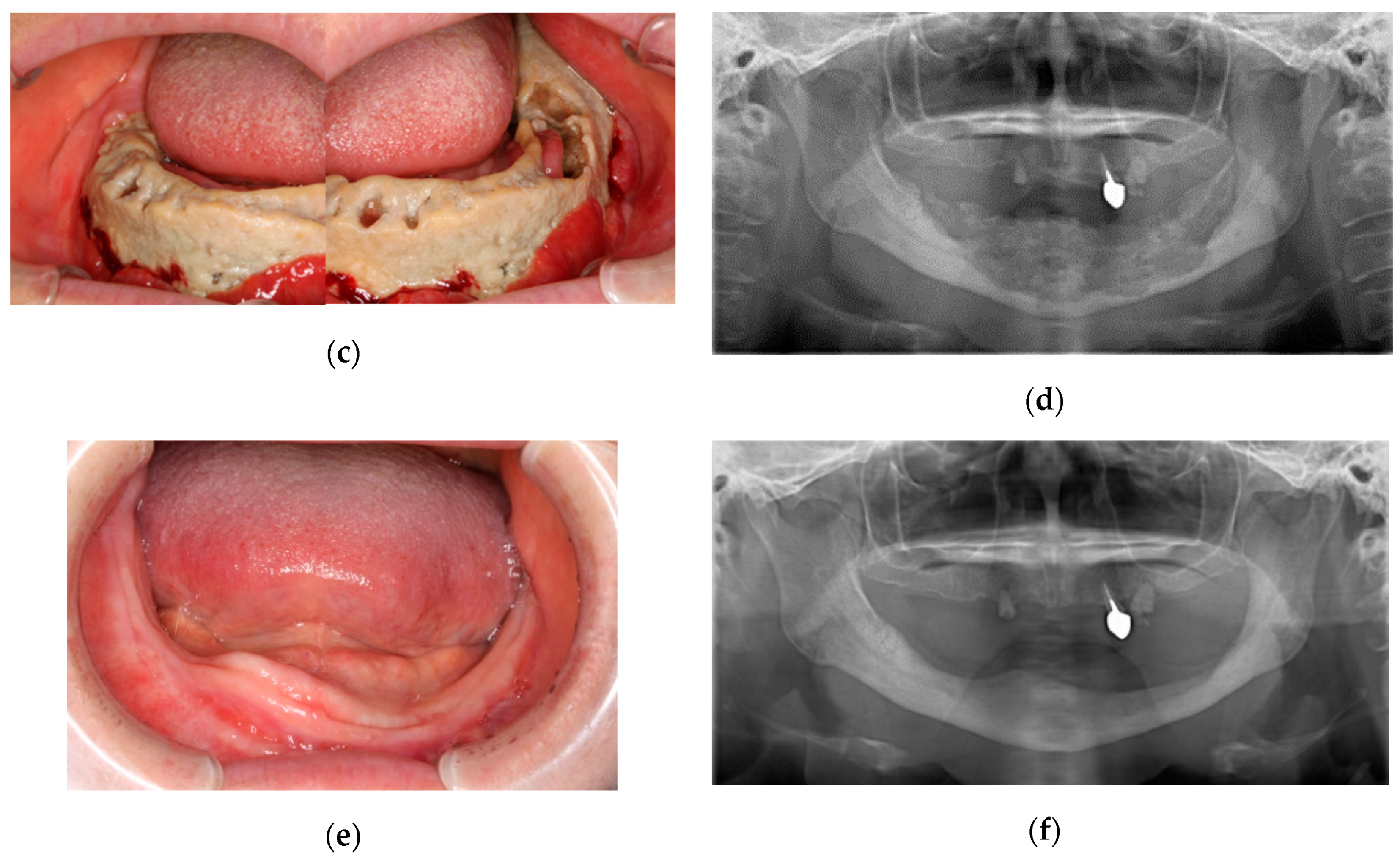
| Description | n (%) or Median (Range) | ||
|---|---|---|---|
| Demographic factors | Sex | Male | 57 (46.3) |
| Female | 66 (53.7) | ||
| Age (years) | 68.0 (25.0–95.0) | ||
| Performance status | 0 | 0 (0.0) | |
| 1 | 11 (8.9) | ||
| 2 | 51 (41.5) | ||
| 3 | 52 (42.3) | ||
| 4 | 9 (7.3) | ||
| Height (cm) | 158.0 (130.0–178.0) | ||
| Weight (kg) | 51.4 (27.6–77.2) | ||
| BMI 1 | 20.6 (13.3–31.0) | ||
| Oral nutrition | Yes | 122 (99.2) | |
| Brinkman index | 0.0 (0.0–2460.0) | ||
| Alcohol consumption | Yes | 10 (8.1) | |
| Medical history | Diabetes mellitus | Yes | 16 (13.0) |
| Rheumatoid arthritis | Yes | 4 (3.3) | |
| Hypocalcemia | Yes | 60 (48.8) | |
| Hypercalcemia | Yes | 0 (0.0) | |
| Hypothyroidism | Yes | 4 (3.3) | |
| Osteoporosis | Yes | 7 (5.7) | |
| Vitamin B deficiency | Yes | 1 (0.8) | |
| Anemia | Yes | 85 (69.1) | |
| Antithrombotic therapy | Yes | 19 (15.4) | |
| Blood parameters | Hemoglobin (g/dL) | 11.4 (6.3–17.7) | |
| Total protein (g/dL) | 6.7 (4.1–8.4) | ||
| Albumin (g/dL) | 3.4 (1.5–4.6) | ||
| Cholesterol (mg/dL) | 180.0 (53.0–337.0) | ||
| Calcium (mg/dL) | 8.9 (6.2–10.2) | ||
| C-reactive protein (mg/dL) | 1.6 (0.0–23.7) | ||
| Underlying disease | Cancer type | Breast | 32 (26.0) |
| Lung | 24 (19.5) | ||
| Prostate | 16 (13.0) | ||
| Colon | 13 (10.6) | ||
| Pancreas | 6 (4.9) | ||
| Kidney | 7 (5.7) | ||
| Liver | 8 (6.5) | ||
| Uterus | 2 (1.6) | ||
| Stomach | 6 (4.9) | ||
| Bladder | 3 (2.4) | ||
| Other | 6 (4.9) | ||
| Bone metastasis | Yes | 123 (100) | |
| Multiple metastases | Yes | 67 (54.5) | |
| Chemotherapy and/or molecular targeted drug | Yes | 34 (27.6) | |
| Angiogenesis inhibitor | Yes | 0 (0.0) | |
| Tyrosine kinase inhibitor | Yes | 0 (0.0) | |
| Hormonal therapy | Yes | 23 (18.7) | |
| Intraoral findings | Number of teeth | 18.0 (0.0–32.0) | |
| Denture use | Yes | 48 (39.0) | |
| Apical periodontitis | Yes | 44 (35.8) | |
| Periodontal disease | Yes | 54 (43.9) | |
| Denosumab | Administration period (months) | 4 (2–52) | |
| Reason for dropout | Continuing | 40 (32.5) | |
| Cancelled | 2 (1.6) | ||
| Deceased | 81 (65.9) | ||
| Follow-up (months) | 4 (2–52) | ||
| DRON J 2 | DRONJ 2 stage [18] | 0 | 0 (0.0) |
| 1 | 3 (2.4) | ||
| 2 | 5 (4.1) | ||
| 3 | 6 (4.9) | ||
| Variables | n (%) or Median (Range) | ||||
|---|---|---|---|---|---|
| Control (n = 109) | DRONJ (n = 14) | p-Value | |||
| Background factor | Sex | Male | 55 (50.5) | 2 (14.3) | 0.011 * |
| Female | 54 (49.5) | 12 (85.7) | |||
| Age (years) | 68.0 (25.0–95.0) | 64.0 (51.0–77.0) | 0.155 | ||
| Performance status | 0 | 0 (0.0) | 0 (0.0) | 0.422 | |
| 1 | 10 (9.2) | 1 (7.1) | |||
| 2 | 43 (39.4) | 8 (57.1) | |||
| 3 | 48 (44.0) | 4 (28.6) | |||
| 4 | 8 (7.3) | 1 (7.1) | |||
| Height (cm) | 160.0 (130.0–178.0) | 152.0 (145.0–164.0) | 0.010 * | ||
| Weight (kg) | 51.4 (27.6–77.2) | 53.3 (31.0–72.5) | 0.469 | ||
| BMI 1 | 20.4 (13.3–28.7) | 21.7 (13.4–31.0) | 0.055 | ||
| Oral nutrition | Yes | 108 (99.1) | 14 (100) | 1.000 | |
| Brinkman index | 0.0 (0.0–2460.0) | 0 (0.0–0.0) | 0.001 ** | ||
| Alcohol consumption | Yes | 10 (9.2) | 0 (0.0) | 0.602 | |
| Medical history | Diabetes mellitus | Yes | 15 (13.8) | 1 (7.1) | 0.692 |
| Rheumatoid arthritis | Yes | 3 (2.8) | 1 (7.1) | 0.387 | |
| Hypocalcemia | Yes | 55 (50.5) | 5 (35.7) | 0.397 | |
| Hypercalcemia | Yes | 0 (0.0) | 0 (0.0) | - | |
| Hypothyroidism | Yes | 4 (3.7) | 0 (0.0) | 1.000 | |
| Osteoporosis | Yes | 5 (4.6) | 2 (14.3) | 0.181 | |
| Vitamin B deficiency | Yes | 1 (0.9) | 0 (0.0) | 1.000 | |
| Anemia | Yes | 76 (69.7) | 9 (64.3) | 0.761 | |
| Antithrombotic therapy | Yes | 17 (15.6) | 2 (14.3) | 1.000 | |
| Blood examination | Hemoglobin (g/dL) | 11.2 (6.3–17.7) | 11.9 (8.3–15.9) | 0.097 | |
| Total protein (g/dL) | 6.7 (4.1–8.4) | 7.0 (5.6–7.8) | 0.170 | ||
| Albumin (g/dL) | 3.4 (1.5–4.6) | 3.3 (2.6–4.5) | 0.385 | ||
| Cholesterol (mg/dL) | 179.0 (53.0–337.0) | 201.5 (146.0–334.0) | 0.232 | ||
| Calcium (mg/dL) | 8.9 (6.2–10.0) | 9.2 (6.4–10.2) | 0.187 | ||
| C-reactive protein (mg/dL) | 1.7 (0.0–23.7) | 1.0 (0.0–4.7) | 0.136 | ||
| Underlying disease | Cancer type | Breast | 21 (10.3) | 11 (78.6) | - |
| Lung | 24 (22.0) | 0 (0.0) | |||
| Prostate | 14 (12.8) | 2 (14.3) | |||
| Colon | 12 (11.0) | 1 (7.1) | |||
| Pancreatic | 6 (5.5) | 0 (0.0) | |||
| Kidney | 7 (6.4) | 0 (0.0) | |||
| Liver | 8 (7.3) | 0 (0.0) | |||
| Uterus | 2 (1.8) | 0 (0.0) | |||
| Stomach | 6 (5.5) | 0 (0.0) | |||
| Bladder | 3 (2.8) | 0 (0.0) | |||
| Other | 6 (5.5) | 0 (0.0) | |||
| Bone metastasis | Yes | 109 (100) | 14 (100) | - | |
| Multiple metastases | Yes | 56 (51.4) | 11 (78.6) | 0.085 | |
| Chemotherapy or molecular targeted drug | Yes | 26 (23.9) | 8 (57.1) | 0.021 * | |
| Angiogenesis inhibitor | Yes | 0 (0.0) | 0 (0.0) | - | |
| Tyrosine kinaseinhibitor | Yes | 0 (0.0) | 0 (0.0) | - | |
| Hormonal therapy | Yes | 16 (14.7) | 7 (50.0) | 0.005 ** | |
| Intraoral findings | Number of teeth | 17.0 (0.0–32.0) | 21.5 (0.0–32.0) | 0.717 | |
| Denture use | Yes | 41 (37.6) | 7 (50.0) | 0.395 | |
| Apical periodontitis | Yes | 34 (31.2) | 10 (71.4) | 0.006 ** | |
| Periodontal disease | Yes | 42 (38.5) | 12 (85.7) | 0.001 ** | |
| Denosumab | Administration period (months) | 4 (2–52) | 10 (7–45) | 0.001 ** | |
| Drop out reason | Continuing | 32 (29.4) | 8 (57.1) | - | |
| Cancelled | 1 (0.9) | 1 (7.1) | |||
| Deceased | 76 (69.7) | 5 (35.7) | |||
| DRONJ 2 | DRONJ 2 stage [18] | 0 | 0 (0.0) | 0 (0.0) | - |
| 1 | 0 (0.0) | 3 (21.4) | |||
| 2 | 0 (0.0) | 5 (35.7) | |||
| 3 | 0 (0.0) | 6 (42.9) | |||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio (CI) | Significance | Odds Ratio (CI) | Significance | |
| Hormonal therapy | 5.81 (1.80–18.81) | 0.003 | 22.07 (2.86–170.24) | 0.003 |
| Chemotherapy/molecular target drug | 4.26 (1.35–13.40) | 0.013 | 18.61 (2.54–136.27) | 0.004 |
| Apical periodontitis | 5.52 (1.62–18.84) | 0.006 | 22.75 (3.20–161.73) | 0.002 |
| Periodontal disease | 9.57 (2.04–44.91) | 0.004 | ||
| Sex | 6.11 (1.31–28.60) | 0.022 | ||
| Body mass index | 1.18 (1.02–1.37) | 0.024 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuma, S.; Matsuda, Y.; Nariai, Y.; Karino, M.; Suzuki, R.; Kanno, T. A Retrospective Observational Study of Risk Factors for Denosumab-Related Osteonecrosis of the Jaw in Patients with Bone Metastases from Solid Cancers. Cancers 2020, 12, 1209. https://doi.org/10.3390/cancers12051209
Okuma S, Matsuda Y, Nariai Y, Karino M, Suzuki R, Kanno T. A Retrospective Observational Study of Risk Factors for Denosumab-Related Osteonecrosis of the Jaw in Patients with Bone Metastases from Solid Cancers. Cancers. 2020; 12(5):1209. https://doi.org/10.3390/cancers12051209
Chicago/Turabian StyleOkuma, Satoe, Yuhei Matsuda, Yoshiki Nariai, Masaaki Karino, Ritsuro Suzuki, and Takahiro Kanno. 2020. "A Retrospective Observational Study of Risk Factors for Denosumab-Related Osteonecrosis of the Jaw in Patients with Bone Metastases from Solid Cancers" Cancers 12, no. 5: 1209. https://doi.org/10.3390/cancers12051209





