The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas—A Meta-Analysis and Systematic Review of the Literature
Abstract
:1. Introduction
2. Subjects and Methods
3. Results
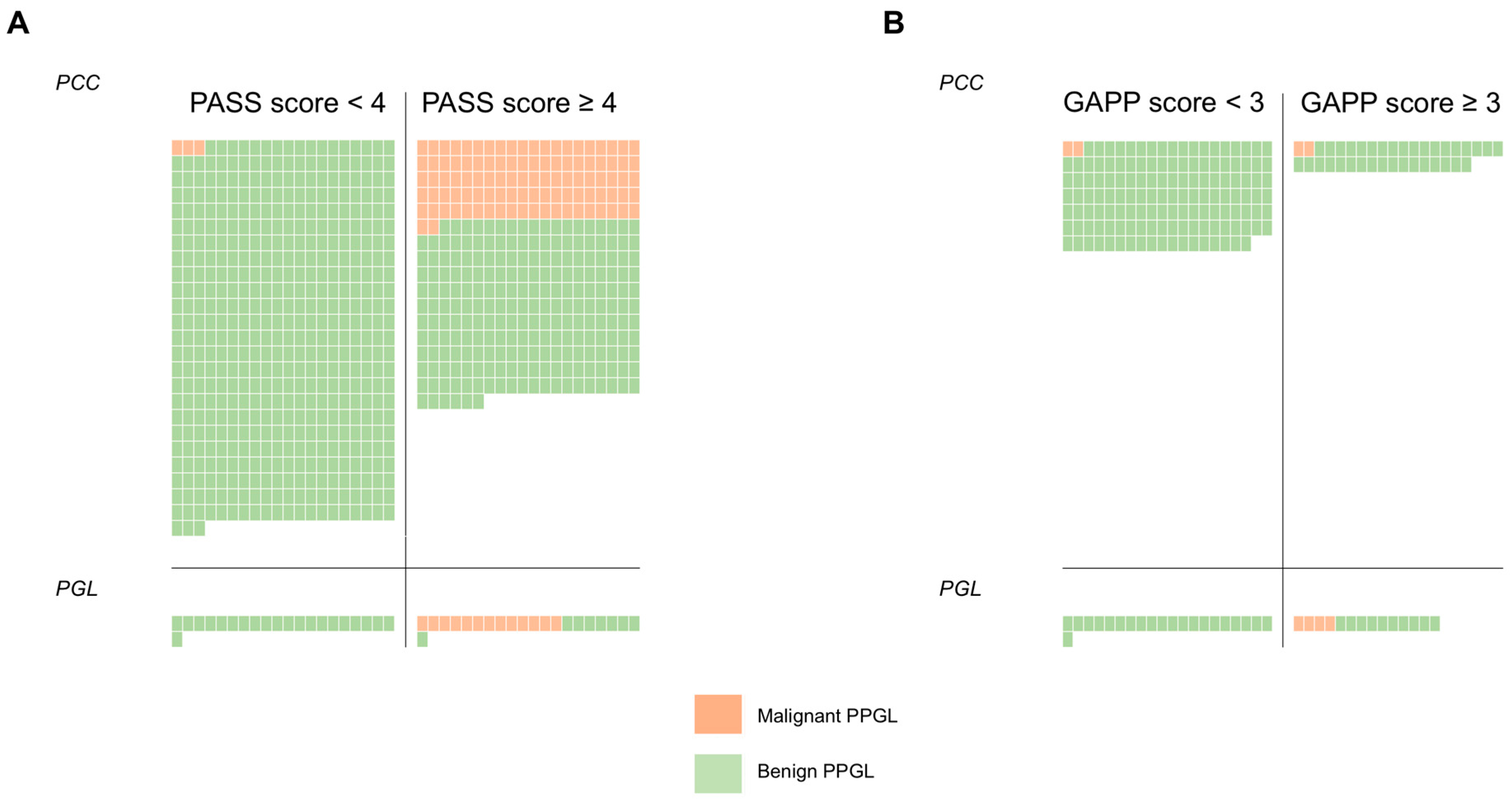
3.1. The PASS Algorithm: Study Selection
3.2. The PASS Algorithm: Meta-Analysis
3.3. The GAPP Algorithm: Study Selection and Meta-Analysis
3.4. Molecular Markers of Malignancy in PPGLs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, L.D.R. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- August, C.; August, K.; Schroeder, S.; Bahn, H.; Hinze, R.; Baba, H.A.; Kersting, C.; Buerger, H. CGH and CD 44/MIB-1 immunohistochemistry are helpful to distinguish metastasized from nonmetastasized sporadic pheochromocytomas. Mod. Pathol. 2004, 17, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajor, M.; Ziaja, J.; Lange, D.; Król, R.; Ciupińska-Kajor, M.; Turska-d’Amico, M.; Maka, B.; Cierpka, L. Analysis of morphology of adrenal pheochromocytoma as regards their potential malignancy. Endokrynol Pol. 2005, 56, 911–916. [Google Scholar] [PubMed]
- Gao, B.; Meng, F.; Bian, W.; Chen, J.; Zhao, H.; Ma, G.; Shi, B.; Zhang, J.; Liu, Y.; Xu, Z. Development and validation of pheochromocytoma of the adrenal gland scaled score for predicting malignant pheochromocytomas. Urology 2006, 68, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bílek, R.; Safarík, L.; Ciprová, V.; Vlcek, P.; Lisá, L. Chromogranin A, a member of neuroendocrine secretory proteins as a selective marker for laboratory diagnosis of pheochromocytoma. Physiol Res 2008, 57 (Suppl. 1), S171–S179. [Google Scholar] [PubMed]
- Strong, V.E.; Kennedy, T.; Al-Ahmadie, H.; Tang, L.; Coleman, J.; Fong, Y.; Brennan, M.; Ghossein, R.A. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery 2008, 143, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tischler, A.S.; Lloyd, R.V.; DeLellis, R.A.; de Krijger, R.; van Nederveen, F.; Nosé, V. Observer variation in the application of the Pheochromocytoma of the Adrenal Gland Scaled Score. Am. J. Surg. Pathol. 2009, 33, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Abdullah, Z.; Kazmi, S.M.B.; Kousparos, G. Pheochromocytomas, PASS, and immunohistochemistry. Horm. Metab. Res. 2009, 41, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mehrotra, P.K.; Jain, M.; Gupta, S.K.; Mishra, A.; Chand, G.; Agarwal, G.; Verma, A.K.; Mishra, S.K.; Singh, U. Size of the tumor and pheochromocytoma of the adrenal gland scaled score (PASS): can they predict malignancy? World J. Surg. 2010, 34, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Szalat, A.; Fraenkel, M.; Doviner, V.; Salmon, A.; Gross, D.J. Malignant pheochromocytoma: predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine 2011, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- de Wailly, P.; Oragano, L.; Radé, F.; Beaulieu, A.; Arnault, V.; Levillain, P.; Kraimps, J.L. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch. Surg. 2012, 397, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Białas, M.; Dyduch, G.; Szpor, J.; Demczuk, S.; Okoń, K. Microvascular density and mast cells in benign and malignant pheochromocytomas. Pol. J. Pathol. 2012, 63, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, R.; Kostadinova-Kunovska, S.; Bogoeva, B.; Spasevska, L.; Petrusevska, G. Histological features, Ki-67 and Bcl-2 immunohistochemical expression and their correlation with the aggressiveness of pheochromocytomas. Prilozi 2012, 33, 23–40. [Google Scholar] [PubMed]
- Mlika, M.; Kourda, N.; Zorgati, M.M.; Bahri, S.; Ben Ammar, S.; Zermani, R. Prognostic value of Pheochromocytoma of the Adrenal Gland Scaled Score (Pass score) tests to separate benign from malignant neoplasms. Tunis Med. 2013, 91, 209–215. [Google Scholar] [PubMed]
- Białas, M.; Okoń, K.; Dyduch, G.; Ciesielska-Milian, K.; Buziak, M.; Hubalewska-Dydejczyk, A.; Sobrinho-Simoes, M. Neuroendocrine markers and sustentacular cell count in benign and malignant pheochromocytomas - a comparative study. Pol. J. Pathol. 2013, 64, 129–135. [Google Scholar] [CrossRef]
- Ocal, I.; Avci, A.; Cakalagaoglu, F.; Can, H. Lack of correlations among histopathological parameters, Ki-67 proliferation index and prognosis in pheochromocytoma patients. Asian Pac. J. Cancer Prev. 2014, 15, 1751–1755. [Google Scholar] [CrossRef]
- Pędziwiatr, M.; Wierdak, M.; Natkaniec, M.; Matłok, M.; Białas, M.; Major, P.; Budzyński, P.; Hubalewska-Dydejczyk, A.; Budzyński, A. Laparoscopic transperitoneal lateral adrenalectomy for malignant and potentially malignant adrenal tumours. BMC Surg. 2015, 15, 101. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Khandeparkar, S.G.S.; Deshmukh, S.D.; Karekar, R.R.; Gaopande, V.L.; Joshi, A.R.; Kesari, M.V.; Shelke, R.R. Risk Stratification in Paragangliomas with PASS (Pheochromocytoma of the Adrenal Gland Scaled Score) and Immunohistochemical Markers. J. Clin. Diagn. Res. 2016, 10, EC01–EC04. [Google Scholar] [CrossRef]
- Lupşan, N.; Resiga, L.; Boşca, A.B.; Georgiu, C.; Crişan, D.; Mirescu, C.; Constantin, A.M.; Şimon, I.; Şovrea, A.S. Diagnostic reevaluation of 17 cases of pheochromocytoma—A retrospective study. Rom. J. Morphol. Embryol. 2016, 57, 651–661. [Google Scholar]
- Suenaga, S.; Ichiyanagi, O.; Ito, H.; Naito, S.; Kato, T.; Nagaoka, A.; Kato, T.; Yamakawa, M.; Obara, Y.; Tsuchiya, N. Expression of Extracellular Signal-regulated Kinase 5 and Ankyrin Repeat Domain 1 in Composite Pheochromocytoma and Ganglioneuroblastoma Detected Incidentally in the Adult Adrenal Gland. Int. Med. 2016, 55, 3611–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Kim, J.H.; Hong, A.R.; Seong, M.-W.; Lee, K.E.; Kim, S.-J.; Kim, S.W.; Shin, C.S.; Kim, S.Y. Disentangling of Malignancy from Benign Pheochromocytomas/Paragangliomas. PLoS ONE 2016, 11, e0168413. [Google Scholar] [CrossRef] [PubMed]
- Maignan, A.; Guerin, C.; Julliard, V.; Paladino, N.-C.; Kim, E.; Roche, P.; Castinetti, F.; Essamet, W.; Mancini, J.; Imperiale, A.; et al. Implications of SDHB genetic testing in patients with sporadic pheochromocytoma. Langenbecks Arch. Surg. 2017, 402, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-M.; Ahn, S.H.; Kim, H.; Kim, B.-J.; Sung, T.-Y.; Kim, Y.H.; Hong, S.J.; Song, D.E.; Lee, S.H. Validation of pathological grading systems for predicting metastatic potential in pheochromocytoma and paraganglioma. PLoS ONE 2017, 12, e0187398. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, C.; Nixon, A.M.; Parianos, C.; Vletsis, G.; Papanastasiou, L.; Markou, A.; Kounadi, T.; Piaditis, G.; Zografos, G.N. Surgery for pheochromocytoma: A 20-year experience of a single institution. Hormones 2017, 16, 388–395. [Google Scholar] [PubMed]
- Stenman, A.; Zedenius, J.; Juhlin, C.C. Over-diagnosis of potential malignant behavior in MEN 2A-associated pheochromocytomas using the PASS and GAPP algorithms. Langenbecks Arch. Surg. 2018, 403, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Muchuweti, D.; Muguti, E.G.; Mbuwayesango, B.A.; Mungazi, S.G.; Makunike-Mutasa, R. Diagnostic and surgical challenges of a giant pheochromocytoma in a resource limited setting-A case report. Int. J. Surg. Case Rep. 2018, 50, 111–115. [Google Scholar] [CrossRef]
- Konosu-Fukaya, S.; Omata, K.; Tezuka, Y.; Ono, Y.; Aoyama, Y.; Satoh, F.; Fujishima, F.; Sasano, H.; Nakamura, Y. Catecholamine-Synthesizing Enzymes in Pheochromocytoma and Extraadrenal Paraganglioma. Endocr. Pathol. 2018, 29, 302–309. [Google Scholar] [CrossRef]
- Stenman, A.; Svahn, F.; Hojjat-Farsangi, M.; Zedenius, J.; Söderkvist, P.; Gimm, O.; Larsson, C.; Juhlin, C.C. Molecular Profiling of Pheochromocytoma and Abdominal Paraganglioma Stratified by the PASS Algorithm Reveals Chromogranin B as Associated with Histologic Prediction of Malignant Behavior. Am. J. Surg. Pathol. 2018. [CrossRef]
- Kim, S.; Han, H.-S.; Choi, Y.; Yoon, Y.-S.; Cho, J.Y. Laparoscopic removal of retroperitoneal tumor with maneuver of hanging inferior vena cava. Surg. Endosc. 2018, 32, 3401. [Google Scholar] [CrossRef]
- Rasquin, L.; Prater, J.; Mayrin, J.; Minimo, C. Simultaneous Pheochromocytoma, Paraganglioma, and Papillary Thyroid Carcinoma without Known Mutation. Case Rep. Endocrinol. 2018, 2018, 6358485. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zhang, J.; Rivera, M.; Erickson, L.A. Urinary Bladder Paragangliomas: Analysis of Succinate Dehydrogenase and Outcome. Endocr. Pathol. 2016, 27, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L.M. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Pacak, K. AACE Adrenal Scientific Committee Precision Medicine: An Update on Genotype/Biochemical Phenotype Relationships in Pheochromocytoma/Paraganglioma Patients. Endocr Pract. 2017, 23, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libé, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crona, J.; Taïeb, D.; Pacak, K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017, 38, 489–515. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Brown, T.C.; Juhlin, C.C.; Andreasson, A.; Wang, N.; Bäckdahl, M.; Healy, J.M.; Prasad, M.L.; Korah, R.; Carling, T.; et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr. Relat. Cancer 2014, 21, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwight, T.; Flynn, A.; Amarasinghe, K.; Benn, D.E.; Lupat, R.; Li, J.; Cameron, D.L.; Hogg, A.; Balachander, S.; Candiloro, I.L.M.; et al. TERT structural rearrangements in metastatic pheochromocytomas. Endocr. Relat. Cancer 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Job, S.; Draskovic, I.; Burnichon, N.; Buffet, A.; Cros, J.; Lépine, C.; Venisse, A.; Robidel, E.; Verkarre, V.; Meatchi, T.; et al. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2019, 25, 760–770. [Google Scholar] [CrossRef]
- Oudijk, L.; Papathomas, T.; de Krijger, R.; Korpershoek, E.; Gimenez-Roqueplo, A.-P.; Favier, J.; Canu, L.; Mannelli, M.; Rapa, I.; Currás-Freixes, M.; et al. The mTORC1 Complex Is Significantly Overactivated in SDHX-Mutated Paragangliomas. Neuroendocrinology 2017, 105, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Chaux, A.; Brimo, F.; Gonzalez-Roibon, N.; Shah, S.; Schultz, L.; Rizk, J.-M.; Argani, P.; Hicks, J.; Netto, G.J. Immunohistochemical evidence of dysregulation of the mammalian target of rapamycin pathway in primary and metastatic pheochromocytomas. Urology 2012, 80, 736.e7-12. [Google Scholar] [CrossRef]
- Murali, R.; Hughes, M.T.; Fitzgerald, P.; Thompson, J.F.; Scolyer, R.A. Interobserver variation in the histopathologic reporting of key prognostic parameters, particularly clark level, affects pathologic staging of primary cutaneous melanoma. Ann. Surg. 2009, 249, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Netto, G.J.; Eisenberger, M.; Epstein, J.I. TAX 3501 Trial Investigators Interobserver variability in histologic evaluation of radical prostatectomy between central and local pathologists: findings of TAX 3501 multinational clinical trial. Urology 2011, 77, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Sloane, J.P.; Amendoeira, I.; Apostolikas, N.; Bellocq, J.P.; Bianchi, S.; Boecker, W.; Bussolati, G.; Coleman, D.; Connolly, C.E.; et al. Causes of inconsistency in diagnosing and classifying intraductal proliferations of the breast. European Commission Working Group on Breast Screening Pathology. Eur. J. Cancer 2000, 36, 1769–1772. [Google Scholar] [CrossRef]

| Study No. | First Author | Year Published | Number of PCCs * | Number of Malignant PCCs * | Definition of Malignant PCCs | Mal PCCs PASS ≥ 4 | Mal PCCs PASS < 4 | Benign PCCs PASS ≥ 4 | Benign PCCs PASS < 4 | SENS | SPEC | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Thompson | 2002 | 100 | 33 | MET | 33 | 0 | 17 | 50 | 100% | 75% | 66% | 100% |
| 2 | August | 2004 | 37 | 14 | MET | 14 | 0 | 23 | 0 | 100% | 0% | 38% | 0% |
| 3 | Kajor | 2005 | 40 | 1 | MET | 1 | 0 | 7 | 32 | 100% | 82% | 13% | 100% |
| 4 | Strong | 2008 | 47 | 5 | MET | 5 | 0 | 10 | 32 | 100% | 76% | 33% | 100% |
| 5 | Agarwal | 2010 | 90 | 6 | MET/DO | 5 | 1 | 27 | 57 | 83% | 68% | 16% | 98% |
| 6 | Szalat | 2010 | 26 | 7 | MET | 6 | 1 | 0 | 19 | 86% | 100% | 100% | 95% |
| 7 | de Wailly | 2012 | 21 | 7 | MET | 7 | 0 | 7 | 7 | 100% | 50% | 50% | 100% |
| 8 | Mlika | 2013 | 11 | 2 | MET | 2 | 0 | 6 | 3 | 100% | 33% | 25% | 100% |
| 9 | Bialas | 2013 | 62 | 5 | REC/MET | 5 | 0 | 29 | 28 | 100% | 49% | 15% | 100% |
| 10 | Ocal | 2014 | 11 | 3 | REC | 3 | 0 | 4 | 4 | 100% | 50% | 43% | 100% |
| 11 | Kulkarni | 2016 | 6 | 1 | MET | 1 | 0 | 2 | 3 | 100% | 60% | 33% | 100% |
| 12 | Lupşan | 2016 | 17 | 13 | MET | 13 | 0 | 2 | 2 | 100% | 50% | 87% | 100% |
| 13 | Suenaga | 2016 | 1 | 0 | REC | 0 | 0 | 1 | 0 | npd | npd | npd | npd |
| 14 | Kim | 2016 | 90 | ns | REC/MET | npd | 0 | npd | 52 | npd | npd | npd | npd |
| 15 | Maignan | 2017 | 65 | 0 | MET | 0 | 0 | 9 | 56 | npd | 86% | npd | npd |
| 16 | Koh | 2017 | 32 | 4 | MET | 3 | 1 | 19 | 9 | 75% | 32% | 14% | 90% |
| 17 | Aggeli | 2017 | 69 | 0 | MET | ns | ns | 31 | 37 | npd | 54% | npd | npd |
| 18 | Stenman | 2018 | 41 | 0 | REC/MET | 0 | 0 | 10 | 31 | npd | 76% | npd | npd |
| 19 | Muchuweti | 2018 | 1 | 0 | MET | 0 | 0 | 1 | 0 | npd | npd | npd | npd |
| 20 | Stenman | 2018 | 81 | 4 | REC/MET | 4 | 0 | 19 | 58 | 100% | 75% | 17% | 100% |
| Summarized | - | 848 | 105 | - | 102 | 3 | 224 | 480 | 97% | 68% | 31% | 99% | |
| Study No. | First Author | Year Published | Number of PGLs * | Number of Malignant PGLs * | Definition of Malignant PGLs | Mal PGLs PASS ≥ 4 | Mal PGLs PASS < 4 | Benign PGLs PASS ≥ 4 | Benign PGLs PASS < 4 | SENS | SPEC | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | August | 2004 | 6 | 6 | MET | 6 | 0 | 0 | 0 | 100% | - | 100% | - |
| 2 | Szalat | 2010 | 1 | 1 | MET | 1 | 0 | 0 | 0 | 100% | - | 100% | - |
| 3 | Kulkarni | 2016 | 4 | 2 | MET | 2 | 0 | 0 | 2 | 100% | 100% | 100% | 100% |
| 4 | Kim | 2016 | 29 | 16 | REC/MET | npd | 0 | npd | 15 | npd | npd | npd | npd |
| 5 | Koh | 2017 | 5 | 0 | MET | 0 | 0 | 3 | 2 | npd | npd | npd | npd |
| 6 | Stenman | 2018 | 11 | 4 | REC/MET | 4 | 0 | 5 | 2 | 100% | 29% | 44% | 100% |
| Summarized | - | 56 | 29 | - | 13 | 0 | 8 | 21 | 100% | 72% | 62% | 100% | |
| Study No. | First Author (Year Published) | Number of PCCs | Number of Malignant PCCs * | Definition of Malignant PCCs * | Mal PCCs GAPP ≥ 3 | Mal PCCs GAPP < 3 | Benign PCCs GAPP ≥ 3 | Benign PCCs GAPP < 3 | SENS | SPEC | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. PCC cohorts stratified by the GAPP algorithm. | ||||||||||||
| 1 | Kimura (2014) | 126 | 24 | MET | npd | npd | 0 | 102 | npd | npd | npd | npd |
| 2 | Koh (2017) | 32 | 4 | MET | 2 | 2 | 19 | 9 | 50% | 32% | 10% | 82% |
| 3 | Stenman (2018) | 41 | 0 | REC/MET | 0 | 0 | 16 | 25 | npd | 61% | npd | npd |
| Summarized | - | 199 | 28 | - | 2 | 2 | 35 | 136 | 50% | 80% | 5% | 99% |
| B. PGL cohorts stratified by the GAPP algorithm. | ||||||||||||
| 1 | Kimura (2014) | 36 | 16 | MET | npd | npd | 0 | 20 | npd | npd | npd | npd |
| 2 | Gupta (2016) | 10 | 4 | MET | 4 | 0 | 6 | 0 | 100% | 0% | 40% | npd |
| 3 | Koh (2017) | 5 | 0 | MET | 0 | 0 | 4 | 1 | npd | 20% | npd | npd |
| Summarized | - | 51 | 20 | - | 4 | 0 | 10 | 21 | 100% | 68% | 29% | 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenman, A.; Zedenius, J.; Juhlin, C.C. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas—A Meta-Analysis and Systematic Review of the Literature. Cancers 2019, 11, 225. https://doi.org/10.3390/cancers11020225
Stenman A, Zedenius J, Juhlin CC. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas—A Meta-Analysis and Systematic Review of the Literature. Cancers. 2019; 11(2):225. https://doi.org/10.3390/cancers11020225
Chicago/Turabian StyleStenman, Adam, Jan Zedenius, and Carl Christofer Juhlin. 2019. "The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas—A Meta-Analysis and Systematic Review of the Literature" Cancers 11, no. 2: 225. https://doi.org/10.3390/cancers11020225





